Abstract
Pure tetragonal and monoclinic phases BiVO4 were prepared from aqueous Bi (NO3)3 and NaVO3 solutions by a rapid microwave-assisted method that employed accurate controlling of microwave irradiation time and power. The highly crystalline phase converted irreversibly from tetragonal to monoclinic BiVO4 with gradually elongated irradiation time gradually, which is further proved by X-ray diffraction, UV–vis and Raman measurements. These variations of phase structures led to different photocatalytic properties under visible light.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bismuth (III) vanadate (BiVO4) is a material that has recently attracted considerable attention for its exhibiting various interesting technological properties, such as ferroelasticity (David et al. 1983; Bierlein et al. 1975; Lu et al. 1986), ionic conductivity (Harota et al. 1992), and pigmentation (Wienand et al. 1998). Recently, the visible-light-responsive property of BiVO4 makes it worthy of consideration as an advanced material for photocatalytic applications (Kudo et al. 1999).
BiVO4 appears in three main crystalline forms: tetragonal zircon, monoclinic scheelite, and tetragonal scheelite structures. It was reported that tetragonal BiVO4 with a 2.9 eV band gap mainly possessing a UV absorption band, while monoclinic BiVO4 with a 2.4 eV band gap had a characteristic visible light absorption band besides the UV band (Kudo et al. 1999; Bhattacharya et al. 1997). Thus, studying the relationship between some properties and different BiVO4 phases is of interest. Over the last few years, it was reported that tetragonal BiVO4 was prepared by a precipitation process from a Bi (NO3)3 nitric acid solution and an aqueous NH4VO3 solution at room temperature (Bhattacharya et al. 1997). Meanwhile, monoclinic BiVO4 usually was obtained by solid state and melting reactions at a high temperature (Lim et al. 1995; Roth et al. 1963; Sleight et al. 1979), and hydrothermal method (Liu et al. 2004). Even more, tetragonal phase can transform into monoclinic at higher temperature by solid state method (Bierlein et al. 1975). Also, the phase transition has been studied by an aqueous process (Kudo et al. 1999) and precipitation method (Tokunaga et al. 2001). However, these techniques need long reaction time, high temperature treatment and complicated processing.
There is a considerable interest in evaluating new methods for the synthesis of very fine BiVO4 powders with high purity homogeneous and ultrafine size, which can improve their photocatalytic properties, regarded as not only the photocatalytic degradation organic compounds, but also photocatalytic O2 evolution from aqueous AgNO3 solution under visible light. Thus, an efficient, simple, and low temperature processing is required to synthesize high quality BiVO4 powders. Microwave-assisted reactions are generally very fast and often occur at lower temperatures than the corresponding reactions in conventional heating procedures.
In this paper, microwave-assisted aqueous process generating phase controlled BiVO4 powders is studied. The properties of the synthesized BiVO4 powders were investigated by X-ray diffraction (XRD), UV–vis, Raman spectroscopies, and transmission electron microscopy (TEM). Meanwhile, the photocatalytic performances of the synthesized BiVO4 powders were also studied.
Experiment
Synthesis
The vanadium compound, NaVO3 stock solution, was used as vanadium source in this study. The NaVO3 stock solution was prepared by stirring stoichiometric amount of V2O5 and NaOH in distilled water for 4 h while heating according to Na/V mole ratio being 1. Aqueous solutions of Bi (NO3)3 · 5H2O dissolved in concentrated HNO3 and NaVO3 were prepared, respectively according to Bi/V molar ratio of 1. Then, cetyltrimethyl ammonium bromide (CTAB) was added to both of the above solutions by the concentration of 9.2 × 10−4 mol/L. After stirring the solutions for 10 min, the solutions were mixed together respectively. During the mixing, the clear solutions would immediately turn to an intensive orange–yellow colour. The pH value was measured as 1. Then, the beaker was placed in the center of a 2,450 MHz microwave oven (Galanz WP 700, 700 W) and was irradiated for 10, 20, 30, 35 and 40 min with power 17% (denoted to sample A, B, C, D and E). After irradiation, the samples were washed by water, filtered, and dried at 350 K for 12 h subsequently, respectively. In a similar way, the BiVO4 powders were also obtained under microwave irradiation for 10 min with 37% and 40% power export, respectively. In addition, it was observed that the solution mixtures were boiling after microwave irradiation for 15 min, which proved that the macroscopic temperature of the mixtures kept unchanged at about 100 °C in the further course of microwave treatment. Therefore, in comparison with the microwave method, the sample was also prepared by the traditional heating method that heated at 100 °C for 40 min.
Characterization
The produced phases were identified by X-ray diffraction (XRD, Germany Brucker D8-advance, Cu Kα radiation). The UV–vis diffuse reflectance spectroscopy (DRS) studies were conducted by using a UV–vis–NIR spectrophotometer (Shimadzu UV-3101PC). Raman spectroscopy measurements were carried out on a Spex 1403 Raman Spectrometer under backscattering geometry. Excitation was taken as the 488 nm line of Ar+ laser. The morphology and particle sizes were determined by Transmission Electron Microscope (TEM, JEOL Ltd. JEM–2010).
Measurements of the photocatalytic properties
The photocatalytic properties of the powders were tested in our house-made instruments. At first, photocatalytic activities of the BiVO4 powders were evaluated by degradation of N, N, N′, N′-tetraethylated rhodamine (RB) under the visible light irradiation (300 W–Xe). An aqueous BiVO4 dispersion was prepared by adding 0.5 g BiVO4 powders with different phases and components into a 100 mL RB solution (2.0 × 10−4 mol dm−3), adding aqueous silver nitrate solutions (AgNO3) (0.05 mol/L) as the load, respectively. The solution was magnetically stirred and irradiated by visible light. During irradiation process for a designated time, the colour of RB solution turned lighter and lighter, and the RB absorption concentration was determined using the 7205 UV–vis spectrometer (Shanghai Xinmao Ltd.).
The photocatalytic O2 evolution of the BiVO4 powders was also evaluated under the visible light irradiation (300 W–Xe). An aqueous BiVO4 dispersion was prepared by adding 0.5 g BiVO4 powder with different phases and components into 150 mL AgNO3 (0. 05 mol/L) as the load, respectively. The solution was magnetically stirred and irradiated by visible light. During irradiation for a designated time, the amount of O2 evolution increased, and the amount of O2 evolution was determined using GC-14C (SHIMADZU)
Results and discussion
Controlled phase transition
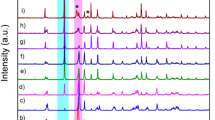
To investigate the formation process of BiVO4 powders, a detailed time course experiment was carried out with power 17% (120 W). Figure 1 shows the XRD patterns of the as-prepared BiVO4 powders obtained over different duration of microwave irradiation. It can be seen that the entire observed diffraction peaks for sample A obtained after microwave irradiation for 10 min match with those of tetragonal BiVO4 (JCPDS card No. 14-0133). No peaks of any other phases or impurities were detected. As the microwave irradiation treatment was prolonged, the peaks corresponding to monoclinic BiVO4 (JCPDS card No. 14-0688) appeared and became more and more dominant, and the peaks for tetragonal BiVO4 gradually disappeared (sample B, C and D). After a reaction time of 40 min, all the peaks in the XRD pattern were indexed to pure monoclinic BiVO4, while the tetragonal BiVO4 peaks were disappeared completely (sample E). The percentile conversion of tetragonal to monoclinic form on irradiation time has been calculated from the normalized ratios of peak area corresponding to (121) peak of monoclinic BiVO4 and that of (200) peak for tetragonal form in the manner of, i.e. αmono = Amono(121)/(Amono(121) + Atetra(200)). The relationship between microwave irradiation time, temperature and the calculated fraction of monoclinic phase transformed are shown in Table 1. It was found that the as-synthesized monophasic tetragonal BiVO4 underwent a phase transition and converted to its monoclinic form over a wide microwave irradiation time from 10 min to 40 min. And longer irradiation of the same sample for 40 min extended the conversion to as much as 100%. From these phenomena, it could be concluded that the precursor first crystallized in the form of tetragonal BiVO4 powders, then transformed to monoclinic BiVO4 gradually through microwave irradiation. Further more, the preparative route employed in the present investigation to produce BiVO4 was found to be sensitive to the reaction time. Therefore, the irradiation time of the microwave-assisted synthesis was clearly an important factor for controlling the phase structure of BiVO4 powder.
In comparison with other soft chemical synthetic methods (Wienand et al. 1998; Kudo et al. 1999; Bhattacharya et al. 1997; Lim et al. 1995; Roth et al. 1963; Sleight et al. 1979; Liu et al. 2004; Tokunaga et al. 2001), this microwave irradiation method was a rapidly process that synthesized both tetragonal and monoclinic BiVO4, and could control the phase transition conveniently only by changing the time of synthesis. The processing time was basically much shorter than the corresponding records reported by studies through other different methods (Wienand et al. 1998; Kudo et al. 1999; Bhattacharya et al. 1997; Lim et al. 1995; Roth et al. 1963; Sleight et al. 1979; Liu et al. 2004; Tokunaga et al. 2001). In order to understand the effect of microwave irradiation, a sample was prepared by traditional heating method, which heated at 100 °C for 40 min. As seen from Fig. 1, bismuth oxide hydroxide nitrate hydrate (Bi6O5(OH)3(NO3)5(H2O)3, JCPDS card No. 70-1226) and vanadium oxide (V2O5, 76-1803) mixtures were obtained by using the traditional heating method. However, the clinobisvanaite feature was observed in the XRD pattern for those synthesized by microwave irradiation for 40 min at almost the same temperature as that in the traditional way. In a word, it was proved that the microwave treatment led to acceleration of synthesizing the pure BiVO4 powders.
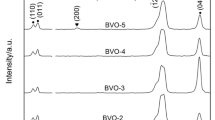
Figure 2 shows the XRD patterns of the BiVO4 powders as-prepared at different microwave irradiation powers. It can be seen that all the diffraction peaks of sample obtained after microwave irradiation for 10 min at 120 W can be indexed to tetragonal BiVO4. As microwave irradiation power was enhanced to a higher magnitude, such as 260 W and even higher power, 280 W, the peaks ascribed to monoclinic BiVO4 can be seen along with the peaks representing tetragonal BiVO4. The percentage of monoclinic phase was calculated by the same formula as above. The 9% of monoclinic could be obtained at 260 W, while it would increase to 18% of monoclinic at 280 W under other similar conditions. The ratio of monoclinic phase BiVO4 is increasing gradually with the powers enhancing. Thus, it would be concluded that the applied microwave powers also do affect the phase structure of BiVO4 powders synthesized by microwave irradiation except the influence of irradiation time.
Photophysical properties of BiVO4 samples
The whole UV–vis DRS spectra of BiVO4 samples prepared for various time are shown in Fig. 3. As can be seen from the figure, the absorption edges of BiVO4 samples (Samples A–E) somehow varied in an orderly fashion. Different compositions of BiVO4 powders result in different band gaps. With the phase transition from tetragonal to monoclinic phase, the band gaps tend to shift from UV region to the visible light region. The band gaps of sample A, B, C, D and E were estimated from the absorption edges to be 2.90 eV, 2.77 eV, 2.64 eV, 2.53 eV and 2.4 eV, respectively. These data clearly demonstrate that the electronic structures of BiVO4 are also changed with the phase transition process which can be controlled by the duration of microwave irradiation.
Raman spectra of BiVO4 samples
Raman spectroscopy is a useful technique for probing the local structure of materials. Figure 4 shows the Raman spectra over the 100 cm−1–1,000 cm−1 region for the series of BiVO4 samples list in above in Table 1. For sample A, in accordance with the interpretation of Hardcastle et al. (1991), in the 750 cm−1–1,000 cm−1 region, the Raman spectrum of tetragonal phase BiVO4 shows two intense bands at 850 cm−1 and 770 cm−1, respectively, which separately attributes to the symmetric V–O stretching mode (Ag symmetry) and the antisymmetric V–O stretching mode (Bg symmetry), similarly to the report (Frost et al. 2006). Compared with the Raman spectrum of tetragonal YVO4 (Sun et al. 2006), that of the tetragonal BiVO4 shows band at 380 cm−1 which is attributed to the O–V–O bending mode (Ag). And the band at 245 cm−1 can be assigned to the Bi–O stretching mode (Eg). With the increasing percentage of monoclinic phase in BiVO4 powders (sample A–E), the Raman bands shift regularly, due to the different bond structure of V–O and Bi–O in monoclinic and tetragonal phases. During the phase transition process from tetragonal to monoclinic BiVO4 (sample B–E), some Raman bands appear, such as 120 cm−1, 208 cm−1, 330 cm−1, 365 cm−1 and 826 cm−1, which are characteristic bands of monoclinic. Meanwhile, the Raman bands of tetragonal phase are weakened gradually. The spectrum of pure monoclinic BiVO4 (sample E) shows a single band at around 826 cm−1 that is normally attributed to the symmetric V–O stretching mode (Ag symmetry). And the bands at around 365 cm−1 and 333 cm−1 are described as the symmetric V–O (Ag) bending mode and antisymmetric V–O (Bg) bending mode of VO4 units, respectively. The external modes (rotation/translation) occur at 208 cm−1 and 120 cm−1. These Raman results provide further evidence that the local structure transition of samples is taken place during the microwave irradiation.
From Fig. 4, it is noticed that the intensity of Raman band of monoclinic at around 826 cm−1 is not stronger than that of tetragonal at around 850 cm−1, although the percentage of monoclinic phase in sample C is 63%, which is even more than tetragonal. We can assume that the tetragonal bonds may be more sensitive than monoclinic.
Morphology and microstructure of the BiVO4 samples
The TEM images of pure tetragonal and monoclinic BiVO4 samples prepared by microwave assisted process are shown in Fig. 5. Some significant differences are observed concerning the morphologies and particle sizes of the BiVO4 samples. The pure tetragonal BiVO4 (sample A) particles, synthesized by microwave irradiation for 10 min at 120 W, demonstrate small particle sizes. At low magnification (Fig. 5a), it is observed that the sample processes a large number of polydisperse nanoparticles. Furthermore, at higher magnification (Fig. 5b), each nanoparticle, with diameters of around 10–20 nm is clearly presented in the micrograph.
The monoclinic BiVO4 (sample E), synthesized by microwave irradiation for 40 min at 120W, appears highly crystallized. At low magnification (Fig. 5c), large sheets (about 1–2 μm in size) and some strips are visible. At higher magnification, the nanostrips can be seen in Fig. 5d, normally 10–30 nm wide and 2–3 μm long. Careful observation shows that these nanostrips aggregate compactly together in parallel lines information.
More details about the structure of a nanostrip captured from a monoclinic BiVO4 (sample E) are investigated by the selected-area electron diffraction (SAED) pattern. The corresponding SAED pattern of a single nanostrip (Fig. 5e) shows that the nanostrip is a single crystal of monoclinic BiVO4, as the highly arrayed diffraction spots in the pattern reveal its single-crystalline nature of the monoclinic BiVO4 nanostrips. In addition, Fig. 5f shows the high-resolution transmission electron microscope (HR-TEM) image taken from the nanostrip of the individual monoclinic BiVO4 (sample E) shown in Fig. 5e. The clear lattice fringes are visible, and the regular spacing of the observed lattice interplanar is about 0.58542 nm, which is consistent with the (002) lattice spacing of monoclinic BiVO4, showing that the nanostrip is of a uniform crystal structure. However, the obvious difference in contrast and the defects at the light-colored area can be clearly observed, which might result from an oriented-attachment growth mode of the nanoplates (Kudo et al. 1999).
Photocatalytic properties of BiVO4 samples
The photocatalytic performance of BiVO4 powders synthesized by microwave irradiation for various time were examined in terms of the photodegradation of RB and the photocatalytic O2 evolution from an aqueous AgNO3 solution under visible-light irradiation (k ≥ 420 nm).
Figure 6 shows that the decrease in absorption intensity of RB, which has a strong absorption at 552 nm, by different BiVO4 catalysts as a function of visible irradiation time. The employed catalysts are BiVO4 powders heated over various microwave irradiation time, sample A, B, C, D and E, respectively. It is clear that the ratio of photodegradation with BiVO4 powders is increasing with the increasing content of monoclinic phase. Moreover, compared sample A with sample B, only a few amounts of monoclinic BiVO4 give great effect on photodegradation RB under visible light irradiation.
Photodegradation RB under visible light irradiation with the role of 0.5 g BiVO4 powders with 0.05 M aqueous AgNO3 under the same condition. (m) blank experiment, 0.05 M aqueous AgNO3 without BiVO4 solution under visible light irradiation; (a) photodegradation RB by sample A with 0.05 M aqueous AgNO3 solution; (b) sample B; (c) sample C; (d) sample D; (e) sample E
The photocatalytic activities of BiVO4 samples for O2 evolution under visible light irradiation are shown in Fig. 7. The amounts of O2 evolution with BiVO4 powders are increasing with the increasing contents of monoclinic phase. It has the same trend with the photodegradation of RB.
The obvious increase in the photocatalytic activity of BiVO4 may result in the variation in phase composition and particle size. The absorption band of monoclinic BiVO4 (sample E) in the visible light region was similar to that of tetragonal BiVO4 (sample A), but monoclinic BiVO4 (sample E) showed higher photocatalytic activity than tetragonal BiVO4. It is assumed that the high activity of monoclinic BiVO4 is probably due to the distortion of the Bi–O polyhedron (Tokunaga 2001), because the major difference in photocatalytic properties between monoclinic BiVO4 and tetragonal is only the distortion (Kudo et al. 1999). The distortion is attributed to 6s2 lone pairs of Bi3+, whose effects have been reported for some bismuth oxide compounds (Jakubowicz 1998; Giraud 1999). It is supposed that the difference in the distortion leads to the different properties. In the present researches, it was found that the photocatalytic activity would also change because of the variations of monoclinic and tetragonal phases, which was also reported by Kudo and so on (Kudo et al. 1999).
Conclusion
To sum up, the phase transition from tetragonal to monoclinic BiVO4 can be easily controlled by microwave irradiation method. With the time of microwave irradiation prolonging, the tetragonal phase transformed into monoclinic phase gradually. The XRD, Raman, TEM and UV–vis spectroscopic characterizations reveals that not only the macrostructure, such as the morphology, surface texture, and grain shape, but also the local structure of the synthesized BiVO4 materials was significantly dependent on the microwave irradiation time and power.
The photocataytic properties strongly depend on the phases and crystal forms. The photodegradation RB activity and the photocatalytic O2 evolution of monoclinic BiVO4 are much higher than that of tetragonal under visible light irradiation, because of their internal structure, such as band gap, the 6s2 lone pair of Bi3+, and so on.
References
Bhattacharya AK, Mallick KK, Hartridage A (1997) Phase transition in BiVO4. Mater Lett 30:7–13
Bierlein JD, Sleight AW (1975) Ferroelasticity in BiVO4. Solid State Commun 16:69–70
David WIF (1983) Ferroelastic phase transition in BiVO4: V. Temperature dependence of Bi3+ displacement and spontaneous strains. Solid State Phys 16:5127–5148
Frost RL, Henry DA, Weier ML, Martens W (2006) Raman spectroscopy of three polymorphs of BiVO4 clinobisvanite, dreyerite and pucherite, with comparisons to (VO4)3– bearing minerals namibite, pottsite and schumacherite. J Raman Spectrosc 37:722–732
Giraud S, Wignacourt JP, Drache M, Nowogrocki G, Steinfink H (1999) The Stereochemical effect of 6s2 lone-pair electrons: the crystal structure of a new lead Bismuth oxyphosphate Pb4BiO4PO4. J Solid State Chem 142:80–88
Hardcastle FD, Wachs IE, Eckert H, Jefferson DA (1991) Vanadium(V) environments in bismuth vanadates: a structural investigation using Raman spectroscopy and solid state 51V NMR. J Solid State Chem 90:194–210
Harota K, Komatsu G, Yamashita M, Takemura H, Yamaguchi O (1992) Formation, characterization and sintering of alkoxy-derived bismuth vanadate. Mater Res Bull 27:823–830
Jakubowicz N, Pe´rez O, Grebille D, Leligny H (1998) Bi3+electronic lone pair configuration in the modulated Bi-2212 type oxides. J Solid State Chem 139:194–199
Kudo A, Omori K, Kato H (1999) A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J Am Chem Soc 121:11459–11467
Lim AR, Choh SH, Jang MS (1995) Prominent ferroelastic domain walls in BiVO4 crystal. J Phys Condens Matter 7:7309–7323
Liu JB, Li KW, Wang H, Zhu MK, Yan H (2004) Hydrotheral preparation of BiVO4 powders. Chem Phys Lett 396:429–432
Lu T, Steele BCY (1986) Electrical conductivity of polycrystalline BiVO4 samples having the scheelite structure. Solid State Ionics 21:339–342
Rao KJ, Vaidhyanathan B, Ganguli M, Ramakrishman PA (1999) Synthesis of inorganic solids using microwaves. Chem Mater 11:882–895 (and references therein)
Roth RS, Waring JL (1963) Synthesis and stability of bismutotantalite, stibiotantalite and chemically similar ABO4 compounds. Am Mineral 48:1348–1356
Sleight AW, Chen H-y, Ferretti A, Cox DE (1979) Crystal growth and structure of BiVO4. Mater Res Bull 14:1571–1581
Sun YJ, Liu HJ, Wang X, Kong XG, Zhang H (2006) Optical spectroscopy and visible upconversion studies of YVO4:Er3+ nanocrystals synthesized by a hydrothermal process. Chem Mater 18:2726–2732
Tokunaga S, Kato H, Kudo A (2001) Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem Mater 13:4624–4628
Wienand H, Adel J (1998) In: Chapter 3—Pigments, Buxbaum G (ed) Industrial inorganic pigments. Wiley-VCH, Weinheim, 113 p
Acknowledgements
The authors appreciate National Natural Science Foundation of China (No. 50602002) and Doctoral Scientific Research Foundation of Beijing University of Technology (No. 52009011200503), for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H.M., Liu, J.B., Wang, H. et al. Rapid microwave-assisted synthesis of phase controlled BiVO4 nanocrystals and research on photocatalytic properties under visible light irradiation. J Nanopart Res 10, 767–774 (2008). https://doi.org/10.1007/s11051-007-9310-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-007-9310-y