Abstract
Hortaea werneckii is a black yeast-like ascomycetous fungi associated with the human superficial infection tinea nigra, which commonly occurs in tropical and subtropical countries. Now, this fungus has been found in the halophilic environment all over the world and recognized as a new model organism in exploring the mechanisms of salt tolerance in eukaryotes. During a survey of endophytic fungi of mangrove forest at South China Sea, two isolates of H. werneckii were recovered from medicinal plant of Aegiceras comiculatum. The isolates were identified by morphological characters and phylogenetic analyses (e.g., ITS rDNA, LSU rDNA and translation elongation factor EF1α). Some physiological tests such as thermotolerance, acid tolerance (pH) and NaCl tolerance as well as pathogenicity test in vitro for the strains of Hortaea were performed. It is the first report that H. werneckii was isolated from medicinal plant of A. comiculatum in south sea of China as the endophytic fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hyphomycete genus Hortaea (Ascomycota) was established in 1984 and presently contains only three species: H. werneckii (type species), H. acidophila [1] and H. thailandica [2]. The genus forms brown, septate, thick-walled hyphae, with ellipsoidal, 0–1-septate (becoming muriformly septate), hyaline to pale brown yeast-like conidia forming directly on the hyphae, via phialides with percurrent proliferation [3]. H. werneckii, previously called Exophiala werneckii or Cladosporium werneckii, is the most studied species in the genus. It can cause non-inflammatory and non-scaling superficial skin infection tinea nigra [4]. Tinea nigra is characterized by brown to black macules and usually occurs on the palms of hands and occasionally found on other parts of the body, such as blood and splenic abscess [5, 6]. Now, H. werneckii has been isolated globally from natural hypersaline environments and recognized as model organism in eukaryotes in exploring conditions of extremotolerance (e.g., oxidative stress, osmotic adaptation and melanization) [7]. Different from H. werneckii, H. acidophila has an ability to grow under extremely acidic conditions, but has low degree of halotolerance [1], while H. thailandica is characterized by conidia with verruculose ornamentation and is described as plant pathogen from Syzygium siamense leaf spots [2].
Recently, some scholars have made efforts to clarify mechanisms of adaptation of H. werneckii to saline environment, including plasma membrane composition and properties [8, 9], melanization of cell wall [10], differences in high osmolarity glycerol (HOG) signaling pathway [11], identification of glycerol synthesis genes [12] and even proteome analysis [13]. These studies indicated that H. werneckii has unique adaption mechanism to halophilic environment in glycerol as “compatible solutes” in the cells of H. werneckii, by modifying cell-wall structure (the cell-wall melanization) instead of lowering the membrane fluidity to reduce glycerol leakage from cell, the existence of the homologue of mitogen-activated protein (MAP) kinase and the unique strategies of Gpd1-regulated glycerol synthesis. The studies about its halotolerant mechanism of H. werneckii are greatly important for elucidation of their function in ecological system.
Mangroves are intertidal forested wetland confined to the tropical and subtropical regions and are considered a dynamic transition zone between terrestrial and marine habitats [14]. Some mangrove species such as Aegiceras corniculatum, Sonneratia caseolaris, Kandelia candel are a valuable source of useful metabolites for medicinal usage [15]. Endophytic fungi hidden in mangrove forest are also recognized as rich sources of bioactive metabolites of multifold importance [16]. In recent years, some new chemical compounds with good biological activity have been isolated from endophytic fungi isolated from the mangrove plant Aegiceras comiculatum [17]. In searching for the endophytic fungi with good bioactivity from mangrove medicinal plant of A. comiculatum, collected from Hainan Province where a region was recognized to possess most of the mangrove species recorded in China [18], two strains of fungi, morphologically similar to genus Hortaea, were isolated from A. comiculatum. In the present study, we identified the fungi by morphological and molecular analyses and also tested their thermotolerance, acid tolerance (pH) and NaCl tolerance as well as pathogenicity in vitro. The fungus will have great potential applications in artificial cultivation of medicinal plants in future.
Materials and Methods
Plant Materials
Aegiceras comiculatum (Fig. 1) was collected from the Conservation Zone of Mangrove Forest in Dong Zhai Gang (110°32′–110°37′E, 19°51′–20°1′N), Hainan Province, China, in March 2008. Plant samples were put in plastic bags and transported to the laboratory in Beijing. Endophytic fungi were isolated in less than 48 h after sampling.
Fungal Isolation
Endophytic fungi were isolated from two individuals of A. comiculatum. Surface sterilization and isolation of endophyte were carried out following the procedure described by Xing et al. [19]. In brief, surface sterilization was carried out in sequence using 75 % ethanol for 1 min, 6 % NaClO for 2 min and 75 % ethanol for 30 s, followed by rinsing in sterile distilled water three times. Approximately 2–5-mm pieces were cut from each root and stem sample. The medium for fungal isolation is seawater–malt agar containing artificial seawater 50 % (v/v) [20], malt extract 1.5 % (Aoboxing, China) and agar 1.4 % (Japan). Five segments were placed in 20 mL seawater–malt agar medium in each Petri dish. Plates were incubated at 25 °C in darkness. After 2 weeks, some pure strains were subcultured on PDA medium for morphological observations. The living cultures were preserved at −80 °C in Biotechnological Center, Institute of Medicinal Plant Development (IMPLD), Chinese Academy of Medical Science (CAMS).
Morphological and Molecular Identification
Endophytic fungi were preliminarily identified based on culture characteristics (colony shape, color of aerial hyphae, growth rate, margin and surface texture), conidiogenous cells and conidia features (color, shape and size) compared with other related species (Table 1).
Sequence-based methods were conducted for specimen identification. Genomic DNA was extracted from pure mycelium with the E.Z.N.A. Fungal DNA kit (Omega Bio-Tek, Doraville, GA, USA) according to the manufacturer’s protocol. Internal transcribed spacer regions (ITS), nuclear large subunit ribosomal RNA (LSU) and translation elongation factor (EF1α) were amplified with the primer pairs ITS1 and ITS4, LROR and LR7 [21] and EF1-983F and EF1-2218R (initially obtained from S. Rehner: ocid.nacse.org/research/deephyphae/EF1primer.pdf), respectively. PCR amplification and sequencing were performed following the method described by Chen et al. [22].
The nucleotide sequences determined in this study were deposited in GenBank, and their accession numbers are given in Table 2. The sequences were visually aligned together with closely similar DNA sequences retrieved from GenBank. The MP tree was constructed in Mega 5.0 using the Close-Neighbor-Interchange algorithm at search level of 1 [23]. The MP trees were estimated by bootstrap values with 1000 replicates. The origin of H. werneckii and related species published in GenBank database and PubMed database including ITS and 28S sequences is listed in Table 2. In phylogenetic analysis, Capronia semiimmersa is used as outgroup for ITS dataset and Mycosphaerella stromatosa as outgroup for LSU dataset.
Physiology
Thermotolerance, acid tolerance (pH) and NaCl tolerance as well as their pathogenicity in vitro of the strain 4263 were tested. The basic PDA medium was composed of potato 200 g/L, glucose 20 g/L and agar 15 g/L. The PDA Petri dish was inoculated with 1.5-mm-diameter agar block cut from colony edges of isolates grown on PDA agar in the following assays. For thermotolerance test, the pure cultures were incubated at 15, 20, 25, 30 and 35 °C for 3 weeks in darkness, respectively. For acid tolerance (pH) test, the initial pH was respectively adjusted to 3.0, 4.0, 5.0, 6.0, 7.0 and 8.0 prior to autoclaving. For NaCl tolerance test, NaCl concentration was set at 1, 2, 3, 4, 5, 10, 15 and 20 %, respectively. After inoculation, assay plate was incubated at 25 °C in darkness. Each experiment was replicated five times.
Pathogenicity Tests
Pathogenicity tests were performed in vitro with cultures of H. werneckii 4263 using the method described by Vettraino et al. [24] with minor modification. Excised leaves of healthy medicinal plants Dendrobium officinale, Anoectochilus roxburghii (Orchidaceae), Zea mays (Poaceae) and Atropa belladonna (Solanaceae) were surface-sterilized in 75 % ethanol for 1 min and then washed in sterile distilled water three times. The tested leaves were put on double-thick filter paper soaked in 3 mL sterile water in Petri dishes and inoculated with 1.5-mm-diameter agar plug cut from colony edges of isolates grown on PDA agar by needle-prickling inoculation method. Then, the tested samples were incubated at 25 °C under 12 h light–12 h dark condition. The agar plug without hyphae was considered as control. Each experiment was replicated five times.
Results
Morphological Examination
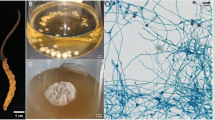
In the present study, 50 strains of culturable endophytic fungi were isolated from total 120 root and stem segments of A. comiculatum and identified based on morphological and molecular methods and included at least seven gena (Cladosporium, Pestalotiopsis, Hortaea, Massarina, Cochliobolus Aureobasidium, Fusarium). In the original 50 cultures, at least two strains (the strain numbers 4261 and 4263) were firstly identified as Hortaea spp. based on morphological characters. The colonies of 4263 and 4261 grow slowly, and three-week-old colonies are up to 5.5 cm in diameter. The colony is initially pale in color, moist, shiny and yeast-like and becoming dark olivaceous aerial mycelia (Fig. 2a, b). In the reverse side, the color is black. Aerial mycelium is pale brown, smooth, septate, branched and thick-walled in the conidiogenous region. Conidiogenous cells integrated, intercalary on hyphae, reduced to short cylindrical loci. The yeast-like conidia are ellipsoid bicellular blastospores, pale brown and usually occur in aggregated masses (6.0–) 7.3–9.5 (12.0) × 3.0–5.7 μm (Fig. 2c, d). Morphological characters of H. werneckii and other related black yeasts species, such as H. acidophila, H. thailandica and Aureobasidium sp., Phaeotheca sp. and Cladosporium sp., are provided in Table 1. By observing black hyphae and bicellular blastospores, combined with its NaCl tolerance (concentration of above 20 %), the strain 4263 was primarily identified as H. werneckii.
Phylogenetic Analyses
For identification, the fungi of 4263 were sequenced for ITS (the accession number JN974881), LSU (JX141471) and EF1a (JX141470). Because only a few reference sequences are present for EF1a of Hortaea in GenBank database, only ITS and LSU rRNA sequences for Hortaea were used for phylogenetic analysis. The ITS sequence analysis showed that the strains 4261 and 4263 are the same species, because none of the base changes were examined; thus, only strain 4263 was chosen for the further analysis. The ITS rDNA sequence alignments contained 32 sequences, of which 30 were downloaded from GenBank. Of the total 540 characters, 227 characters were constant and 202 were parsimony informative. One of the MP trees (158 trees, TL = 121, CI = 0.768, RI = 0.729) is shown in Fig. 3. The MP analyses supported the mangrove endophytic fungus 4263 as a member of the Hortaea genus (BP = 100 % in Fig. 3) and clustered 4263 strain as a member of other H. werneckii forming a subclade (BP = 100 %). Moreover, the 4263 strain showed 99 % similarity in ITS rDNA sequence to the sequence of H. werneckii in GenBank (accession numbers GQ334389) and 99 % similarity in EF1α sequence (JX141470) to that of H. werneckii in GenBank (GU349058).
Phylogenetic analyses of mangrove endophytic fungus 4263 inferred from ITS rDNA. The majority-rule consensus tree derived by MP analyses. Maximum parsimony bootstrap values (>50 %) are shown above the branch. C. semiimmersa is used as outgroup. Bold font shows that the sequences were acquired in our laboratory
The nrLSU dataset contained 17 sequences, 12 taxa and a total of 1,300 characters, of which 153 were parsimony informative. A maximum parsimony analysis (12 trees, TL = 232, CI = 0.763, RI = 0.844) strongly supported 4263 as other form of monophyletic H. werneckii (BP = 100 %) (Fig. 4) though Hortaea genus might be polyphyletic.
Phylogenetic analyses of mangrove endophytic fungus 4263 inferred from LSU rDNA. The majority-rule consensus tree derived by MP analyses. Maximum parsimony bootstrap values (>50 %) are shown above the branch. M. stromatosa is used as outgroup. Bold font show that the sequence was acquired in our laboratory
Physiology and Pathogenicity Tests
The fungus of H. werneckii was able to grow in a broad range of temperatures, with 25 °C being the optimum temperature (Fig. 5). Slow growth was also observed above 35 °C. Below 35 °C, the colonies do not horizontally spread, but grow vertically along the inoculating block, while a number of conidia are produced (Fig. 2c, d). The optimum pH for growth was 6.0, but it can grow in pH of 3 and 8 (Fig. 5). The fungus can tolerate an NaCl concentration of 20 % (Fig. 5).
Results form pathogenicity tests showed that the fungus did not cause conspicuously pathogenic symptom in excised leaves of four tested medicinal plants after the inoculation period of 10 days. And tissue section examination did not find fungal hyphae in the tested spot.
Discussion
Hortaea werneckii was primarily known as the etiological pathogen of human dermatosis called tinea nigra, a superficial infection of the human hand [25, 26], and sometimes it was isolated from some organs of body [27], but now is well known as a model microorganism for studying the mechanisms of salt tolerance in eukaryotes. It has recently been observed that this fungal species is capable of biotransformation of progesterone and human steroid hormone [13].
This species is a common saprophytic fungus in soil, compost, humus and surface and interior of wood submerged in brine [28]. In recently years, it has been isolated from humans and animals with superficial infectious lesions, seawater, limestone and natural or man-made salt pan environments, but its primary environmental ecological niche is hypersaline water (see Table 2). To our knowledge, black yeast H. werneckii has been rarely isolated and superficial mycosis caused by H. werneckii has been rarely reported in China. This is the first report of the organism from the stem of mangrove medicinal plant A. comiculatum collected in the South China Sea coast.
Colony characteristics of H. werneckii are similar and cannot be distinguished from the other black yeasts such as H. thailandica and Exophiala. Molecular analysis is particularly useful for fungal classification. Meanwhile, physiology features such as acid tolerance (pH) and NaCl tolerance are also a diagnostic character for black yeast identification. H. acidophila can grow in extreme acid environment but is unable to grow above 5 % NaCl [1]. Compared with H. acidophila, H. werneckii can grow above the NaCl concentration of 15 %. In addition, H. werneckii morphologically differs from other salt-tolerant yeast-like fungi of gena Phaeotheca and Hyphospora in their monilioid hyphae and blastospores rather than meristematic hyphae and endoconidia.
In the pathogenicity test, the isolate 4263 failed to cause any symptoms on excised leaves of D. officinale and A. roxburghii (Orchidaceae), Z. mays (Poaceae) and A. belladonna (Solanaceae). As it was well known, there are few reports about H. werneckii as plant pathogenic fungi all over the world. No disease symptom was caused by H. werneckii in our experiments, and it was possible that the tested medicinal plants were not suitable host. Due to isolation of H. werneckii from surface-sterilized tissues of healthy A. comiculatum, it appears that the fungi might reside in host plant as endophytes. Although the ecological function of H. werneckii in host plant A. comiculatum is not fully understood, some endophytic fungi can enhance plant growth and plant adaption to stress tolerance and disease resistance [29]. Because of the limited data (only two strains of the fungus were isolated from the host plant), it can be speculated that H. werneckii might enhance tolerance to high salt environments in the host plant A. comiculatum. Further studies of the ecological function of H. wernckii associated with mangrove plants are needed to address these issues.
References
Hölker U, Bend J, Pracht R, Tetsch L, Müller T, Höfer M, de Hoog GS. Hortaea acidophila, a new acid-tolerant black yeast from lignite. Antonie Van Leeuwenhoek. 2004;86(4):287–94.
Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, de Hoog GS, Groenewald JZ. Phylogenetic lineages in the Capnodiales. Stud Mycol. 2009;64:17–47.
Crous PW, Braun U, Groenewald JZ. Mycospharella is polyphyletic. Stud Mycol. 2007;58:1–32.
Perez C, Colella MT, Olaizola C, de Capriles CH, Magaldi S, Mata-Essayag S. Tinea nigra: report of twelve cases in Venezuela. Mycopathologia. 2005;160:235–8.
Bonifaz A, Badali H, de Hoog GS, Cruz M, Araiza J, Cruz MA, Fierro L, Ponce RM. Tinea nigra by Hortaea werneckii, a report of 22 cases from Mexico. Stud Mycol. 2008;61:77–82.
Ng KP, Soo-Hoo TS, Na SL, Tay ST, Hamimah H, Lim PC, Chong PP, Seow HF, Chavez AJ, Messer SA. The mycological and molecular study of Hortaea werneckii isolated from blood and splenic abscess. Mycopathologia. 2005;159:495–500.
Gunde-Cimerman N, Ramos J, Plemenitas A. Halotolerant and halophilic fungi. Mycol Res. 2009;113:1231–41.
Turk M, Abramovic Z, Plemenitaš A, Gunde-Cimerman N. Salt stress and plasma-membrane fluidity in selected extremophilic yeasts and yeast-like fungi. FEMS Yeast Res. 2007;7:550–7.
Turk M, Plemenitaš A, Gunde-Cimerman N. Extremophilic yeasts: plasma-membrane fluidity as determinant of stress tolerance. Fungal Biol. 2011;115:950–8.
Petrovič U, Gunde-Clmerman N, Plemenitaš A. Cellular responses to environmental salinity in the halophilic black yeast Hortaea werneckii. Mol Microbiol. 2002;45:665–72.
Turk M, Plemenitaš A. The HOG pathway in the halophilic black yeast Hortaea werneckii: isolation of the HOG1 homolog gene and activation of HwHog1p. FEMS Microbiol Lett. 2002;216:193–9.
Lenassi M, Zajc J, Gostinčar C, Gorjan A, Gunde-Cimerman N, Plemenitaš A. Adaptation of the glycerol-3-phosphate dehydrogenase Gpd1 to high salinities in the extremely halotolerant Hortaea werneckii and halophilic Wallemia ichthyophaga. Fungal Biol. 2011;115:959–70.
Matis M, Zakelj-Mavric M, Peter-Katalinić J. Global analysis of the Hortaea werneckii proteome: studying steroid response in yeast. J Proteome Res. 2005;4(6):2043–51.
Gopal B, Chauhan M. Biodiversity and its conservation in the Sundarban mangrove ecosystem. Aquat Sci. 2006;68:338–54.
Kathiresan K, Bingham BL. Biology of mangroves and mangrove ecosystem. Adv Mar Biol. 2001;40:81–251.
Lin P. A review on the mangrove research in China. J Xiamen Univ Nat Sci. 2001;40:592–603.
Lin ZJ, Zhu TJ, Fang YC, Gu QQ, Zhu WW. Polyketides from Penicillium sp. JP-1, an endophytic fungus associated with the mangrove plant Aegicera corniculatum. Phytochemistry. 2008;69:1273–8.
Lin W, Li L, Fu H, Sattler I, Huang X, Grabley S. New cyclopentenone derivatives from an endophytic Streptomyces sp. isolated from the mangrove plant Aegiceras comiculatum. J Antibiot. 2005;58:594–8.
Xing XK, Guo SX. Fungal endophyte communities in four Rhizophoraceae mangrove species on the south coast of China. Ecol Res. 2011;26:403–9.
Höller U, Konig GM, Wright AD. Three new metabolites from marine-derived fungi of the genera Coniothyrium and Microsphaeropsis. J Nat Prod. 1999;62:114–8.
White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. RCR protocols: a guide to methods and applications. New York: Academic Press; 1990. p. 315–22.
Chen J, Wang H, Guo SX. Isolation and identification of endophytic and mycorrhizal fungi from seed and root of Dendrobium (Orchidaceae). Mycorrhiza. 2012;22:297–307.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9.
Vettraino AM, Natili G, Anselmi N, Vannini A. Recovery and pathogenicity of Phytophthora species associated with a resurgence of ink disease in Castanea sativa in Italy. Plant Pathol. 2001;50:90–6.
Hughes JR, Moore MK, Pembroke AC. Tinea nigra palmaris. Clinical Exp Dermatol. 1993;18:481–3.
Ng KP, Soo-Hoo TS, Na SL, Tay ST, Hamimah H, Lim PC, Chong PP, Seow HF. Tinea nigra palmaris: a clinical case in Argentina. Rev Argent Microbiol. 2007;39:218–20.
Chavez AJ, Messer SA. The mycological and molecular study of Hortaea werneckii isolated from blood and splenic abscess. Mycopathologia. 2005;159:495–500.
Zalar P, de Hoog GS, Gunde-Cimerman N. Ecology of halotolerant dothideaceous black yeasts. Stud Mycol. 1999;43:38–48.
Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, von Wettstein D, Franken P, Kogel KH. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. PNAS. 2005;102:13386–91.
Schoch CL, Crous PW, Groenewald JZ et al. A class-wide phylogenetic assessment of Dothideomycetes. Stud Mycol 2009;64:1–15S10
Sharmin S, Haritani K, Tanaka R et al. The first isolation of Hortaea werneckii from a household guinea pig. Nippon Ishinkin Gakkai Zasshi, 2002;43(3):175–80.
Burgaud G, Arzur D, Durand L, Cambon-Bonavita MA,BarbierG. Marine culturable yeasts in deep-sea hydrothermal vents: species richness and association with fauna. FEMS Microbiol Ecol 2010;73(1):121–33.
De Leo F, Urzi C, de Hoog GS.A new meristematic fungus, Pseudotaeniolina globosa. Antonie Van Leeuwenhoek 2003;83(4):351–60.
Abliz P, Fukushima K, Takizawa K, Miyaji M, Nishimura K. Specific oligonucleotide primers for identification of Hortaea werneckii, a causative agent of tinea nigra. Diagn Microbiol Infect Dis 2003;46(2):89–93.
Rakeman JL, Bui U, Lafe K, Chen YC, Honeycutt RJ, Cookson BT. Multilocus DNA sequence comparisons rapidly identify pathogenic molds. J Clin Microbiol 2005;43(7):3324–33.
Cantrell SA, Casillas-Martinez L, Molina M. Characterization of fungi from hypersaline environments of solar salterns using morphological and molecular techniques. Mycol Res 2006;110:962–70.
Liu WC, Li CQ, Zhu P, Yang JL, Cheng KD. Phylogenetic diversity of culturable fungi associated with two marine sponges: Haliclona simulans and Gelliodes carnosa, collected from the Hainan Island coastal waters of the South China Sea. Fungal Divers 2010;42(1):1-15.
Cantrell SA, Casillas-Martinez L, Molina M. Characterization of fungi from hypersaline environments of solar salterns using morphological and molecular techniques. Mycol Res 2006;110:962–70.
Abliz P, Fukushima K, Takizawa K, Nieda N, Miyaji M, Nishimura K. Rapid identification of the genus fonsecaea by PCR with specific oligonucleotide primers. J Clin Microbiol 2003;41(2):873–6.
Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, de Hoog GS, Groenewald JZ. Phylogenetic lineages in the Capnodiales. Stud Mycol 2009;64:17–47S7.
Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. 4th ed. Minnesota, USA: The American Phytopathological Society Press; 1998.
Zalar P, de Hoog GS, Gunde-Cimerman N. Taxonomy of the endoconidial black yeast genera Phaeotheca and Hyphospora. Stud Mycol 1999;43:49–56.
De Hoog GS, Zeng JS, Harrak MJ, Sutton DA. Exophiala xenobiotica sp. nov., an opportunistic black yeast inhabiting environments rich in hydrocarbons. Antonie van Leeuwenhoek 2006:90(3):257–68.
Acknowledgments
This investigation was supported by the National Natural Science Foundation of China (30830117, 30900004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Xing, XK., Zhang, LC. et al. Identification of Hortaea werneckii Isolated from Mangrove Plant Aegiceras comiculatum Based on Morphology and rDNA Sequences. Mycopathologia 174, 457–466 (2012). https://doi.org/10.1007/s11046-012-9568-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-012-9568-1