The effect of heating temperature and stirring time during preparation of foam aluminum alloy A356 on its relative porosity is studied. The optimum amount of the foam-forming agent, i.e., titanium hydride TiH2, facilitating uniform distribution of pores throughout the whole cross section of a hardened casting is determined. Optimum conditions are established for foam formation in a melt during stirring using a mixer are described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In various branches of industry there is currently use of syntactic foams, i.e., new composite materials consisting of micro-capsules (microspheres of different materials: glass ceramic, and light alloys. Syntactic foams are especially effective under conditions of compressive loads since particles formed within them become supporting elements under compression. They have a high stress plateau under compression, which corresponds compaction of the material and gives considerable energy absorption as a result [1,2,3].
Recently interest in use of foam metals has increased, especially foam aluminum in automobile and railway transport, and aerospace technology, where a reduction in weight and security are required. With use of foam aluminum with closed pores data are important about properties in tension, fatigue, and impact failure [4]. Aluminum alloy A356Footnote 1 (6.5 – 7.5% Si, 0.20 – 0.45% Mg) is a well-studied cast alloy used extensively in industry. A reduction in iron content in the alloy prevents formation of intermetallic Al – Fe systems aimed at influencing mechanical properties [5,6,7]. In a cast condition the microstructure of these alloys consists mainly of solid solution dendrites based on aluminum, surrounded by Al – Si eutectic. Coarse lamellar Al – Si-eutectic is modified with introduction of Sr, Na, or Sb [8, 9]. Astudy of foam aluminum in recent years has been mainly devoted to mechanical properties and energy absorption processes. Little attention has been devoted to acoustic properties, including the sound insulating properties of foam aluminum Al – Si with isolated cells. Due to features of the structure, foam aluminum has great potential for use as a sound insulating and noise insulating material [10, 11]. The foam forming agent in foam aluminum production used is titanium hydride. Heat treatment of titanium hydride TiH2 in air at 400 – 500°C delays its decomposition. According to published data it is effective with addition of this hydride into metal melts. Titanium hydride decomposes at around 465°C, which is lower than the melting temperature for alloys of the Al – Si system (570 – 615°C), and therefore in order to prepare foam aluminum the chemical reaction TiH2(sol) → Ti(sol) + H2(g) is used, where (sol) is solid state and (g) is gaseous state. Strength in compression of foam material is determined by foam density, as a result of which strength may be controlled within certain limits. However, density should be changed arbitrarily, and it is necessary to control other parameters including alloy composition, foam morphology (cell size and shape) and structural state of matrix metal. During forma formation from Al – Si melt, especially in the cooling stage, pores are elongated [12,13,14,15,16].
The aim of this work is to study the effect of titanium hydride content as a foam-forming agent and exposure temperature on relative porosity, pore size, and structure uniformity through the cross section of foam aluminum based on alloy A356.
Methods of Study
Aluminum alloy was studied of the following chemical composition, wt.%: 7.00 Si; 0.45 Mg; ≤ 0.2 Cu; ≤ 0.1 Zn; ≤ 0.2 Fe; ≤ 0.2 Ti; ≤ 0.1 Mn, balance Al. According to calculations in a Pro-Cast package the liquidus temperature of this hypereutectic alloy was 625°C. The Pro-Cast package makes it possible to calculate the liquidus temperature of hypo- and hypereutectic alloys based on their chemical composition.
The experimental procedure for foam formation of alloy A356 consists of the following operations: 1) alloy melting; 2) addition of Al powder; 3) addition of titanium hydride TiH2; 4) extraction from a crucible; 5) foam cooling.
Alloy A356 weighing 1 kg was melted in an argon atmosphere in a steel crucible with an Al2O3 coating. In order to increase melt viscosity 6% of aluminum powder was added of purity better than 99.9% Al (particle size 55 μm) with stirring and a constant blade rotation rate of 450 rpm. Aluminum alloy viscosity was determined before the instant of spindle rotation.
Mixing was performed in order to obtain uniform distribution of foam forming agent within the met and improve foam consistency. For this a stirrer was used in the form of disc of stainless steel 2 inches in diameter with notches in four or five locations, where bending was fulfilled so that a multiblade ventilator was obtained. The stirrer was placed at the end of a rod 3/8 of an inch in diameter and 18 inches long with a drive from an electric motor with a rotation rate of 400 – 1200 rpm. The foam forming agent added was 0.5 – 2.5% titanium hydride powder (purity more than 99%, particle diameter 40 μm) when the torsional moment of the stirrer reached a value of 0.35 N∙m. Three minutes after this the rotation rate of the stirrer reached 1200 rpm and foam forming action of the titanium hydride commenced, and the melt was gradually converted into foam. For complete decomposition of the foam forming agent the melt was held in a furnace at 575°C. In this stage gas bubble within the melt grow continuously with time forming a cellular structure. Liquid metal is mixed in an argon atmosphere heated to 90°C with a maximum flow rate of 2150 cm3/min. Argon is added directly to the melt in order to control oxidation in the course of foam formation. This controlled atmosphere reduces magnesium loss to a minimum. In addition, magnesium loss was balanced by adding Al – 10% Mg alloy immediately before addition of foam forming agent. This time range is called the foam delay stage, which may be of different duration. Finally, the crucible is extracted from the furnace, foam melt is cooled in air and solidified.
Foam prepared by the procedure described has closed porosity. The density of a foam specimen ρ* was measured by the Archimedes method. Relative density was determined as ρ*/ρs, where ρs is matrix density. Volumetric porosity (Pr b) specifies the volume fraction of all pores in final foamed alloy A356. It is normally calculated in relation to weight W or volume V s of a specimen. In this the volume of pores V i is determined, specimen volume V and specific gravity ρ of the matrix. In order to monitor completeness of hydrogen liberation in the range 20 – 1000°C it is recommended to use thermogravimetric analysis whose results are then compared with a theoretical pore volume. Morphology of the cellular structure of pores was studied in transverse sections of foam material by means of light and scanning electron microscopy.
It should be noted that an important parameter of porous materials is structure and porosity uniformity throughout the length of a workpiece, which requires special study and is not considered in this work.
Results and Discussion
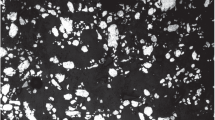
During the foam formation stage melt structure varies with stirring, which was established using a quenching method. The structure of foam aluminum A356 is shown in Fig. 1 after stirring for different times. Research has shown (Fig. 2) that the number of pores in foam aluminum increases with an increase in stirring time, and their size decreases. This is explained by formation of a considerable number of gas bubbles with an increase in free hydrogen volume. Astirrer rotating at a considerable rate causes a reduction in bubble size and simultaneously a significant part of the hydrogen emerges from melt into the atmosphere. Gradually an approximate balance is achieved between the amount of hydride hydrogen liberated during melting remaining in the melt and released to the atmosphere. As a result of this porosity of foam aluminum A356 remains constant, the amount pores during stirring increases, and diameter decreases. The final size of bubbles and overall pore volume are directly connected with the gaseous hydrogen content within melt.
Foam aluminum microstructure is shown in Fig. 3 prepared with different stirring temperatures. It is seen that pore size depends on foam formation temperature. At 565°C the cellular structure is uniform, which corresponds to the optimum conditions for melting titanium hydride at this temperature. The range of alloy A356 solidification is an important factor controlling foam formation kinetics, the rate of hydrogen liberation, and coalescence.
It has been shown (Fig. 4) that addition of 0.5% TiH2 in preparing foam aluminum is inadequate. A nonuniform cellular structure may be seen in Fig. 4 a. Addition of 1.0 and 1.5% TiH2 leads to greater inhomogeneity of spherical pores (Fig. 4 b and c). Uniform porosity is achieved with addition of 2% TiH2 to a foam aluminum melt (Fig. 4 d). In this case free gaseous hydrogen is entirely absorbed by melt that leads to an increase in foam aluminum volume. A relationship is shown in Fig. 5 between A356 foam aluminum density and the amount of added TiH2 with an exposure temperature of 565°C. It is seen that porosity increases significantly with addition 1.0% TiH2. With addition of 1.0 – 1.5 wt.% TiH 2 uniform spherical porosity is observed in combination with a favorable reduction in bulk density.
Results are shown in Fig. 6 for scanning electron microscopy of foam aluminum A356 prepared with a different amount of added TiH2. It is seen that the average thickness of a thin intercellular wall is about 20 μm, and walls are easily broken or bent. These thin walls formed with excess foaming agent. This experimental result demonstrates that the optimum TiH2 content and higher liberation of gaseous hydrogen facilitates an increase in pore volume and foam forming efficiency. With an increase in gas liberation it may emerge into the atmosphere through melt, which reduces foam formation efficiency. Consequently, the optimum amount of titanium hydride is 1.0 – 1.5%. In this case there is formation of a uniform cellular pore structure throughout the whole foam aluminum cross section.
With low melt viscosity gaseous hydrogen is released to the atmosphere before foam formation that leads to formation of foam with low porosity. Under these conditions in the bottom part there is formation of a zone free from bubbles, since the surface tension of the melt causes liquid drainage from foam. On the other hand, increased viscosity slows down drainage and facilitates bubble retention, as a result of which foam will have greater porosity.
In view of this in order to stabilize liquid foam adequate viscosity is required. Gaseous hydrogen to melt expansion due to which the internal gas pressure \( {P}_{{\mathrm{H}}_2} \) becomes sufficiently high in order to overcome external forces, i.e., pressure P c in a bubble, caused by interphase bubble – melt energy and atmospheric pressure P a. For hydrogen bubble continuous growth in a melt the following relationship should be fulfilled:
where P c = 2σ/r (σ is melt surface tension, r is bubble radius). As a result of this the smaller a bubble, the greater will be pressure within it. With a bubble radius of 0.05 mm the pressure forming a bubble in molten A356 is about 178 atmospheres (about 18 MPa). Correspondingly for uniform generation of finer bubbles a very high pressure is required. In practice this pressure is unachievable in a melt. However, within a melt there is formation of many bubbles, i.e., the barrier for their generation is readily overcome, since it there is heterogeneous generation. TiH2 powder is wetted by melt and acts with it very rapidly after contact formation, and the melt becomes supersaturated with hydrogen. Hydrogen bubbles stick to the surface of remaining TiH2 particles and act as generation venters in the course of the TiH2 decomposition reaction. Gradually the main part of hydrogen bubbles is released and passes through melt to a free surface with continuous reaction of TiH2 particles. Formation of coarser pores may be due to diffusion of hydrogen in a melt saturated with hydrogen. The degree of hydrogen diffusion from surrounding liquid depends significantly on rate of melt solidification. Formation of small, almost spherical pores, starts to occur with generation of hydrogen bubbles at the solid–liquid phase interface.
Conclusions
-
1.
Thermal decomposition of titanium hydride TiH2 (foam forming agent) is the main mechanism of foam formation in molten aluminum and provides a uniform porous structure in solidified A356 foam aluminum.
-
2.
A uniform pore structure in A356 foam aluminum may be prepared by controlling viscosity and melt crystallization.
-
3.
The volume faction of pores and their size depend on titanium hydride TiH2 content, since liberated gaseous hydrogen is absorbed entirely by melt and increases the volume of A356 foam aluminum. An increase in melt stirring duration leads to an increase in number of pores and a reduction in size.
-
4.
Melt viscosity may be controlled adding aluminum powder to a melt.
-
5.
A uniform cellular structure of foam aluminum is achieved at 565°C. With addition of 1.0 – 1.5% TiH2 to a A356 melt facilitates achievement of good foam uniform spherical porosity and sufficient reduction in bulk density.
Notes
Here and subsequently through the article text element content is given in weight fractions, expressed as a %.
References
L. Licitra, D. D. Luong, O. M. Strbik, and N. Gupta, Mater. Design, 1 – 12 (2014).
H. Dengwei, Y. Juan, Z. Xiangyang, et al., Trans. Nonferrous Met. Soc. China, 22, 85 – 89 (2012).
H. Fathi, E. Emadoddin, and A. Habibolahzadeh, Iranian J. Mater. Sci. Eng., 9, 40 – 48 (2012).
N. V. Ravi Kumar, N. Ramachandra Rao, et al., Mater. Sci. Eng., A527, 6082 – 6090 (2010).
A. Byakova, I. Kartuzov, S. Gnyloskurenko, and T. Nakamura, Adv. Mater. Sci. Eng., 1 – 9 (2014).
D. E. Palmer, in: University of Wisconsin-Milwaukee (2014), pp. 32 – 36.
S. A. Al Kahtani, Adv. Mater. Sci. Appl., 22(4) 144 – 153 (2013).
M. Yongliang, Y. Guangchun, and L. Hongjie, Mater. Design, 31, 1567 – 1569 (2010).
L. E. G. Cambronero, I. Canadas, D. Martinez, and J. M. Ruiz-Roman, Sol. Energy, 84, 879 – 887 (2010).
Z. Sedigh Sarajan, Mater. Manuf. Proc., 24, 561 – 593 (2009).
P. Lorenzo, A. Massimiliano, and P. Marco, Int. J. Impact Eng., 35, 644 – 658 (2008).
Y. Mu and G. Yao, Mater. Sci. Eng., A527, 1117 – 1119 (2010).
Z. Wang, Z. Li, J. Ning, and L. Zhao, Mater. Design, 30, 977 – 982 (2009).
A. Kim and I. Kim, Acta Mech. Solida Sin., 21, 4 – 10 (2008).
R. Edwin Raj and B. S. S. Daniel, Mater. Manuf. Proc. 22, 525 – 545 (2007).
D. H. Yang, B. Y. Hur, D. P. He, and S. R. Yang, Mater. Sci. Eng. A, 445 – 446, 415 – 426 (2007).
The authors thank the Islamic University Yazd Branch (Grant title: Study of A356 Alloy Foaming Fabrication by Melting Method Using Titanium Hydride) for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 6, pp. 27 – 32, June, 2017.
Rights and permissions
About this article
Cite this article
Sarajan, Z. Preparation of A356 Foam Aluminum by Means of Titanium Hydride. Met Sci Heat Treat 59, 352–356 (2017). https://doi.org/10.1007/s11041-017-0155-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-017-0155-4