Abstract
Calcineurin is a heterodimeric protein phosphatase complex composed of catalytic (CnaA) and regulatory (CnaB) subunits and plays diverse roles in regulating fungal stress responses, morphogenesis, and pathogenesis. Fungal pathogens utilize the calcineurin pathway to survive in the host environment and cause life-threatening infections. The immunosuppressive calcineurin inhibitors (FK506 and cyclosporine A) are active against fungi, making calcineurin a promising antifungal drug target. Here, we review novel findings on calcineurin localization and functions in Aspergillus fumigatus hyphal growth and septum formation through regulation of proteins involved in cell wall biosynthesis. Extensive mutational analysis in the functional domains of A. fumigatus CnaA has led to an understanding of the relevance of these domains for the localization and function of CnaA at the hyphal septum. An evolutionarily conserved novel mode of calcineurin regulation by phosphorylation in filamentous fungi was found to be responsible for virulence in A. fumigatus. This finding of a filamentous fungal-specific mechanism controlling hyphal growth and virulence represents a potential target for antifungal therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive fungal infections are a leading cause of death in immunocompromised patients [1]. With a 40–60 % mortality rate, invasive aspergillosis, caused by the filamentous fungus Aspergillus fumigatus, is the most frequent cause of death among mold infections [2]. The calcineurin pathway is an important signaling cascade in eukaryotes and calcineurin is a promising antifungal target due to the distinct mode of action from other antifungal classes, activity against drug resistant strains, and synergism with existing antifungals [3]. However, currently available calcineurin inhibitors lead to host immunosuppression and limit potential therapeutic effectiveness [4]. It is therefore important to identify targets that specifically inhibit fungal calcineurin, resulting in fungal killing without host immune suppression.
Calcineurin is a Ca2+/calmodulin (CaM)-dependent protein phosphatase that is ubiquitous and conserved among the eukaryotes [5–8]. It is composed of a catalytic (CnaA) and a regulatory (CnaB) subunit. As a protein phosphatase, CnaA interacts with phosphorylated substrates through its amino-terminal catalytic domain. The other highly conserved domains in CnaA include a carboxy-terminal regulatory domain containing the CnaB-binding helix (CnBBH), the CaM-binding domain (CaMBD), and an auto inhibitory domain (AID) [9–11] (Fig. 1a). After binding to its regulatory subunit, CnaB, which contains four EF hand Ca2+-binding motifs (EF hand motif is a helix-loop-helix structural domain found in the family of calcium-binding proteins), CnaA is activated in the presence of Ca2+ and CaM [11]. In contrast to the calcineurin gene multiplicity observed in mammals [11], lower eukaryotes such as the budding yeast Saccharomyces cerevisiae contain two genes encoding the catalytic subunit (CNA1 and CNA2) and a single gene for the regulatory subunit (CNB1) [12]. The fission yeast Schizosaccharomyces pombe, as well as filamentous fungal species, contain one gene encoding each calcineurin subunit [13].
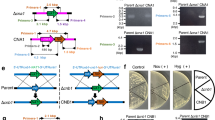
A. fumigatus CnaA domain organization and targeted mutations. a The various domains in CnaA and mutations in the important domains are shown. The PxIxIT linker region mutation (shown in green; 352NIR354 to alanines) affects substrate-binding, combined mutation of Thr359Pro (T359P), His361Leu (H361L) and Leu365Ser170 (L365S) close to the PxIxIT-binding motif (THL-PLS) reduces CnaA enzyme activity, the V371D mutation in the calcineurin B-Binding Helix (CnBBH; shown in blue with the V371 residue mutated to Asp) blocks CnaB binding to CnaA. The 4 serine residues (S406, S408, S410 and S413) in the novel serine–proline-rich region (SPRR; shown in yellow; 404PTSVSPSAPSPPLP417) are mutated to alanines to investigate the importance of CnaA phosphorylation for its function and activity. The key residues 442RVF444 in the calmodulin-binding domain (CaMBD; shown in purple) are mutated to alanines to block calmodulin binding. b Comparative sequence alignment of the unique serine–proline-rich region is shown. This serine–proline-rich region containing 14 amino acids is completely absent in the human calcineurin A and not conserved in the yeasts. The autoinhibitory domain (AID) is shown in red. (Color figure online)
Calcineurin plays a central role in the regulation of cation homeostasis, morphogenesis, cell wall integrity, and pathogenesis in fungi [8, 14, 15]. It regulates growth at alkaline pH and at higher temperatures, membrane stress, mating, and virulence in both Candida albicans and Cryptococcus neoformans [16–20]. In addition, its role in morphogenesis, spindle body organization and membrane trafficking has been well described in S. pombe [13, 21, 22]. Previous reports in the filamentous fungi have implicated calcineurin in cell cycle progression [23], hyphal branching [24], stress adaptation [25], sclerotial development [26], and appressorium formation [27]. Although calcineurin signaling is conserved among fungi, recent studies indicate important divergences in calcineurin-dependent functions among different human fungal pathogens. For example, while in the model yeast, S. cerevisiae, calcineurin (CNA1) null mutant was able to grow at higher temperature, the C. neoformans CNA1 disruption strain was non-viable in host environment mimicking conditions (37 °C, 5 % CO2 or alkaline pH) and was avirulent [28]. In contrast to C. neoformans, calcineurin was dispensable for survival of C. albicans at 37 or 42 °C, and the C. albicans cnb1 mutant strains had no defects in germination and filamentous growth [20, 29]. Host niche also seems to be an important factor for calcineurin control over virulence, as demonstrated in vaginal or pulmonary candidiasis models [30]. Therefore, critical understanding of the calcineurin pathway in A. fumigatus will pave the way for devising new drug targets for combating invasive aspergillosis. In this review, we summarize recent results on the functional analysis of the calcineurin complex in A. fumigatus hyphal growth and septation.
Aspergillus fumigatus Calcineurin Mutants Exhibit Defects in Germination, Hyphal Morphology and Septum Formation
Analysis of cnaA deletion mutant in A. fumigatus revealed the importance of calcineurin for growth and virulence [31]. To distinguish the relevance of the catalytic and regulatory subunits of calcineurin for hyphal growth and septation in A. fumigatus, calcineurin single (ΔcnaA; ΔcnaB) and double deletion (ΔcnaAΔcnaB) strains were generated. While the ΔcnaB strain showed a compact colony morphology indistinguishable from the ΔcnaA strain, revealing the absolute requirement of CnaB regulatory subunit for calcineurin function, the ΔcnaAΔcnaB strain showed more delayed germination and a greater radial growth defect [32]. In contrast to the wild-type strain with fully extended hyphae, the ΔcnaA strain showed a compact colony with blunt hyphae and irregular branching at the tips, while the ΔcnaB and the ΔcnaAΔcnaB strains formed hyphae with fewer branches. Partial growth remediation of the ΔcnaA strain in the presence of sorbitol indicated probable differences in the cell wall components of the individual ΔcnaA and ΔcnaB mutants or an osmotic defect in the ΔcnaA strain. Involvement of calcineurin in osmotic stress response pathways through the PKC and HOG pathways has previously been reported in fungi [33, 34]. These differing phenotypes resulting from deletion of individual calcineurin subunits and the entire complex suggested a previously unsuspected complexity in their individual functions.
Calcineurin Complex Coordinates Hyphal Cell Wall Organization
While both the ΔcnaA and ΔcnaB strains showed abnormal septa, the ΔcnaAΔcnaB strain had curved or wavy septa, sometimes incomplete or even broken indicating a disorganization of β-glucan assembly at the septum upon deletion of both calcineurin subunits. Extracellular web-like material observed by scanning electron microscopy in all the calcineurin mutant strains further suggested the possibility of highly disordered cell wall architecture [32]. While the nature of the extracellular fibrous material is yet unknown, it might be a mixture of polysaccharides and mannoproteins that are improperly assembled due to defects in cell wall synthesis resulting from the deletion of the calcineurin genes.
Morphological analysis of the cell wall by transmission electron microscopy confirmed the requirement of calcineurin complex for proper cell wall architecture. While the cell wall in the wild-type strain was uniformly electron-dense, all of the calcineurin mutants displayed a thicker cell wall. The inner layer, which mostly consists of glucan, seemed enlarged, and the outer layer, which contains mannoproteins, was thicker. Septum formation in the ΔcnaAΔcnaB strain was not coordinated properly from both sides of the hyphal wall, resulting in incomplete septum formation. The two sides of the septum were not formed at the same time, which resulted in improper co-ordination of septation from the two ends. While both the ΔcnaA and ΔcnaB strains showed abnormal septa, the ΔcnaA ΔcnaB strain had curved often wavy, incomplete or even broken septa. Aniline blue, which stains cell wall β-glucan, did not show septal staining in the ΔcnaA ΔcnaB strain [32]. These results indicated that, in comparison with the single deletion strains, deletion of both the subunits of calcineurin is more deleterious and results in greater abnormality of the cell wall and septa. The calcineurin complex may therefore be important for the correct deposition of new cell wall material at the septum and for normal cell wall structure. Collectively, these results indicated that the calcineurin mutants have an inherent defect in the composition of their cell walls.
The growth defect and septation abnormalities observed in the double mutant may be either due to the lack of proper synthesis of the major cell wall components, chitin and β-glucan, or an improper assembly of these components. The β-glucan content in all the calcineurin mutants was reduced by ∼40 % when compared to the wild-type strain [32]. In contrast to the decreased β-glucan content of the calcineurin mutants, compensatory increase in the chitin levels was noted in all the mutants, with the ΔcnaAΔcnaB strain showing an increase of ∼40 % and each ΔcnaA and ΔcnaB strain showing ∼20 % increase when compared to the wild-type strain. Evidence to clearly implicate calcineurin control of both β-glucan and chitin is not yet available but the increase in chitin content is a compensatory response to reduced β-glucan. Such compensatory increases in the chitin contents of strains treated with caspofungin, an inhibitor of β-1,3-glucan synthase, were also noted earlier [35]. Despite an increased growth defect in the ΔcnaAΔcnaB strain compared to the single mutants, there were no statistically significant variations in the major cell wall components when comparing the single and double mutants.
While we do not have clear evidence on calcineurin impact on chitin levels, the β-glucan levels are controlled by calcineurin through the downregulation on fksA gene. Analysis of the transcriptional profiles of eight chitin synthase genes (chsA, chsB, chsC, chsD, chsE, chsF, chsG and chsEb) in the ΔcnaB and ΔcnaAΔcnaB strains showed a downregulation of all the chitin synthase genes, as was previously reported in the ΔcnaA strain [36]. The abnormality in the assembly of the cell wall components in the calcineurin mutants may result from the impaired incorporation of chitin in the cell wall due to the decreased proportion of β-glucan. Previous results have indicated an ∼twofold decrease in the transcription of fksA, encoding the catalytic subunit of β-1,3-glucan synthase, in the ΔcnaA strain [36], which coincides with decreased β-glucan levels in all the calcineurin mutants. Model depicting calcineurin control over cell wall biosynthetic genes and hyphal growth is shown in Fig. 2.
Model showing calcineurin-mediated regulation of hyphal growth, cell wall integrity and virulence in A. fumigatus. Calcineurin, comprising of the catalytic subunit (CnaA) and the regulatory subunit (CnaB), is activated by Ca2+-calmodulin (CaM). CnaA is phosphorylated at four serine residues in the serine–proline-rich region (SPRR) and also at two serine residues in the C-terminus, and two serine residues in the N-terminus of CnaB by the activity of kinases (GSK-3β, CK1, CDK1, MAPK). Calcineurin is inhibited by the binding of the immunophilin–immunosuppressant complex (FK506-FKBP12). The phosphorylated calcineurin complex may dephosphorylate the transcription factor CrzA and translocate it into the nucleus to activate the transcription of cell wall biosynthesis-related genes (chsA, chsC and fksA). Similarly, the phosphorylated calcineurin complex may also interact with cell wall proteins directly in a phosphorylation-dependent manner to regulate their activity and cell wall homeostasis. (Color figure online)
Localization of the Calcineurin Complex at the Hyphal Septum is Required for Regular Septation and Proper Hyphal Growth
CnaA localizes as punctate dot-like structures at the hyphal tips and in developing conidiophores [37]. CnaA also concentrates as a disk around the septal pore in both newly formed and mature septa. The ΔcnaB strain showed a similar growth phenotype as deletion of cnaA [31], indicating a cooperative regulation between the catalytic and regulatory subunits [32], and fluorescence microscopy revealed the co-localization of mcherry-CnaB and CnaA-EGFP at the septa. Time-lapse microscopy of the calcineurin complex revealed that the dot-like structures initially present in the swollen conidium concentrated at the point of germ tube emergence and remained at the tip of the germling as hyphal extension occurred. Retrograde movement of the vesicular structures, containing the calcineurin subunits, from the hyphal tip toward the septation initiation sites and concentration at the center of the septum was evident during septum formation. The calcineurin complex was present throughout the process of septum formation. The presence of calcineurin during the initial germination phase and then during hyphal extension and septation indicated a diverse role for calcineurin in morphogenetic control. Treatment with FK506 or cyclosporine A did not affect localization of the calcineurin complex at the septum, although the treatment resulted in a phenotype that resembled a calcineurin subunit deletion. In the absence of CnaA, CnaB remained in dot-like structures which were evenly distributed in the hyphal compartments, without septal localization. However, CnaA localized to the hyphal septum even in the absence of CnaB. Although CnaA localizes at the septum independent of CnaB, the ∆cnaB phenotype could not be restored to that of the wild-type and showed septation defects similar to the ∆cnaA strain, indicating the absolute requirement of CnaA complexing with CnaB for normal calcineurin function at the hyphal septum. Furthermore, this indicated that CnaA may localize at the septum by binding to other as yet undefined proteins.
Important Domains Required for Calcineurin Function and Septal Localization
Complementation experiment involving the transformation of truncated cnaA that consisted of only the N-terminal catalytic domain into the ∆cnaA strain did not restore hyphal growth and septal localization which revealed that the N-terminal catalytic domain (1-347 aa) does not contain the determinants required for septal localization; however, the inclusion of the CnBBH and CaMBD regions efficiently localized CnaA at the septum and restored proper hyphal growth [38]. Surprisingly, CaM, the well-known calcineurin interactor and activator, is not required for septal targeting of CnaA. It is possible that targeting CnaA to the hyphal septum occurs either independently or by binding to other unknown protein(s).
Binding studies with the human calcineurin previously revealed the PxIxIT motif as a common binding site for calcineurin on its substrates [39] (Fig. 1a). In S. cerevisiae, mutation of the calcineurin residues (N366 I367 R368) in contact with the PxIxIT motif resulted in defective substrate interaction [40]. Recent structural studies of Ca2+ /CaM bound to a 25-residue peptide spanning the CaMBD in the human calcineurin catalytic subunit also revealed that R408, V409, and F410 play a major role in rigidity and stabilization of the central helix of CaM bound to calcineurin [41]. In A. fumigatus transformation of the full length cnaA harboring the mutated PxIxIT-binding NIR residues (NIR-AAA) into the ∆cnaA strain to verify for complementation, only partially restored hyphal growth and completely mislocalized CnaA indicating that septal localization of CnaA occurs through binding to other protein(s). On the contrary, similar complementation experiment after mutation of the critical Ca2+ /CaM-binding RVF residues in the CaMBD to alanines (RVF-AAA) had partial hyphal growth restoration but did not affect CnaA septal localization (Fig. 1a; Table 1). Calmodulin is a well-known activator of calcineurin. Calmodulin binds to the calmodulin-binding domain (CaMBD) in CnaA to displace the AID and activates calcineurin. The observed growth defect with the RVF-AAA mutation may be due to the inability of CaM to bind to CnaA, and as a result, the AID remains bound to the regulatory domain, leading to continued inhibition of calcineurin activity (Table 1). Although CaM localizes at the hyphal tip and septum in A. nidulans [42], which was confirmed in A. fumigatus, these results, coupled with the truncational analyses, confirmed that CnaA localization at the septum is CaM-independent.
Critical regions controlling calcineurin function in S. cerevisiae have been identified by substitution of V385 with an aspartic acid that disrupted the interaction between the catalytic and the regulatory subunit, and also by random mutagenesis of three residues (S373, H375, and L379) that led to loss of calcineurin activity but did not disrupt calcineurin A binding to Ca2+ /CaM or to calcineurin B [43] (Fig. 1a). The importance of these domains for septal localization in A. fumigatus was analyzed by mutation of V371 to aspartic acid (V371D) and the T359, H361, and L365 to proline, leucine and serine (THL-PLS), respectively. Both mutations had a significant effect on hyphal growth, calcineurin activity, but neither affected CnaA septal localization (Table 1). The V371D mutation confirmed that although CnaB is not required for CnaA septal localization, it is required for CnaA function and growth. The THL-PLS mutation had an effect on the catalytic activity, and therefore, it is possible that although CnaA is localized at the hyphal septum, it is catalytically inactive. The reduction in calcineurin activity due to these mutations and the lack of caspofungin-mediated paradoxical growth recovery (caspofungin at high concentrations reverses the growth inhibition of A. fumigatus, a process known as the “paradoxical effect”) established that catalytic site residues and CnaB-binding activity of CnaA do not influence its septal localization, yet active calcineurin is required at the hyphal septum to direct proper hyphal growth.
The Unique Serine–Proline-Rich Region (SPRR) Identified in A. fumigatus CnaA is Phosphorylated and Required for Proper Hyphal Growth and Virulence
By analyzing the conserved domains in CnaA, we identified a filamentous fungal-specific novel linker between the highly conserved CnBBH and the CaMBD [38] (Fig. 1a). Clustalw alignments confirmed the presence of the SPRR (404-PTSVSPSAPSPPLP-417) within the 23-residue linker that is completely absent in the human calcineurin α-catalytic subunit (Fig. 1b). Phylogenic analysis of this region clearly distinguished the filamentous fungal calcineurins from other organisms, indicating the evolutionarily importance of SPRR for filamentous hyphal growth. Phosphoproteomic analysis revealed the phosphorylation of all 4 clustered serines in the SPRR (S406, S408, S410 and S413) and two additional serine residues in the C-terminus at positions 537 and 542. Phosphorylation of CnaA was also examined in the presence of a specific inhibitor, FK506, to correlate phosphorylation versus activity. Twofold decrease in the phosphorylation of S406 in the CnaA SPRR and a 1.2- and 1.8-fold increase in the phosphorylation of S537 and S542, respectively, was noted in the C-terminus compared to the untreated control [38]. These results suggested a previously unknown link between FK506-mediated inhibition of calcineurin activity and CnaA phosphorylation, including in the novel SPRR.
Heterologous expression of CnaA homologs from other closely related filamentous fungi, N. crassa and M. grisea, also revealed the phosphorylation of serine residues within the SPRR providing further evidence that filamentous fungal calcineurins have diverged from the yeasts and other organisms. Complementation experiments with S. cerevisiae calcineurin A (CNA1) did not restore/complement the A. fumigatus cnaA mutant (unpublished results) which indicated that the filamentous fungal calcineurins may have diverged. We postulate that conservation of this unique SPRR domain in CnaA among filamentous fungi is evolutionarily significant; filamentous fungi may have acquired this unique domain and that phosphorylation in this domain is another novel mode of calcineurin regulation. Based on these novel findings in the regulation of A. fumigatus calcineurin, we expect that calcineurin interacts with its substrates in a phosphorylated/dephosphorylated state to regulate different cellular functions (Fig. 2). Furthermore in vitro phosphorylation assays revealed GSK3β, CK1, CDK1 and MAPK as potential kinases that might phosphorylate CnaA in vivo. Based on a recent report on the inactivation of GSK-3 by calcineurin inhibitors cyclosporine A and tacrolimus (FK506) in renal tubular cells [44], and our result demonstrating the phosphorylation of CnaA by GSK-3β and CK1 [38], it is possible that FK506 inhibits the activity of GSK-3β, resulting in its inability to phosphorylate CnaA. Post-translational modifications involving protein phosphorylation/dephosphorylation are important events regulating protein function in vivo, by either activation or inhibition of activity of the protein. Few studies have focused on phosphorylation of calcineurin and the in vivo consequence of mutations in its key domains, but none of these residues are conserved in A. fumigatus and other filamentous fungi [45–49].
In comparison with the wild-type strain, the CnaAmt-4SA strain, in which the 4 phosphorylated serine residues within the SPRR were mutated to alanine (S406A, S408A, S410A and S413A) to block phosphorylation, exhibited a significant growth defect but did not affect septal localization (Table 1). Supporting these observations, calcineurin activity was also decreased by ~70 % in the CnaAmt-4SA strain compared to the wild-type strain, indicating that phosphorylation plays an important role in the regulation of calcineurin activity. The mortality associated with CnaAmt-4SA strain infection in a persistently neutropenic murine inhalational model of invasive aspergillosis was significantly lower (10 %) in comparison with the wild-type strain (90 %), indicating that phosphorylation in this novel SPRR is critical for calcineurin function and virulence. Analyzing phosphorylation-dependent interactants of calcineurin will help identify target proteins that can be exploited as additional fungal-specific therapeutic targets.
References
Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the transplant-associated infection surveillance network (TRANSNET) database. Clin Infect Dis. 2010;50(8):1091–100.
Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan S-P, Meier-Kriesche H-U, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012;65(5):453–64.
Steinbach WJ, Reedy JL, Cramer RA, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol. 2007;5(6):418–30.
Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, et al. The Mechanism of Action of Cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80(3):S40–5.
Stewart AA, Ingebritsen TS, Manalan A, Klee CB, Cohen P. Discovery of A Ca2+-and calmodulin-dependent protein phosphatase. FEBS Lett. 1982;137(1):80–4.
Cyert MS. Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu Rev Genet. 2001;35(1):647–72.
Sugiura R, Sio SO, Shuntoh H, Kuno T. Calcineurin phosphatase in signal transduction: lessons from fission yeast. Genes Cells. 2002;7(7):619–27.
Fox DS, Heitman J. Good fungi gone bad: the corruption of calcineurin. BioEssays. 2002;24(10):894–903.
Hemenway C, Heitman J. Calcineurin. Cell Biochem Biophys. 1999;30(1):115–51.
Aramburu J, Rao A, Klee CB. Calcineurin: from structure to function. Curr Top Cell Regul. 2000;36:237–95.
Rusnak F, Mertz P. Calcineurin: form and Function. Physiol Rev. 2000;80(4):1483–521.
Cyert MS, Kunisawa R, Kaim D, Thorner J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci USA. 1991;88(16):7376–80.
Yoshida T, Toda T, Yanagida M. A calcineurin-like gene ppb1 + in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J Cell Sci. 1994;107(7):1725–35.
Fox DS, Cruz MC, Sia RAL, Ke H, Cox GM, Cardenas ME, et al. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12–FK506 in Cryptococcus neoformans. Mol Microbiol. 2001;39(4):835–49.
Kraus PR, Heitman J. Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochem Biophys Res Commun. 2003;311(4):1151–7.
Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, Cardenas ME, et al. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 2002;21(4):546–59.
Bader T, Bodendorfer B, Schroppel K, Morschhauser J. Calcineurin Is Essential for Virulence in Candida albicans. Infect Immun. 2003;71(9):5344–54.
Reedy JL, Filler SG, Heitman J. Elucidating the Candida albicans calcineurin signaling cascade controlling stress response and virulence. Fungal Genet Biol. 2010;47(2):107–16.
Chen YL, Kozubowski L, Cardenas ME, Heitman J. On the roles of calcineurin in fungal growth and pathogenesis. Curr Fungal Infect Rep. 2010;4:244–55.
Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16(10):2576–89.
Zhang Y, Sugiura R, Lu Y, Asami M, Maeda T, Itoh T, et al. Phosphatidylinositol 4-phosphate 5-kinase Its3 and calcineurin Ppb1 coordinately regulate cytokinesis in fission yeast. J Biol Chem. 2000;275(45):35600–6.
Kita A, Sugiura R, Shoji H, He Y, Deng L, Lu Y, et al. Loss of Apm1, the μ1 subunit of the clathrin-associated adaptor-protein-1 complex, causes distinct phenotypes and synthetic lethality with calcineurin deletion in fission yeast. Mol Biol Cell. 2004;15(6):2920–31.
Rasmussen C, Garen C, Brining S, Kincaid RL, Means RL, Means AR. The calmodulin-dependent protein phosphatase catalytic subunit (calcineurin A) is an essential gene in Aspergillus nidulans. EMBO J. 1994;13(16):3917–24.
Prokisch H, Yarden O, Dieminger M, Tropschug M, Barthelmess IB. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol Gen Genet. 1997;256:104–14.
Juvvadi PR, Kuroki Y, Arioka M, Nakajima H, Kitamoto K. Functional analysis of the calcineurin-encoding gene cnaA from Aspergillus oryzae: evidence for its putative role in stress adaptation. Arch Microbiol. 2003;179(6):416–22.
Harel A, Bercovich S, Yarden O. Calcineurin Is Required for Sclerotial Development and Pathogenicity of Sclerotinia sclerotiorum in an Oxalic Acid-Independent Manner. Mol Plant Microbe Interact. 2006;19(6):682–93. doi:10.1094/mpmi-19-0682.
Choi J, Kim Y, Kim S, Park J, Lee Y-H. MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae. Fungal Genet Biol. 2009;46(3):243–54.
Liu J, Farmer JD Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66(4):807–15.
Blankenship JR, Wormley FL, Boyce MK, Schell WA, Filler SG, Perfect JR, et al. Calcineurin Is Essential for Candida albicans Survival in Serum and Virulence. Eukaryot Cell. 2003;2(3):422–30. doi:10.1128/ec.2.3.422-430.2003.
Bader T, Schröppel K, Bentink S, Agabian N, Köhler G, Morschhäuser J. Role of calcineurin in stress resistance, morphogenesis, and virulence of a candida albicans wild-type strain. Infect Immun. 2006;74(7):4366–9.
Steinbach WJ, Cramer RA Jr, Perfect BZ, Asfaw YG, Sauer TC, Najvar LK, et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 2006;5(7):1091–103.
Juvvadi PR, Fortwendel JR, Rogg LE, Burns KA, Randell SH, Steinbach WJ. Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus. Mol Microbiol. 2011;82(5):1235–59.
Garrett-Engele P, Moilanen B, Cyert M. Calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar H(+)-ATPase. Mol Cell Biol. 1995;15(8):4103–14.
Delgado-Jarana J, Sousa S, Gonzalez F, Rey M, Llobell A. ThHog1 controls the hyperosmotic stress response in Trichoderma harzianum. Microbiology. 2006;152(6):1687–700.
Fortwendel JR, Juvvadi PR, Pinchai N, Perfect BZ, Alspaugh JA, Perfect JR, et al. Differential effects of inhibiting chitin and 1,3-{beta}-D-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob Agents Chemother. 2009;53(2):476–82.
Cramer RA Jr, Perfect BZ, Pinchai N, Park S, Perlin DS, Asfaw YG, et al. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot Cell. 2008;7(7):1085–97.
Juvvadi PR, Fortwendel JR, Pinchai N, Perfect BZ, Heitman J, Steinbach WJ. Calcineurin localizes to the hyphal septum in Aspergillus fumigatus: implications for septum formation and conidiophore development. Eukaryot Cell. 2008;7(9):1606–10.
Juvvadi PR, Gehrke C, Fortwendel JR, Lamoth F, Soderblom EJ, Cook EC, et al. Phosphorylation of calcineurin at a novel serine-proline rich region orchestrates hyphal growth and virulence in Aspergillus fumigatus. PLoS Pathog. 2013;9(8):e1003564.
Li H, Zhang L, Rao A, Harrison SC, Hogan PG. Structure of calcineurin in complex with pvivit peptide: portrait of a low-affinity signalling interaction. J Mol Biol. 2007;369(5):1296–306.
Roy J, Li H, Hogan PG, Cyert MS. A conserved docking site modulates substrate affinity for calcineurin, signaling output, and in vivo function. Mol Cell. 2007;25(6):889–901.
Ye Q, Wang H, Zheng J, Wei Q, Jia Z. The complex structure of calmodulin bound to a calcineurin peptide. Proteins Struct Funct Bioinform. 2008;73(1):19–27.
Chen S, Song Y, Cao J, Wang G, Wei H, Xu X, et al. Localization and function of calmodulin in live-cells of Aspergillus nidulans. Fungal Genet Biol. 2010;47(3):268–78.
Jiang B, Cyert MS. Identification of a novel region critical for calcineurin function in vivo and in vitro. J Biol Chem. 1999;274(26):18543–51.
Berzal S, Alique M, Ruiz-Ortega M, Egido J, Ortiz A, Ramos AM. GSK3, snail, and adhesion molecule regulation by cyclosporine a in renal tubular cells. Toxicol Sci. 2012;127(2):425–37.
Singh TJ, Wang JH. Phosphorylation of calcineurin by glycogen synthase (casein) kinase-1. Biochem Cell Biol. 1987;65(10):917–21.
Hashimoto Y, Soderling TR. Regulation of calcineurin by phosphorylation. Identification of the regulatory site phosphorylated by Ca2+/calmodulin-dependent protein kinase II and protein kinase C. J Biol Chem. 1989;264(28):16524–9.
Martensen TM, Martin BM, Kincaid RL. Identification of the site on calcineurin phosphorylated by calcium/CaM-dependent kinase II: modification of the CaM-binding domain. Biochemistry. 1989;28(24):9243–7.
Hashimoto Y, King MM, Soderling TR. Regulatory interactions of calmodulin-binding proteins: phosphorylation of calcineurin by autophosphorylated Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci. 1988;85(18):7001–5.
Kume K, Koyano T, Kanai M, Toda T, Hirata D. Calcineurin ensures a link between the DNA replication checkpoint and microtubule-dependent polarized growth. Nat Cell Biol. 2011;13(3):234–42.
Acknowledgments
These studies were funded by NIH/NIAID 1 R56 AI077648-01A2 and 1 R21 AI097541-01A1 Grants to WJS. FL is supported by the Swiss Foundation for Medical-Biological Grants and the Swiss National Science Foundation (P3SMP3-151742).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Juvvadi, P.R., Lamoth, F. & Steinbach, W.J. Calcineurin-Mediated Regulation of Hyphal Growth, Septation, and Virulence in Aspergillus fumigatus . Mycopathologia 178, 341–348 (2014). https://doi.org/10.1007/s11046-014-9794-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-014-9794-9