Scanning electron microscopy and confocal laser scanning microscopy are used to study a fracture surface of hydrogen-charged low-carbon steel, which has been deformed in air at room temperature to 12% residual strain and then crushed in liquid nitrogen. It is shown that the quasi-cleavage facets formed during the room-temperature deformation have a strongly curved surface in contrast to the flat cleavage facets formed under the loading in liquid nitrogen. It is inferred that the quasi-cleavage facets in the hydrogen-charged steel do not form by the mechanism of cleavage in the deformed structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen embrittlement (HE) has been an important problem for the oil, gas, chemical and other industries for tens of years. Despite the many-year history of investigation, the nature of HE still remains an object of discussion. One of the most complicate disputed topics is the mechanism of fracture of steels [1, 2]. It has been shown that depending on a number of conditions, fracture of a steel after its saturation with hydrogen may be accompanied by intragrain cracking [3, 4], transgranular cleavage [5], quasi-cleavage [5,6,7], formation of an unusual dimple relief [1], appearance of flakes in fractures [8], “fish eye” flaws [9, 10], etc. Specifically, an actively discussed topic of today is the role of hydrogen in the mechanism of formation of fractures with a quasi-cleavage morphology, also termed cleavage-like or quasi-brittle one [7, 11,12,13]. The term “quasi-cleavage” is used frequently to describe steels fractures with different microstructure including the cases not connected with HE [14, 15]. In accordance with the concept of M. A. Shtremel [15], “quasi-cleavage is a collective term for fractures having the form of a number of terraces, i.e., approximately flat transgranular areas without definite orientation with respect to the grain lattice as a whole.” He assumes that formation of such a relief is a consequence of cleavage in a structure with high dislocation density. Cleavage in bcc irons and steels occurs over crystallographic planes of the {001} family [15], and therefore is accompanied in an annealed microstructure by formation of flat facets with the same crystallographic orientation. However, in accordance with [15], flat facets are impossible in a strongly strained structure, because at a high dislocation density the crystallographic cleavage planes are turned by a large angle, which transforms a cleavage facet into a smooth curvilinear surface. It has been shown in a recent work [16] with the help of confocal laser scanning microscopy (CLSM) that quasi-cleavage facets on the surface of “fish eye” flaws in a hydrogen-charged low-carbon steel have a curvilinear profile in contrast to the flat cleavage facets in the fracture of the same steel broken in liquid nitrogen. Since the hydrogen-charged specimens in [16] have undergone higher deformation before the fracture than those tested in liquid nitrogen, it may be expected, in accordance with the arguments of Shtremel, that the curved quasi-cleavage facets in the hydrogen-charged steel are cleavage facets in deformed ferrite grains. However, there is a steady opinion that the mechanism of formation of a quasi-cleavage relief in the case of HE does not concern cleavage fracture [7, 11,12,13]. For example, the authors of [7] assume that a quasi-cleavage facet morphology in a hydrogen-charge ferrite steel forms by a ductile mechanism due to formation and growth of micropores generated at the intersection of slip bands. Since the data on this topic are controversial, we aimed the present work at determining whether the cleavage in a structure with high dislocation density is a mechanism of formation of quasi-cleavage facets on the surface of “fish eye” flaws in a hydrogen-charged low-carbon ferritic steel.
Methods of Study
We studied steel S235JR with chemical composition (in wt.%) 0.140 C, 0.022 Si, 0.463 Mn, 0.089 Cr, 0.037 Ni,
0.068 Cu, 0.059 Al, < 0.005 V, 0.015 P, 0.011 S, the matrix Fe. A flat smooth sample for testing for uniaxial tension with size 15 × 4 × 2.5 mm was cut from a hot-rolled sheet using an electrospark mill. All the faces of the sample were ground in succession with emery paper of different grain sizes up to number 2500. Then the sample was annealed in vacuum at 950°C for 30 min.
The sample was charged with hydrogen by an electrolytic method in a 5% solution of H2SO4 with a 1.5 g/liter addition of thiourea to stimulate the charging with hydrogen at current density 400 mA/cm2 for 1 h. Right after the hydrogen charging, the sample was washed with running cold water, dried elaborately, and fixed in the grips of an H50KT universal testing machine (Tinius Olsen); the lower grip was equipped with a vessel for pouring liquid nitrogen. The sample was extended at room temperature to 12% residual strain at a constant speed of the beam equal to 5 mm/min. Then the sample was unloaded and the vessel was filled with liquid nitrogen fully covering the sample. When the nitrogen stopped to boil, the sample was loaded again until failure.
One more identical sample saturated with hydrogen by the same method was placed into a Galileo G8 (Bruker) gas analyzer right after hydrogen charging to determine the concentration of the diffusion-mobile hydrogen. The gas analysis was conducted by the method of extraction heating in a flow a nitrogen carrier gas. The sample was heated to 200°C at a rate of 17 K\min and then held at this temperature for 15 min. The residual concentration of hydrogen in the sample was determined by the method of melting in a nitrogen carrier gas with the help of the same device.
Fracture surfaces were studied using a SIGMA (Zeiss) scanning electron microscope and a Lext OLS4000 (Olympus) CLSM.
Results
According to the results of the gas analysis, the total content of hydrogen in the sample after hydrogen charging was 32 ppm, and the concentrations of the diffusion-mobile and strongly bonded hydrogen were 30 and 2 ppm respectively.
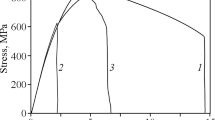
As a result of the mechanical tests by the method described above, we plotted a stress-strain diagram (Fig. 1), from which we may infer that the sample tested in liquid nitrogen has fractured in the range of quasi-elastic deformations without any additional plastic strain. However, when a similar sample was tested in liquid nitrogen without subjecting it to preliminary charging with hydrogen and plastic deformation, the stress-strain diagram exhibited a well manifested yield plateau and a short region of the stage of strain hardening [16].
Analysis of the fracture surfaces has shown the presence of several “fish eye” flaws in the form of round regions on the fracture surface a with faceted quasi-cleavage morphology (Fig. 2a). The orientation of the river lines decorating the surface of the quasi-cleavage facets indicates that the “fish eyes” have formed in a radial direction from the center (Fig. 2a – c). Outside the “fish eyes” the fracture was fully represented by cleavage facets. The images obtained by SEM and CLSM exhibit a well-manifested interface between the “fish eye” flaw and the cleavage surface (Figs. 2 and 3). It can be seen from Figs. 2 and 3 that the quasi-cleavage facets have a different contrast and a much more developed surface relief than the cleavage facets. At the same time, like the cleavage facets, they possess well-manifested boundaries and remind ferrite grains in the microstructure of the steel in the size and in the shape. Adistinguishing feature of the morphology of the quasi-cleavage facets is the presence of numerous detachment ridges on their surface and of bands oriented almost perpendicularly to the river lines and resembling fatigue striations (marked with the arrows in Fig. 2d).
“Fish eye” flaw with quasi-cleavage morphology surrounded with cleavage facets (SEM): a) appearance; b – d ) magnified regions on the cleavage/quasi-cleavage interface; the arrows in Figs. c and d mark sources of nucleation of cleavage facets and a band perpendicular to the river lines, respectively; a – c) SE detector; d ) in lens detector.
Relief of a fracture surface on a cleavage/quasi-cleavage interface: a) 2D CLSM image; b ) the same in 3D; c) profile over line 1 in Fig. 3, a.
By the data of the CLSM study, the relief of the fracture surface within a “fish eye” flaw and outside its boundary differs substantially. As an example, we present in Fig. 3c the profile of a fracture surface along a secant plane over line 1 in Fig. 3a so that it passes both through the surface of a “fish eye” and through the cleavage surface intersecting their interface (Fig. 3b ). It can be seen in Fig. 3c that there is a well manifested boundary of transition from a smoothly curved profile region to a region described by a broken line on the quasi-cleavage/cleavage interface (the vertical dashed line). Thus, the data obtained show that the surface of a quasicleavage facet has a strongly curved profile, whereas the cleavage facets are flat. Outside the “fish eye” flaw some grains break forming two or more cleavage facets (Fig. 3). In all these cases the facets are oriented at an angle of 90° to each other (one such angle is marked in Fig. 3c), and their interface is a straight line (dashed line 2 in Fig. 3a). We have not detected facets of this type on the surface of the “fish eye” flaws.
Discussion
It has been shown reliably that the appearance of “fish eye” flaws in fractures due to static testing of steels is connected with the presence of hydrogen in the steel [9, 10, 16]. It is also known that such flaws start to form in the annealed structure of low-carbon steel under uniaxial tension simultaneously with the beginning of plastic straining [9, 17]. At least, they are observed in the structure already in the early stage of strain hardening [17]. In the present work, the first loading of the hydrogen-charged sample conducted at room temperature was stopped at a residual strain of 12%, i.e., in the final stage of strain hardening for a steel charged with hydrogen under such conditions. It is obvious that the “fish eye” flaws detectable in the fracture have been formed under the loading at room temperature. As a result of cooling to the temperature of liquid nitrogen, hydrogen looses its diffusion mobility and stops to participate in the fracture process. The “fish eye” flaws are “frozen” in the stage of their development when the loading is stopped. At a low temperature, when plastic straining of the steel is difficult, the specimen is in an embrittled condition, and the flaws play the role of sharp notches from which cleavage cracks start. This is confirmed by the diverging fan-like river lines on the cleavage facets neighboring quasi-cleavage facets (the arrows in Fig. 2c ). As a consequence, the sample fractures in liquid nitrogen by a brittle mechanism in the stage of quasi-elastic straining (Fig. 1). Thus, the straining of the microstructure under the surface in the fracture region represented by cleavage facets and under the surface of the “fish eye” flaws should be about the same. However, according to the results of the study of the topography of the fracture surface, the quasi-cleavage facets of the “fish eye” flaw have a strongly curved profile, whereas the cleavage facets remain virtually flat. Had the curvilinear profile of the surface of the quasicleavage facet formed as a result of growth of a cleavage crack in a strained structure, the cleavage facets formed under loading of the sample in liquid nitrogen would have had a similar profile. In this case the “fish eye” flaws can hardly be observable, because the morphology of their surface should not differ from the rest part of the fracture. However, the experiment has given an opposite picture. Consequently, the straining of the microstructure could not have been the cause of curving of the quasi-cleavage facets. It seems that the 12% strain is not sufficient for noticeable curving of the surface of even those facets which have surely been formed by the cleavage mechanism. It seems that the strain for this should be higher. Basing ourselves on the results obtained and on the reasoning presented above, we may conclude that the cracks causing formation of “fish eye” flaws in the fracture grow by a mechanism differing from the cleavage one. If we admit, in accordance with the data of [15], that “quasi-cleavage” is a fracture surface formed as a result of cleavage in a composite structure (multiphase, fine dispersed, with a high dislocation density, etc.), the facets on the surface of the “fish eye” flaws are not exactly quasi-cleavage ones, because the mechanism of their formation seems to be of another nature. There are several alternative hypotheses on this nature, which imply in this or that way that formation of a quasi-cleavage surface in hydrogen-charged steels is a result of ductile fracture [7, 11,12,13].
In accordance with the theory of hydrogen-excited localized plasticity (HELP), hydrogen interacts with dislocations, promotes their mobility, lowers the Peierls barrier, intensifies planar slip, and blocks transverse slip [1, 2, 18]. It is assumed that these effects are responsible for the fact that hydrogen concentrated in the range of maximum tensile stresses in front of the mouth of a crack or any other stress concentrator promotes intensification and localization of plastic strain at these places. Thus, the process of formation and nucleation of micropores in the hydrogen-enriched regions starts substantially earlier than in the rest of the volume of the metal. With allowance for this theory, for the results of the study of the dislocation structure under the surface of a facet, and for the fact that hydrogen is carried by dislocations, the authors of [7] put forward a hypothesis that quasi-cleavage facets form in a ferritic steel due to crack nucleation at intersections of slip bands, their subsequent growth and merging. In this case, the regular structure of detachment ridges on the surface of the facets is explainable by regularity of the structure of the slip bands.
S. P. Lynch has suggested a theory of adsorption-induced dislocation emission (AIDE) in accordance with which hydrogen adsorbed on the surface of a crack and dissolved at a depth of several atomic layers underneath lowers the surface energy and thus promotes flow of dislocations from the crack mouth [1, 19]. It is assumed that in this case the crack grows chiefly due to emission of dislocations from its mouth, while the transverse slip in the plastic zone in front of the crack becomes less favorable from the standpoint of energy. In the opinion of the author of [1, 19], this process may cause formation of relatively smooth fracture surfaces, consisting, however, of quite fine nanometer dimples not detectable by SEM due to their size.
Indeed, if a crack forms in a hydrogen-saturated steel due to formation and merging of nanosize pores, i.e., by a ductile mechanism, we may expect substantial deviations of the trajectory of its growth from a rectilinear direction, because the path of the crack inside a grain is not related rigidly to the crystallography, like in the case of cleavage. A consequence of crack growth by such a mechanism is formation of regions with a relatively smooth and curved surface in the fracture. However, the results obtained in the present work are not exhaustive for proving or disproving these hypotheses. It is obvious that the topic requires additional investigation.
Conclusions
-
1.
Quasi-cleavage facets form on the surface of “fish eye” flaws in a hydrogen-charged steel by a mechanism differing from that of cleavage in a structure with enhanced dislocation density.
-
2.
Presumably, the curving of the surface of quasi-cleavage facets in a hydrogen-charged low-carbon steel may be a consequence of propagation of a ductile crack, the growth of which is controlled by the hydrogen accumulated near its mouth, which intensifies and localizes plastic strain in this region.
References
S. P. Lynch, “Hydrogen embrittlement phenomena and mechanisms,” Corros. Rev., 30, 63 – 133 (2012) (doi: https://doi.org/10.1515/corrrev-2012-0502).
I. M. Robertson, P. Sofronis, A. Nagao, et al., “Hydrogen embrittlement understood,” Metall. Mater. Trans. A, 46, 2323 – 2341 (2015) (doi: https://doi.org/10.1007/s11661-015-2836-1).
M. Koyama, H. Springer, S. V. Merzlikin, et al., “Hydrogen embrittlement associated with strain localization in a precipitation-hardened Fe – Mn – Al – C light weight austenitic steel,” Int. J. Hydrogen Energy, 39, 4634 – 4646 (2014) (doi: https://doi.org/10.1016/j.ijhydene.2013.12.171).
S. Wang, M. L. Martin, P. Sofronis, et al., “Hydrogen-induced intergranular failure of iron,” Acta Mater., 69, 275 – 282 (2014) (doi: https://doi.org/10.1016/j.actamat.2014.01.060).
L. P. Botvina, T. V. Tetyueva, and A. V. Ioffe, “Stage nature of multiple fracture of low-alloy steels in hydrogen environment,” Metalloved. Term. Obrab. Met., No. 2, 14 – 22 (1998).
T. Neeraj, R. Srinivasan, and J. Li, “Hydrogen embrittlement of ferritic steels: Observations on deformation microstructure, nanoscale dimples and failure by nanovoiding,” Acta Mater., 60, 5660 – 5171 (2012) (https://doi.org/10.1016/j.actamat.2012.06.014).
M. L. Martin, J. A. Fenske, G. S. Liu, et al., “On the formation and nature of quasi-cleavage fracture surfaces in hydrogen embrittled steels,” Acta Mater., 59, 1601 – 1606 (2011) (doi: https://doi.org/10.1016/j.actamat.2010.11.024).
D. Ya. Povolotskii and A. N. Morozov, Hydrogen and Flakes in Steel [in Russian], Metallurgizdat, Moscow (1959), 182 p.
E. Merson, A. Vinogradov, and D. L. Merson, “Application of acoustic emission method for investigation of hydrogen embrittlement mechanism in the low-carbon steel,” J. Alloys Compd., 645, 460 – 463 (2015) (doi: https://doi.org/10.1016/j.jallcom.2014.12.083).
M. R. Louthan, “Hydrogen embrittlement of metals: a primer for the failure analyst,” J. Fail. Anal. Prev., 8, 289 – 307 (2008) (doi: https://doi.org/10.1007/s11668-008-9133-x).
A. Nagao, C. D. Smith, M. Dadfarina et al., “Interpretation of hydrogen-induced fracture surface morphologies for lath martensitic steel,” Proc. Mater. Sci., 3, 1700 – 1705 (2014) (doi: https://doi.org/10.1016/j.mspro.2014.06.274).
M. L. Martin, I. M. Robertson, and P. Sofronis, “Interpreting hydrogen-induced fracture surfaces in terms of deformation processes: A new approach,” Scr. Mater., 59, 3680 – 3687 (2011) (doi: https://doi.org/10.1016/j.actamat.2011.03.002).
S. P. Lynch, “Interpreting hydrogen-induced fracture surfaces in terms of deformation processes: A new approach,” Scr. Mater., 65, 851 – 854 (2011) (doi: https://doi.org/10.1016/j.scriptamat.2011.06.016).
A. Kumar, A. J. Wilkinson, and S. G. Roberts, “Quasi-cleavage fracture planes in spheroidized A533B steel,” J. Microsc., 227, 248 – 253 (2007) (doi: https://doi.org/10.1111/j.1365-2818.2007.01808.x).
M. A. Shtremel, Fracture, Book 2 [in Russian], Izd. Dom. MISiS, Moscow (2015), 975 p.
E. Merson, A. V. Kudrya, V. A. Trachenko, et al., “Quantitative characterization of cleavage and hydrogen-assisted quasi-cleavage fracture surfaces with the use of confocal laser scanning microscopy,” Mater. Sci. Eng. A, 665, 35 – 46 (2016) (doi: https://doi.org/10.1016/j.msea.2016.04.023).
E. D. Merson and V. A. Poluyanov, “Stage nature of growth of “fish eye”-type cracks under uniaxial tension of low-carbon steel saturated with hydrogen,” in: Proc. XVI Int. Sci.-Eng. Ural Workshop of Young Metal Scientists [in Russian], Ekaterinburg (2015), pp. 343 – 346.
M. Naguma, Fundamentals of Hydrogen Embrittlement, Springer Singapore, Singapore (2016) (doi: https://doi.org/10.1007/978-981-10-0161-1).
S. P. Lynch, “Environmentally assisted cracking: Overview of evidence for an adsorption-induced localised-slip process,” Acta Metall., 36, 2639 – 2661 (1988) (https://doi.org/10.1016/0001-6160(88)90113-7).
The work has been performed with financial support of the Russian Foundation for Basic Research (Grant 17-08-01033).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 3, pp. 53 – 57, March, 2019.
Rights and permissions
About this article
Cite this article
Merson, E.D., Poluyanov, V.A., Merson, D.L. et al. About the Nature of Quasi-Cleavage in Low-Carbon Steel Embrittled with Hydrogen. Met Sci Heat Treat 61, 191–195 (2019). https://doi.org/10.1007/s11041-019-00399-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-019-00399-x