Abstract
Allogeneic hematopoietic cell transplant (HCT) recipients are at increased risk of invasive fungal infections (IFI), which are associated with a high mortality rate. We evaluated the impact of IFI in allogeneic HCT patients. In total, 541 consecutive allogeneic HCT recipients were included. The cumulative incidence of any IFI and mold infections at 1-year post-HCT was 10 and 7%, respectively. Median times to IFI and mold infection were 200 and 210 days, respectively. There was a trend toward fewer IFI and mold infections in the last several years. Both acute graft-versus-host disease (GVHD) (OR 1.83, p = 0.05) and corticosteroid duration (OR 1.0, p = 0.026) were significantly associated with increased risk of IFI, acute GVHD (OR 2.3, p = 0.027) emerged as the most important association with mold infections. Any IFI [HR 4.1 (2.79–6.07), p < 0.0001] and mold infections [HR 3.34 (2.1–5.1), p < 0.0001] were independently associated with non-relapse mortality (NRM). This association persisted in the setting of both acute and chronic GVHD. Corticosteroid treatment for >90 days was also significantly associated with higher NRM [HR 1.9 (1.3–2.6), p < 0.0001]. This study highlights the impact of IFI on NRM among HCT patients. The decrease in number of IFI and mold infections over the last several years may reflect the benefit of prophylaxis with mold-active antifungal agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients undergoing allogeneic hematopoietic cell transplantation (HCT) are at high risk of invasive fungal infections (IFI), which are associated with a high mortality rate [1]. Over the last 3 decades, the use of fluconazole prophylaxis in this population has decreased the incidence of Candida infections, while invasive mold infections, such as invasive aspergillosis, mucormycosis and fusariosis have emerged as the major cause of IFI [2]. Newer generation antifungal agents active against Aspergillus and other molds have allowed more effective prophylactic and therapeutic strategies among HCT recipients [3]. However, universal anti-mold prophylaxis remains controversial and is not standardized [4, 5]. In 2008, the EORCT/MSG diagnostic criteria for IFI were updated to reflect advances in the field by introducing the nonculture-based test for galactomannan as a criterion for probable invasive aspergillosis [6]. In many institutions, nonculture-based tests have not been readily available until recently and they have not been routinely used.
We evaluated the incidence, risk factors and impact of IFI in a cohort of 541 patients who underwent allogeneic HCT from 2007 to 2012. During this time period, significant advances in the detection, prophylaxis and treatment of mold infections were introduced into clinical practice. In our institution, routine use of antifungal prophylaxis was codified and galactomannan assays were added to the algorithm for the diagnosis of invasive aspergillosis.
Methods
Patients
All adult patients ≥18 years-old who underwent allogeneic HCT with a peripheral blood stem cell or bone marrow graft at the University of Michigan Blood and Marrow Transplant (BMT) Program between January 2007 and December 2012 were included in this retrospective study and were followed for 2 years post-transplantation. This study was approved by the University of Michigan Institutional Review Board.
Transplantation
The majority of patients (n = 398, 74%) received a fludarabine-based preparative regimen, in most cases in combination with busulfan (n = 365, 67%). Other preparative regimens included busulfan with cyclophosphamide in 16 patients (3%), a combination of carmustine, etoposide, cytarabine, and melphalan in 10 (0.2%) and total body irradiation (1200 Gy) with cyclophosphamide in 40 (7.4%). All patients received antiviral prophylaxis with acyclovir from day 0 up to 1 year post-HCT and antibacterial prophylaxis with levofloxacin while neutropenic or on corticosteroids. There was no consistent, targeted antifungal prophylaxis until 2011. Before 2011, most patients received prophylaxis with either fluconazole or voriconazole, depending on transplant physician preference. Starting in 2011, patients undergoing fully matched HCT without graft-versus-host disease (GVHD) received fluconazole prophylaxis at a standard dose of 100 mg orally daily per institutional guidelines. HLA-mismatched patients and those who developed GVHD requiring systemic therapy received oral voriconazole or posaconazole (oral solution) prophylaxis until they were completely off immunosuppressive drugs.
Data Collection and Definitions
Demographic data were obtained from the University of Michigan BMT Database. Informatics assistance with data abstraction and the electronic medical record search engine (EMERSE) tool were provided by the University of Michigan Cancer Center’s Biomedical Informatics Core with partial support from the National Institutes of Health (NIH) Support Grant (CA46592).
We reviewed clinical, microbiological, pathological and radiological data on all patients who had an IFI. Each documented IFI within the first 2 years post-HCT was recorded and classified as proven, probable or possible according to EORTC-MSG criteria [6], and only those categorized as proven or probable were included in this analysis. When available, galactomannan measurement in bronchoalveolar lavage fluid was included as part of the diagnostic criteria for invasive aspergillosis.
Nadir and duration of absolute neutrophil count in this patient population was very homogenous and predictable based on underlying disease and chemotherapy received during conditioning prior to receiving HCT. Therefore, neutropenia was not expected to impact the rate of occurrence of IFI in these patients, and nadir and duration of neutropenia were not included in the analysis.
Acute GVHD was defined and scored according to Glucksberg criteria [7], and chronic GVHD was defined and scored according to NIH Consensus Criteria for clinical trials [8].
Corticosteroid duration was measured as the total number of days the patient was on high-dose corticosteroids (≥0.3 mg/kg prednisolone or equivalent for >3 weeks) for any reason within the first 2 years after HCT. In patients with steroid-refractory acute GVHD, second line therapy was chosen at the discretion of the treating physician. Patients were divided into 2 groups according to the duration of steroid therapy (<90 or >90 days) for survival analysis.
Statistical Methods
Pairwise comparisons were performed, in which the χ 2 or Fisher’s exact test was used for categorical variables and the Wilcoxon rank-sum test was used for continuous variables. Logistic regression was used to estimate associations of patient characteristics with development of IFI, while cumulative incidence methods were used to estimate associations of patient characteristics and infection with non-relapse mortality, for which relapse is a competing event. Statistical significance was defined as a p value less than 0.05. All computations were done with the statistical package R, version 3.0.1.
Results
Patient Characteristics
A total of 541 consecutive patients who underwent allogeneic HCT were included in the study (Table 1). The median age was 54 (range 18–73), and 322 (60%) were women. Fifty percent of the transplants were from related donors, and the source was peripheral blood stem cells in the vast majority of patients (n = 507, 94%). The underlying hematological diseases are summarized in Table 1.
Fungal Infections
Sixty-one patients (11.3%) had an IFI after allogeneic HCT, and 45 of those 61 patients had an invasive mold infection. The median time to occurrence of an IFI was 200 days (8–644 days). The median time to occurrence of an invasive mold infection was 210 days (9–644 days). The cumulative incidence of any IFI and mold infections at 1 year after HCT was 10 and 7%, respectively.
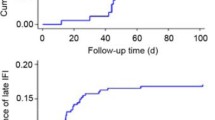
Two-thirds of the IFI (41 of 61 patients) occurred early in the study period between 2007 and 2010. We observed a decrease in the number of IFI after 2010 following the introduction of institutional guidelines for antifungal prophylaxis (Fig. 1). The occurrence of IFI due to Mucorales or other uncommon molds was sporadic throughout the 8 years of the study.
Distribution of 61 invasive fungal infections over time among adult patients who underwent allogeneic HCT with a peripheral blood stem cell or bone marrow graft at the University of Michigan Blood and Marrow Transplant Program between January 2007 and December 2012. Patients were followed for 2 years post-transplantation
*Other: Blastomyces spp. (1 episode), Pneumocystis jiroveci (3 episodes)
Among the 45 patients who had invasive mold infections, 5 had proven invasive infections. These included invasive rhinosinusitis caused by Mucorales in 3 patients and disseminated infection caused by Scedosporium apiospermum and Alternaria spp. in 1 patient each. The remaining 40 patients had probable mold infections, including invasive pulmonary aspergillosis in 29 (64%). Of these 29 patients, 18 had a diagnosis of probable invasive pulmonary aspergillosis established by EORTC/MSG criteria using BAL galactomannan. Other causes of probable invasive pulmonary mold infection included Mucorales in 3 patients and Paecilomyces spp. in 2 patients. One patient had probable rhinosinusitis caused by a Mucorales, and 4 patients had probable invasive pulmonary infection caused by molds that could not be identified.
Twelve patients were diagnosed with yeast infection, of which 6 were fungemias, 3 with C. glabrata, and 1 each with C. albicans, C. parapsilosis, and Saccharomyces cerevisiae. Six others had non-severe focal infections caused by Candida spp. One patient had pulmonary blastomycosis. Three patients, one of whom was receiving monthly pentamidine prophylaxis, developed Pneumocystis jiroveci pneumonia.
Forty of the 61 patients with IFI were receiving antifungal prophylaxis with azoles or micafungin at the time of diagnosis (Table 2). There were more breakthrough fungemias with yeasts in the micafungin group. Mold infections were scattered among patients receiving any of the 3 different prophylactic regimens.
Risk Factors for IFI and Invasive Mold Infections
On univariate analysis, acute GVHD (OR 2.04, p = 0.019) and a longer duration of corticosteroid therapy (OR 1.0, p = 0.008) were significantly associated with the development of any IFI. Transplantation of peripheral blood stem cells showed a significant association with IFI when compared with bone marrow grafts (p = 0.029). However, this finding is difficult to interpret as only 34 (6.2%) patients received bone marrow grafts (Table 3).
For mold infections, both acute GVHD (OR 2.5, p = 0.012) and duration of corticosteroid therapy (OR 1.0, p = 0.03) were significant risk factors. Peripheral blood stem cell transplantation was strongly associated with development of mold infection when compared with bone marrow grafts (p < 0.0001). Again, this finding is difficult to interpret because of the small number of patients who received a bone marrow graft (Table 4).
In order to assess the relative impact of GVHD and duration of corticosteroid therapy on the incidence of any IFI and on mold infections, in particular, we performed bivariate analysis. Both acute GVHD (OR 1.83, p = 0.05) and corticosteroid duration (OR 1.0, p = 0.026) were significantly associated with increased risk of IFI. In the case of mold infections, acute GVHD (OR 2.3, p = 0.027) emerged as the most important association. Chronic GVHD was not significantly associated with a higher risk of IFI or mold infections (Table 5).
Risk Factors for Non-Relapse Mortality
The cumulative incidence of non-relapse mortality for all patients was 16 and 21% at 1 and 2 years, respectively. IFI were significantly associated with a higher 2-year mortality rate [62 vs. 21%, HR 3.2 (2.2–6), p < 0.0001], as were mold infections [58 vs. 23%, HR 2.6 (1.9–4.3), p < 0.0001] when compared with patients who did not have IFI or mold infections.
Corticosteroid treatment duration of more than 90 days was associated with significantly higher mortality [HR 1.9 (1.3–2.6), p < 0.0001]. This association was present in patients with and without acute or chronic GVHD. The mortality associated with IFI and mold infections, in particular, was significantly higher in patients receiving higher prednisone dose equivalent in mg/kg at the time of the infection [HR 2.1 (1.3–3.4) p = 0.002 and HR 2.05 (1.2–3.5), p = 0.008, respectively].
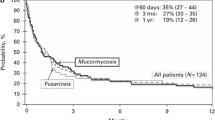
In patients with acute GVHD, having any IFI was associated with an increased 2-year mortality rate of 72% relative to 27% in patients with acute GVHD and no IFI [HR 2.9 (1.9–4.5), p < 0.0001]. Similarly, mold infections were associated with an increased 2-year mortality rate of 68% compared to 29% in patients with acute GVHD and no mold infection [HR 2.7 (1.7–4.2), p = 0.0001)] (Fig. 2). In patients with chronic GVHD, the presence of any IFI, but mold infections in particular, were associated with a significant increase in 2-year mortality (p < 0.0001) (Fig. 3).
Discussion
Invasive fungal infections and mold infections in particular are a major cause of morbidity and mortality not related to relapse in allogeneic HCT recipients [2, 9]. Classically, severe neutropenia secondary to aggressive conditioning regimens has been identified as a risk factor for fungal infections early after transplantation (<30 days). Immunosuppressive therapy for GVHD is typically identified as a major risk factor for fungal infections late after transplantation (>100 days) [10, 11].
Similar to prior reports, we found a rate of development of IFIs of 10% and of mold infections of 7% [12–15]. Previous studies reported the median time to development of an IFI or mold infection after allogeneic HCT to be about day +100 [11, 16–18]. We observed that most infections occurred later after HCT at a median time of 200 and 210 days, for all IFI and for mold infections, respectively. This shift toward IFIs occurring later in the post-transplant course perhaps could be explained by the increasing use of antifungal prophylaxis typically until day +100.
In our study, 19 of 28 cases of probable invasive pulmonary aspergillosis had a positive BAL galactomannan. The number of allogeneic HCT remained steady during the study period. We suspect the peak number of cases of aspergillosis occurring in 2010 was likely related to the introduction of Aspergillus galactomannan testing on BAL samples as a microbiological criterion for IPA in the EORTC/MSG guidelines published in 2009 [6].
We also observed a decrease in the number of IFI after 2010 following the introduction of institutional guidelines for antifungal prophylaxis that recommended using voriconazole for all unrelated donor HCT through day +100 and restarting this agent when steroids were given at total daily dose ≥0.3mg/kg.
In recent years, there has been an increased incidence of infections due to unusual fungi in HCT recipients [19, 20]. These changes have been attributed, in part, to the use of certain agents for antifungal prophylaxis, as well as more aggressive immunosuppression [1, 14]. In our HCT recipients, there were 7 patients who were infected with Mucorales, and 4 of them had been on voriconazole prophylaxis. These numbers, as in previous reports, are small, and it is difficult to assess causation, but these data add to the speculation of a voriconazole effect [21, 22]. We also noted disseminated infection with Alternaria, Paecilomyces, and Scedosporium, but there were no infections caused by Fusarium spp., which are increasingly reported in HCT recipients [2, 23].
The use of peripheral stem cells has been associated with a faster neutrophil recovery but more chronic GVHD when compared with bone marrow or cord blood transplantation [24]. In our study, transplantation of peripheral stem cells showed a significant association with IFI when compared with bone marrow transplants. This finding differs from prior published data, but might be explained by the small proportion of patients who received bone marrow grafts in our series.
Acute GVHD is a common clinical complication, occurring in up to 50% of patients early after allogeneic HCT. Corticosteroids remain the backbone in the management of patients with GVHD. Corticosteroids are widely recognized as a significant risk factor for development of IFI. On bivariate analysis, we found that acute GVHD and >90 day-exposure to high-dose corticosteroids were two independent variables that were associated with a significantly increased risk for IFI and for mold infections. Corticosteroids are known to suppress the phagocytic and killing activity of neutrophils and macrophages [25]. In vitro and in vivo studies have shown that corticosteroid exposure promotes mold and yeast infection and also could enhance the virulence of these pathogens [26–28].
Acute GVHD is a known risk factor for IFI [29–31]. However, the question remains whether the role of GVHD may reflect the effect of corticosteroids and other immunosuppression used for the management of these patients. Our data confirm the findings of others that acute GVHD is an independent risk factor for late onset IFI [31, 32]. However, the exact mechanisms leading to a higher risk of IFIs in acute GVHD have not been elucidated. Acute GVHD is a complex process that encompasses mucosal damage, release of pro-inflammatory cytokines, and T cell differentiation followed by target tissue damage. There has been an increasing appreciation of the role of Th17 in the severity of GVHD [33]. Recent data suggest that Th17 is associated with extended inflammation and defective clearance of fungi [34]. In mice, a Th17 response driven by IL-23 was an important negative regulator of the Th1 immune response against fungi [35]. Moreover, the Th17 pathway was associated with an extended inflammatory response and impaired pathogen clearance in Aspergillus and Candida infections [36]. Further studies are needed to elucidate the association between acute GVHD and IFIs.
Chronic GVHD has a distinctive end-organ pathology causing fibrosis and has a strong association with T cell differentiation along the Th17 pathway [37, 38]. Chronic GVHD has been previously identified as an independent risk factor for IFI [31], but we did not find this association in our HCT recipients.
Significant advances in the management of some IFI, especially aspergillosis, have led to improved outcomes with overall mortality rates of 35–57% [1, 39–41]. In our study, IFIs and mold infections were independently associated with increased 2-year non-relapse mortality, particularly among those patients who were receiving high-dose corticosteroids at the time of development of IFI or mold infection. Two-year non-relapse mortality was higher in those patients who had acute or chronic GVHD associated with an IFI or mold infection.
This study has several limitations. Most importantly, it is a single center study reflecting the approach to HCT at only one transplant center. Additionally, the data were collected retrospectively. The role of antifungal prophylaxis in decreasing the number of mold infections was inferred, but not specifically studied.
In spite of the limitations, our study documents the epidemiology and impact of IFI on mortality among HCT patients. Most IFI and mold infections occurred late after transplantation (>200 days) and were associated with acute GVHD and prolonged use of corticosteroids. Development of an IFI or mold infection significantly increased non-relapse mortality among patients who had acute or chronic GVHD.
References
Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48:265–73.
Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–100.
Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–47.
Malani AN, Kerr LE, Kauffman CA. Voriconazole: How to use this antifungal agent and What to expect. Semin Respir Crit Care Med. 2015;36:786–95.
Clark NM, Grim SA, Lynch JP 3rd. Posaconazole: use in the prophylaxis and treatment of fungal infections. Semin Respir Crit Care Med. 2015;36:767–85.
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis Off Publ Infect Dis Soc Am. 2008;46:1813–21.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56.
Akan H, Antia VP, Kouba M, Sinko J, Tanase AD, Vrhovac R, et al. Preventing invasive fungal disease in patients with haematological malignancies and the recipients of haematopoietic stem cell transplantation: practical aspects. J Antimicrob Chemother. 2013;68:iii5–16.
Bow EJ. Invasive fungal infection in haematopoietic stem cell transplant recipients: epidemiology from the transplant physician’s viewpoint. Mycopathologia. 2009;168:283–97.
Omer AK, Ziakas PD, Anagnostou T, Coughlin E, Kourkoumpetis T, McAfee SL, et al. Risk factors for invasive fungal disease after allogeneic hematopoietic stem cell transplantation: a single center experience. Biol Blood Marrow Transplant. 2013;19:1190–6.
Slavin S, Naparstek E, Nagler A, Ackerstein A, Kapelushnik J, Or R. Allogeneic cell therapy for relapsed leukemia after bone marrow transplantation with donor peripheral blood lymphocytes. Exp Hematol. 1995;23:1553–62.
Baddley JW, Stroud TP, Salzman D, Pappas PG. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin Infect Dis. 2001;32:1319–24.
Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–17.
Jantunen E, Ruutu P, Niskanen L, Volin L, Parkkali T, Koukila-Kahkola P, et al. Incidence and risk factors for invasive fungal infections in allogeneic BMT recipients. Bone Marrow Transplant. 1997;19:801–8.
Martino R, Bautista G, Parody R, Garcia I, Esquirol A, Rovira M, et al. Severe infections after single umbilical cord blood transplantation in adults with or without the co-infusion of CD34+ cells from a third-party donor: results of a multicenter study from the Grupo Espanol de Trasplante Hematopoyetico (GETH). Transpl Infect Dis. 2015;17:221–33.
Junghanss C, Marr KA, Carter RA, Sandmaier BM, Maris MB, Maloney DG, et al. Incidence and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem cell transplantation: a matched control study. Biol Blood Marrow Transplant. 2002;8:512–20.
Mikulska M, Raiola AM, Bruno B, Furfaro E, Van Lint MT, Bregante S, et al. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant. 2009;44:361–70.
Miceli MH, Diaz JA, Lee SA. Emerging opportunistic yeast infections. Lancet Infect Dis. 2011;11:142–51.
Miceli MH, Lee SA. Emerging moulds: epidemiological trends and antifungal resistance. Mycoses. 2011;54:e666–78.
Kauffman CA. Zygomycosis: reemergence of an old pathogen. Clin Infect Dis. 2004;39:588–90.
Park BJ, Pappas PG, Wannemuehler KA, Alexander BD, Anaissie EJ, Andes DR, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg Infect Dis. 2011;17:1855–64.
Horn DL, Freifeld AG, Schuster MG, Azie NE, Franks B, Kauffman CA. Treatment and outcomes of invasive fusariosis: review of 65 cases from the PATH Alliance registry. Mycoses. 2014;57:652–8.
Rowley S, Friedman TM, Korngold R. Hematopoietic stem cell transplantation for malignant diseases. In: Rich R, editor. Clinical immunology principles and practice. 3rd ed. Philadelphia: Mosby-Elsevier; 2008. p. 1223–36.
Buttgereit F, Seibel MJH, Bijlsma JWJ. Glucocorticoids. In: Rich R, editor. Clinical immunology principles and practice. 3rd ed. Philadelphia: Mosby-Elsevier; 2008. p. 1293–305.
Gyetvai A, Emri T, Fekete A, Varga Z, Gazdag Z, Pesti M, et al. High-dose methylprednisolone influences the physiology and virulence of Candida albicans ambiguously and enhances the candidacidal activity of the polyene antibiotic amphotericin B and the superoxide-generating agent menadione. FEMS Yeast Res. 2007;7:265–75.
Farmakiotis D, Shirazi F, Zhao Y, Saad PJ, Albert ND, Roilides E, et al. Methylprednisolone enhances the growth of Exserohilum rostratum in vitro, attenuates spontaneous apoptosis, and increases mortality rates in immunocompetent Drosophila flies. J Infect Dis. 2014;210:1471–5.
Cordonnier C, Ribaud P, Herbrecht R, Milpied N, Valteau-Couanet D, Morgan C, et al. Prognostic factors for death due to invasive aspergillosis after hematopoietic stem cell transplantation: a 1-year retrospective study of consecutive patients at French transplantation centers. Clin Infect Dis. 2006;42:955–63.
Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–66.
Bhatti Z, Shaukat A, Almyroudis NG, Segal BH. Review of epidemiology, diagnosis, and treatment of invasive mould infections in allogeneic hematopoietic stem cell transplant recipients. Mycopathologia. 2006;162:1–15.
Girmenia C, Raiola AM, Piciocchi A, Algarotti A, Stanzani M, Cudillo L, et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant. 2014;20:872–80.
Hou CY, Xu LL, Chen H, Liu N, Jiang M, Wang GQ, et al. Intestinal aGVHD and infection after hematopoietic stem cell transplantation. Med Sci Monit. 2013;19:802–6.
Henden AS, Hill GR. Cytokines in graft-versus-host disease. J Immunol. 2015;194:4604–12.
Shoham S, Levitz SM. The immune response to fungal infections. Br J Haematol. 2005;129:569–82.
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8.
Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–706.
Malard F, Bossard C, Brissot E, Chevallier P, Guillaume T, Delaunay J, et al. Increased Th17/Treg ratio in chronic liver GVHD. Bone Marrow Transplant. 2014;49:539–44.
Serody JS, Hill GR. The IL-17 differentiation pathway and its role in transplant outcome. Biol Blood Marrow Transplant. 2012;18:S56–61.
Gregg KS, Kauffman CA. Invasive aspergillosis: epidemiology, clinical aspects, and treatment. Semin Respir Crit Care Med. 2015;36:662–72.
Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–40.
Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis. 2010;50:1559–67.
Acknowledgement
Funding was provided by National Institutes of Health (Grant No. CA46592).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Marisa H. Miceli, Tracey Churay, Thomas Braun, and Carol A. Kauffman have no conflicts to report. Daniel R. Couriel is an active member of Merck, Inc. Advisory Board.
Additional information
Marisa H. Miceli and Tracey Churay have contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Miceli, M.H., Churay, T., Braun, T. et al. Risk Factors and Outcomes of Invasive Fungal Infections in Allogeneic Hematopoietic Cell Transplant Recipients. Mycopathologia 182, 495–504 (2017). https://doi.org/10.1007/s11046-017-0115-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-017-0115-y