Abstract
In an attempt to clarify the potential role of endophytic fungi in integrated pest management, the compatibility of an endophytic isolate of Lecanicillium lecanii (Zimmermann) Gams & Zare (Hyphomycetes) with nine insecticides used against Aphis gossypii Glover (Homoptera : Aphididae) was examined both in vitro over 14 days and in planta. In the laboratory, most insecticides partially or completely inhibited the germination of conidia and growth of hyphae in nutrient-rich conditions. Endosulfan completely inhibited the germination of conidia and hyphal growth. In contrast, all insecticides were compatible with L. lecanii in planta, and the fungus was readily recovered from inoculated, colonized leaves. These data support the hypothesis that endophytic L. lecanii will be unaffected by insecticides and could be integrated in the management of pests in cotton.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aphis gossypii Glover (Cotton aphid) (Homoptera: Aphididae) is a polyphagous species of aphid widely distributed in tropical, subtropical and temperate regions. A. gossypii is a key pest of cotton, citrus and cucurbits, and in temperate regions, it principally attacks vegetables in fields and greenhouses [1, 2]. The cotton aphid is emerging as a major pest; it has developed resistance to a broad range of insecticides [3–5] and is a vector of Cotton bunchy top virus [6]. The use of entomopathogens might provide an effective complementary approach in the integrated management of this pest in cotton.
Lecanicillium lecanii (Zimmermann) Gams & Zare is a cosmopolitan fungus associated with insects. L. lecanii is pathogenic to several species of aphids and white flies [7–9]. Commercial formulations of Lecanicillium are available as Mycotal® and Vertalec® against whiteflies and aphids, respectively [10]. L. lecanii can colonize the leaves of cotton and an endophyte [11] and possibly many other plant species [12, 13]. Presence of the endophytic fungus may enhance plant resistance to aphids through a variety of potential mechanisms including increased feeding deterrence, induced plant defence responses, antibiosis due to fungal metabolites, as well as providing a source of inoculum to enable direct infection of aphids [11]. However, the establishment of L. lecanii as an endophyte or as the casual agent of an aphid epizootic in the field might be affected by a wide range of insecticides directed against aphids. Compatibility between entomopathogens and insecticides used against aphids in cotton would enable their complementary use to be considered as part of an integrated control strategy.
Studies of in vitro compatibility of pesticides with the entomopathogenic fungi L. lecanii, Beauveria bassiana (Balsamo) Vuillemin, Metarhizium anisopliae ((Metchnikoff), Paecilomyces farinosus (Holmsk. exGray) A. H. S. Br. & G. Sm., P. fumosoroseus (Wize) Brown & Smith, Scopulariopsis brevicaulis Bainier, Conidiobolus thromboides Drechsler, C. coronatus (Constantin) Batko and Pandora nouryi (Remaudiere and Hennebert) Hubner [14–24] indicated variable effects of direct exposure to pesticides on the growth and sporulation of the fungus and germination of the conidia. Few studies have addressed the effect of pesticides on the entomopathogenic fungi when the fungus was on the plant. The dry residues of insecticides on tomato and verbena plants had no significant effects on the control of Bemisia tabici Gennadius by the fungus L. muscarium, though they significantly reduced the spore germination in the laboratory [23]. A strain of Verticillium sp DSP degraded the residues of chlorpyrifos in contaminated soil [25].
No studies to date have examined the effects of pesticides on entomopathogenic fungi when the fungus is endophytic in the crop plant or whether the use of pesticides influences the initiation or spread of fungal colonization of leaves. Laboratory studies indicate that pesticides may severely reduce the initiation of colonization through inhibition of germination of conidia, reduce the spread of colonization through inhibition of hyphal tip function and indeed reduce the size of a colony when the fungus is established in the leaf. Inhibition of the fungus by insecticides would reduce the value of the endophytic stage of these entomopathogens. Therefore, the present investigation aimed to determine the in vitro and in planta compatibility of insecticides used against cotton aphids in Australia with the entomopathogen L. lecanii.
Materials and Methods
Lecanicillium lecanii was isolated from within asymptomatic leaves of cotton from a cotton variety trial at Narrabri, New South Wales, Australia [26], and maintained on potato dextrose agar (PDA). Morphological identifications were supplemented by sequence data from the ITS region of nuclear ribosomal DNA. Both the primers ITS 1 and ITS 4 were used for PCR amplification and sequenced in both directions [27]. ITS sequences from the isolate were matched to reference sequences based on similarity to other known nucleotide sequences using a BLAST search (http://www.ncbi.nlm.nih.gov/BLAST/). Sequence data from the isolate used in this study were deposited in GenBank as accession number GU953211.
Mycelial plugs and conidia required for the experiments were obtained from 14-day-old L. lecanii cultures maintained in the laboratory at 24°C. The insecticides used in this study (Table 1) are recommended for use against cotton aphid in Australia.
In Vitro Studies
The effect of each insecticide on hyphal elongation in vitro was tested at three concentrations, (R = manufacturer’s recommendation for field operations, R/2 = Half the recommended concentration and 2R = 2-fold recommended concentration). Each insecticide was separately added to molten cooled PDA media. A 6-mm mycelial plug was taken using a cork borer from the edges of a 14-day-old colony and was placed in the centre of 5 replicate plates and the plates were incubated at 24°C. The radial elongation of L. lecanii was measured from the edge of the plugs 7 and 14 days after inoculation (DAI) and the data compared with growth on untreated PDA.
Because growth on solid media might limit the contact between mycelia of the fungus and the insecticide, the effect of insecticides on mycelial growth in liquid suspension was examined. The insecticides at R and R/2 concentrations were added to 100 ml of Czapek-Dox (composition: MgSO4.7H2O 0.5 g, FeSO4 0.01 g, KCl 0.5 g, K2HPO4 1 g, NaNO3 3 g, sucrose 30 g, distilled water 1000 ml) solution in a 250-ml conical flask. The flasks were inoculated with 5 mycelial plugs (cut using a No. 5 cork borer) from 14-day-old colonies. Growth of five replicates of each treatment was compared with growth in media lacking insecticide. Flasks were placed in a completely randomized design and incubated on a rotary shaker at 120 rpm at 20°C. After 7-day incubation, the content of the flask was filtered using Whatman filter paper. The resulting mycelial mats were dried in an oven at 80°C for 2 days and weighed.
To determine the effect of insecticides on conidial germination, conidia of L. lecanii were harvested from 14-day-old cultures on PDA by scraping the surface with a sterile scalpel after flooding the plates with sterile water containing 0.02% Triton ×100. The conidial suspension was filtered to remove the hyphal debris. The conidial concentration was determined using a Neubauer haemocytometer and adjusted to 1 × 108 conidia/ml with sterile water. A suspension of 1 ml of 1 × 108 conidia/ml was added to 9 ml of each of 5 replicates of insecticide concentration (R, R/2 and 2R). For the control treatment, 1 ml of 1 × 108 conidia/ml was added to 9 ml of sterile water. After incubation for 1 h at 20°C, 100 μl of each suspension was inoculated to the surface of a fresh plate of PDA. The inoculated plates were incubated for 24 h at 20°C. Percent conidial germination was assessed by counting the proportion of germinated conidia out of 100 in randomly selected fields of view on each plate. Conidia were considered as germinated when germ tubes exceeded half of the length of the conidium.
In Planta Studies
Surface-sterilized seeds of cotton var. Sicala V2 were sown 5 cm below the surface of a potting mix (a mixture of a black clay soil and sand-peat mixture in a 1:1 ratio) in a 1.5-l plastic pot. There was only one plant per pot. Single seedlings were maintained in a growth cabinet with a 12-h day (26°C) and 12-h night (20°C). The plants in pots were watered twice a week and fertilized with 20 ml of Hoagland’s solution [28] weekly.
To study the endophytic establishment of the fungus in cotton plants treated with insecticides, only one concentration (R) was used. Seedlings with three fully expanded leaves were sprayed with the recommended concentration of insecticides to run off. The control plants were sprayed to run off with water only. After 24 h, the second fully expanded leaf from the top was inoculated with L. lecanii by placing an entire 14-day culture (4 cm diameter) on agar on the moistened upper surface of the leaf [26]. There were five plants for each insecticide treatment in a randomized complete block design. The negative control plants were treated with an equal surface area of agar without fungus. The experiment was repeated twice. The leaves thus inoculated were covered with cling wrap for 48 h to maintain relative humidity. Endophytic colonization was confirmed by re-isolation of the fungus from the leaves 1 week after inoculation. Re-isolation was chosen because the fungus is difficult to visualize when in leaf tissue. Re-isolation is only possible if the fungus retains viability. Leaves from the inoculated and uninoculated control plants were excised, surface-sterilized with 70% ethanol for 2 min, bleached (2% Chlorine) for 3 min and rinsed with sterile distilled water three times. The leaves were aseptically cut into 1 × 1 cm segments, and all segments were placed on the PDA plates. The efficacy of surface sterilization was ascertained by pressing a sub-sample of the surface-sterilized leaves on a PDA plate and also by spreading 100 μl of sterile distilled water used for rinsing on a PDA plate. The plates were incubated at 20°C. Endophyte establishment was determined in five randomly selected replicate leaves from each treatment 10 days after inoculation to PDA. The data were expressed as colonization frequencies where colonization frequency = 100× (number of plant pieces colonised/total number of plant pieces).
Statistical Analysis
Data on colony diameter, mycelial weight and conidial germination were analysed with two-way ANOVA, whereas in planta study data were analysed by one-way ANOVA using Genstat® for Windows (11th edition 2009). The percentage conidial germination values were subjected to arcsine transformation to stabilize variance. Treatment effects were analysed for significance using an F-test (P = 0.05). Significant treatment effects were further analysed using Duncan’s multiple range test (DMRT).
Results
In Vitro Studies
Colony diameter on agar differed significantly between the insecticides (F = 21.57, df = 9, P < 0.001 and F = 124.16, df = 9, P < 0.001 at 7 and 14 DAI, respectively) and insecticide concentration (F = 7.51, df = 2, P < 0.001 and F = 55.19, df = 2, P < 0.001 at 7 and 14 DAI, respectively). The effect of the interaction between insecticide and concentration was also significant at 7 and 14 DAI (F = 3.38, df = 18, P < 0.001 and F = 4.35, df = 18, P < 0.001, respectively: Table 2).
At 7 DAI, imidacloprid and acetamiprid did not significantly inhibit the colony diameter at all the concentrations tested. Endosulfan and Thiodicarb significantly inhibited the colony growth in all the concentrations (Table 2).
At 14 DAI, only acetamiprid did not cause any significant reduction in colony diameter at 2R and R. Thiodicarb, primicarb, omethoate and endosulfan significantly inhibited the colony diameter at all the concentrations tested (Table 2).
Mycelial growth in liquid media differed significantly between the insecticides (F = 10.69, df = 9, P < 0.001) and concentrations (F = 46.52, df = 1, P < 0.001). Endosulfan completely inhibited the mycelial growth at both the concentrations. At the recommended field concentration, all the insecticides significantly inhibited the mycelial growth. However, at R/2, omethoate and pymetrozine caused no significant effect on the mycelial growth (Table 3).
Conidial germination differed between the insecticides (F = 2965.07, df = 9, P < 0.001) and concentrations (F = 58.85, df = 2, P < 0.001). The interaction effects (F = 8.59, df = 18, P < 0.001) were also significant indicating that proportional conidial germination decreased with an increase in concentration of insecticide, except with omethoate, and acetamiprid where there were no significant differences. All the insecticides significantly inhibited the conidial germination at 2R and R. Thiamethoxam, imidacloprid and pymetrozine did not cause any significant reduction in conidial germination at R/2 (Table 4).
In Planta Studies
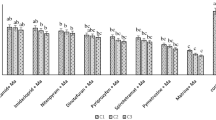
The endophytic establishment of L. lecanii was highly variable and declined in treated leaves, but the differences were not statistically significant (P = 0.066). Proportional isolation varied from a mean 22% in omethoate to 47% in the positive control (Fig. 1). Lecanicillium lecanii was not isolated from the negative control plants.
Discussion
Lecanicillium sp. is an important entomopathogen used for the control of aphids and white flies [7]. L. lecanii may infect the cotton aphid from both conidia and endophytic colonies [11]. Compatibility with insecticides commonly used against the pest is essential if L. lecanii is to be used in IPM. Previous studies have indicated the importance of testing the compatibility of insecticides and entomopathogens under both laboratory and field conditions. The present study highlights differences in the effects of insecticides on the fungus when observed in vitro versus temporal effect in planta.
In laboratory tests, significant differences in the effects on the fungus were observed among the insecticides, concentrations and their interactions at 7 and 14 DAI. Endosulfan completely inhibited hyphal elongation on agar and in liquid media and the germination of conidia. Other fungi inhibited by endosulfan include Hirsutella nodulosa (Petch) [29] and B. bassiana [22, 30]. The insecticide thiodicarb also significantly reduced the hyphal growth and germination of conidia. While we are not aware of any studies that compare the compatibility of thiodicarb with entomopathogens, similar inhibition might be predicted. The other insecticides did not significantly affect the fungal growth. Increased colony growth over control was observed in imidacloprid treatment at 2R. Similar observations were recorded for Paecilomyces sp. [31]. A sustained increase in fungal elongation may indicate that substances present in the formulation are being used for nutrients by the fungus [31]. However, only limited supplementation was evident and then on only one harvest of the treatment with imidacloprid.
Treatment with imidacloprid had no effect on colony diameter of B. bassiana [29, 31]. However, contrasting results were observed for all the acetamiprid and thiomethoxam treatments [31]. The insecticide pirimicarb did not significantly affect the radial growth of Paecilomyces fumosoroseus [20] and V. lecanii [10]. Pymetrozine slightly inhibited the radial growth of P. fumosoroseus at recommended concentration, while it was similar to the control at lower concentrations [20]. At 14 DAI, only acetamiprid was on par with the control treatment at R and R/2. This corroborates reports that the effect of pesticides in the media may decline overtime [20].
In general, the insecticides reduced the germination of conidia, though thiomethoxam and pymetrozine were not statistically different from the control treatment at R/2. Imidacloprid reduced the germination of L. muscarium [23] and B. bassiana conidia [29]. There was no significant inhibition of conidia germination of B. bassiana, M. anisopliae and Paecilomyces sp by acetamiprid, imidacloprid and thiomethoxam treatments [31]. Pymetrozine slightly inhibited the germination of conidia of P. fumosoroseus at the recommended concentration but was similar to the control treatment at lower concentrations [20]. Conidial germination was more severely affected than the radial growth of the fungus overall [10, 20], but contradictory results were observed by other authors [32, 33]. Thus, these data broadly support the hypothesis that insecticides reduce fungal growth and germination of conidia at recommended rates of use.
In contrast to the in vitro studies, L. lecanii established in plants sprayed with all of the insecticides used. Only a few studies have compared the compatibility of pesticides and fungus between laboratory and more natural conditions. For instance, the fungicides that reduced colony diameter and germination of M. anisopliae in the laboratory had no significant effects on the CFU in bulk soil [32]. The fungicides that inhibited the fungal colony of M. anisopliae in the laboratory were compatible in the glasshouse possibly due to the spatial separation of the fungicides and conidia [35]. The reduction in the effects of insecticides on plants might be because less insecticide is intercepted by the plant surface and also that systemic insecticides are distributed throughout the plant, thereby diluting the insecticide [36]. Mycelial growth of L. muscarium was previously observed to be similar to the control when the fungus was applied to plants containing residues of insecticides [23, 34]. These studies broadly indicate protection from insecticides when then the fungi are in more natural conditions. Although this study examined only one isolate of L. lecanii, a similar degree of protection from insecticides may be expected for other isolates and probably most fungi.
The impact of various insecticides on germination of conidia and fungal growth in vitro indicates a direct “cost” of these compounds to L. lecanii. However, in plants, regeneration of the fungus from inoculated leaves was statistically similar across insecticides. The major “cost” in vitro was not evident in living plant tissue. Recovery of the fungus indicates successful establishment and initiation of colonies in leaves of cotton.
The potential use of L. lecanii as an endophyte is a novel approach to the management of cotton aphids in an integrated pest management programme. Addition of the fungus to IPM would complement chemical approaches as the fungus does not react significantly to the use of insecticides. Potential problems remain with the use of natural and introduced parasitoids as they may also be infested by the fungus. L. lecanii is known only to infest sap-sucking insects, most of which are pests of crop plants. Thus, the potential impact on non-target insects is likely to be quite small. The advantage of IPM is that each element of the strategy may add to the overall reduction of the pest while reducing the chances of the pest developing resistance to one particular component of the approach. Additional elements in IPM will enable more effective and long-term control of insect pests in agriculture and horticulture. The present study shows that none of the insecticides had a significant effect on the establishment of L. lecanii as an endophyte in cotton seedlings although the insecticides severely reduced the germination of conidia and fungal growth in the laboratory. Some further issues remain to be clarified, including survival of the fungus in plants and complementarity of entomopathogens and other elements of IPM on survival of aphids.
References
Blackman RL, Eastop VF. Aphids on the world’s crops, an identification guide. Chichester: John Wiley & Sons; 1985.
Leclant F, Deguine JP. Cotton aphids. In: Mathews GA, Tunstall JP, editors. Insect pests of cotton. UK: C. A. B; 1994. p. 285–323.
Herron GA, Rophail J, Powis K. Insecticide resistance in cotton aphid. In: ‘Cotton on to the future’: the eighth Australian cotton conference, Conrad Jupiters, Broadbeach, Gold Coast, Queensland, 1996; 14–16 Aug 1996. pp. 141–146.
Wang KY, Liu TX, Yu CH, Jiang XY, Yi MQ. Resistance of Aphis gossypii (Homoptera: Aphididae) to fenvalerate and imidacloprid and activities of detoxification enzymes on cotton and cucumber. J Econ Entomol. 2002;95:407–13.
Ahmad M, Arif MI, Denholm I. High resistance of field populations of the cotton aphid Aphis gossypii Glover (Homoptera: Aphididae) to pyrethroid insecticides in Pakistan. J Econ Entomol. 2003;96:875–8.
Reddall A, Ali A, Able JA, Stonor J, Tesoriero L, Wright PR, Rezaian MA, Wilson LJ. Cotton bunchy top: an aphid and graft transmitted cotton disease. Australas Plant Pathol. 2004;33:197–202.
Cuthbertson AGS, Walters KFA. Pathogenicity of the entomopathogenic fungus, Lecanicillium muscarium, against the sweetpotato whitefly Bemisia tabaci under laboratory and glasshouse conditions. Mycopathologia. 2005;160:315–9.
Kim JJ, Goettel MS, Gillespie DR. Potential of Lecanicillium species for dual microbial control of aphids and the cucumber powdery mildew fungus, Sphaerotheca fuliginea. Biol Control. 2007;40:327–32.
Kim JJ, Goettel MS, Gillespie DR. Evaluation of Lecanicillium longisporum, Vertalec (R) for simultaneous suppression of cotton aphids, Aphis gossypii, and, cucumber powdery mildew, Sphaerotheca fuliginea, on potted cucumbers. Biol Control. 2008;45:104–9.
Hall RA. The fungus Verticillium lecanii as a microbial insecticide against aphids and scales. In: Burges HD, editor. Microbial control of pests and diseases 1970–1980. London: Academic Press; 1981. p. 483–98.
Anderson CMT, McGee PA, Nehl DB, Mensah RK. The fungus Lecanicillium lecanii colonises the plant Gossypium hirsutum and the aphid Aphis gosypii. Aust Mycol. 2007;26:65–70.
Pampapathy G, Sword GA, Murdoch G, McGee PA. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol Control. 2010;55:34–41.
Vega F. Insect pathology and fungal endophytes. J Invertebr Pathol. 2008;98:277–9.
Khalil SK, Shah MA, Naeem M. Laboratory studies on the compatibility of entomopathogenic fungus Verticillium lecanii with certain pesticides. Agric Ecosyst Environ. 1985;13:329–34.
Majchrowicz I, Paprawski TJ. Effects in vitro of nine fungicides on growth of entomopathogenic fungi. Biocontrol Sci Technol. 1993;3:321–36.
Paprawski TJ, Majchrowicz I. Effects of herbicides on in vitro vegetative growth and sporulation of entomopathogenic fungi. Crop Prot. 1995;14:81–7.
Saito T, Yabuta M. Laboratory studies on effect of pesticides on entomopathogenic fungus, Verticillium lecanii. Jpn J Appl Entomol Zool. 1996;40:71–6.
Todorova SI, Coderre D, Duchesne RM, Cote JC. Compatibility of Beauveria bassiana with selected fungicides and herbicides. Environ Entomol. 1998;27:427–33.
Andalo V, Moino A, Santa-Cecilia LVC, Souza GC. Compatibility of Beauveria bassiana with chemical pesticides for the control of the coffee root mealybug Dysmicoccus texensis tinsley (Hemiptera: Pseudococcidae). Neotropical Entomol. 2004;33:463–7.
Er MK, Gokce A. Effects of selected pesticides used against glasshouse tomato pests on colony growth and conidial germination of Paecilomyces fumosoroseus. Biol Control. 2004;31:398–404.
Li W, Wang XF, Sheng CF. Impact of sixteen chemical pesticides on conidial germination of two entomopathoralean fungi: Conidiobolus thromboides and Pandora nouryi. Biocontrol Sci Technol. 2004;14:737–41.
Oliveira CN, Neves PMOJ, Kawazoe LS. Compatibility between the entomopathogenic fungus Beauveria bassiana and insecticides used in coffee plantations. Sci Agric. 2003;60:663–7.
Cuthbertson AGS, Waltera KFA, Deppe C. Compatibility of the entomopathogenic fungus Lecanicillium muscarium and insecticides for eradication of sweetpotato whitefly, Bemisia tabaci. Mycopathologia. 2005;160:35–41.
Samson PR, Milner RJ, Sander ED, Bullard GK. Effect of fungicides and insecticides applied during planting of sugarcane on viability of Metarhizium anisopliae and its efficacy against white grubs. Biocontrol. 2005;50:151–63.
Fang H, Xiang YQ, Hao YH, Chu XQ, Pan XD, Yu JQ, Yu YL. Fungal degradation of chlorpyrifos by Verticillium sp DSP in pure cultures and its use in bioremediation of contaminated soil and pakchoi. Int Biodeterior Biodegra. 2007;61:294–303.
McGee PA. Reduced growth and deterrence from feeding of the insect pest Helicoverpa armigera associated with fungal endophytes from cotton. Aust J Exp Agric. 2002;42:995–9.
White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego, CA: Academic Press; 1990. p. 315–22.
Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Univ. of Calif. Agric. Exp. Station, Berkeley 1950; Circ. 347.
Alizadeh A, Samih MA, Khezri M, Riseh RS. Compatibility of Beauveria bassiana (Bals.) Vuill. with several pesticides. Int J Agric Biol. 2007;9:31–4.
Tkaczuk C, Labanowska BH, Mietkiewski R. The influence of pesticides on the growth of fungus Hirsutella nodulosa (Petch)-Entomopathogen of strawberry mite (Phytonemus pallidus ssp. fragariae Zimm.). J Fruit Ornam Plant Res. 2004;12:119–26.
Neves PMOJ, Hirose E, Tehujo PT, Moino AM Jr. Compatibility of entomopathogenic fungi with neonicotinoid insecticides. Neotropical Entomol. 2001;30:263–8.
Bruck DJ. Impact of fungicides on Metarhizium anisopliae in the rhizosphere, bulk soil and in vitro. Biocontrol. 2009;54:597–606.
Moorhouse ER, Charnely AK, Gillespie AT. Review of the biology and control of the vine weevil, Otiorhynchus sulcatus (Coleoptera: Curculionidae). Ann Appl Biol. 1992;121:431–54.
Cuthbertson AGS, Blackburn LF, Northing P, Luo W, Cannon RJC, Walters KFA. Further compatibility tests of the entomopathogenic fungus Lecanicillium muscarium with conventional insecticide products for control of sweetpotato whitefly, Bemisia tabaci on poinsettia plants. Insect Sci. 2008;15:355–60.
Chandler D, Davidson G. Evaluation of entomopathogenic fungus Metarhizium anisopliae against soil-dwelling stages of cabbage maggot (Diptera: Anthomyiidae) in glasshouse and field experiments and effect of fungicides on fungal activity. J Econ Entomol. 2005;5:1856–62.
Latteur G, Jansen JP. Effects of 20 fungicides on the infectivity of conidia of the aphid entomopathogenic fungus Erynia neoaphidis. Biocontrol. 2002;47:435–44.
Acknowledgments
We thank Syngenta Crop Protection Pty Limited, Bayer Crop Science and Dupont for providing the insecticides for the study. The primary author would also like to thank the University of Sydney for providing the scholarship to carry out his PhD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gurulingappa, P., Mc Gee, P. & Sword, G.A. In Vitro and In Planta Compatibility of Insecticides and the Endophytic Entomopathogen, Lecanicillium lecanii . Mycopathologia 172, 161–168 (2011). https://doi.org/10.1007/s11046-011-9410-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-011-9410-1