The kinetics of formation of austenite in steel 08G2B under continuous heating at a rate of 0.3 and 90 K/sec to the intercritical temperature range is studied with the help of x-ray diffraction, dilatometric and calorimetric methods of analysis. The tests are performed for specimens in two initial conditions, i.e., after recrystallization-free controlled rolling with accelerated cooling and after water quenching from 1000°C. It is shown that the α – γ transformation under the conditions studied may be treated as a diffusion-controlled isokinetic reaction occurring in two stages with rapid formation of austenite nuclei primarily over the boundaries of initial austenite grains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The required level of functional properties of a large group of steel articles and the changes in their shape under hot deformation are provided by heating to a temperature of the austenitic range and subsequent cooling. In this connection, the α – γ phase transformations in steels have been a key moment presenting practical and scientific interest since the appearance of the science of metals and until present.
A substantial contribution into the development of this problem has been made by the works of A. A. Popov [1, 2], who was often the first to consider the key problems of formation of austenite such as the places of nucleation and the rate of growth of the nuclei, the roles of carbon diffusion, of the composition and initial structure of the steel, of the heating rate, etc. Popov has predicted the possibility of nucleation of austenite under metastable conditions by a “diffusion-free” way, when the appearing regions of austenite do not differ in the chemical composition from the initial ferrite.
The works of A. A. Popov remain important today, because consideration of formation of new phases under metastable conditions, i.e., at high heating rates, decelerated diffusion and rearrangement of defects fixed by nanoparticles of carbonitrides in complexly alloyed steels, becomes a priority in the science of metals. This requires deep understanding of the mechanisms and kinetics of the processes occurring in crystallization for virtually precise control of the structure and phase composition of steels.
Recrystallization of steels under heating is a very complex phenomenon in the science of metals, because the α – γ transition is accompanied by a large group of processes developing in parallel [3,4,5,6,7], i.e.,
-
precipitation and dissolution of cementite, special carbides and intermetallics;
-
diffusion redistribution of carbon and alloying elements (Ni, Mn, Cr, Si, etc.) between the phases;
-
rearrangement the crystal structure defects inherited from ferrite or newly arising, which causes cell formation or recrystallization;
The intensity of these closely related processes differs in different stages of formation of austenite and depends on the action of internal (initial structure, density of crystal structure defects) and external (rate and temperature of the heating, duration of isothermal hold, stresses applied to the body) factors, which gives a large field of work to researchers and producers.
In most structural steels austenite forms at a high temperature, which creates considerable difficulties for researchers, whereas at room temperature the products of decomposition of supercooled austenite are available for study. However, all the characteristics of the products of decomposition of austenite depend on its condition by the moment of cooling. This relation between the characteristics (the structure, the composition, etc.) of the initial austenite and the products of its decomposition forms the essence of the topic considered.
The aim of the present work was to investigate the kinetics of formation of austenite by dilatometric and calorimetric methods and to study the structure of steel 08G2B after continuous heating at different rates to the temperatures of the intercritical range and subsequent accelerated cooling.
Methods of Study
We studied commercial steel 08G2B with chemical composition (in wt.%) 0.08 C, about 2 Mn, 0.2 Mo, about 0.15 Σ(Ti, V, Nb), 0.004 N, 0.04 Al, 0.004 S, 0.007 P.
Specimens with cross section 10 × 10 mm were cut from the middle of a sheet with a thickness of 27.7 mm in the direction perpendicular to that of rolling. The sheet was fabricated by crystallization-free controlled rolling with accelerated cooling. Apart of the specimens was quenched in water after a 30-min hold at 1000°C (the two initial conditions of the sheet will be denoted CR and Q, respectively).
To study the microstructure, the specimens were heated in laboratory furnaces to 650, 730, 760, and 860°C at a rate of about 0.3 K/sec without isothermal holding and quenched in water.
The metallographic analysis was conducted using a Nikon Epiphot 200 light microscope. The fine structure was studied on foils using a JEM 2100 transmission electron microscope.
The dilatometric analysis was performed using a Linseis RITAL 78 quenching dilatometer at heating rates 0.3 and 90 K/sec, which were close to the rates of furnace and induction heating, respectively. The calorimetric studies of specimens with a diameter of 5 mm and a height of 3 mm were conducted in an argon atmosphere in a STA-449 Jupiter differential scanning calorimeter; the rates of the heating and of the cooling were ~ 0.3 K/sec.
The critical points Ac 1 and Ac 3 were determined on the dilatometric and calorimetric curves by the method of separation of tangents. The content of the austenite formed after heating at different rates was determined by the lever rule in the form of functions q A = f (T h) replotted to an equation
where τ = T i /v h is the time of attainment of temperature T i under continuous heating at rate v h, k is a constant, and n is the kinetic exponent.
Function (1) is known as Avrami’s equation, which is widely used for analyzing isothermal reactions [8, 9]. Functions y = f (τ) or y = f (T) can be used in the case of not isothermal heating for isokinetic reactions occurring under special conditions [8]; for the case of formation of austenite this will be considered in the discussion of the results of this study.
In rectifying coordinates ln ln \( \frac{1}{1-{q}_{\grave{A}}} \) – ln τ dependence (1) allowed us to single out the stages of the α – γ transformation, the kinetic coefficient n in each stage and the temperature A t of transition from one stage to another.
Results
Kinetics of Formation of Austenite
In accordance with the results of the dilatometric study (Table 1), growth in the heating rate from 0.3 to 90 K/sec raises the critical point Ac 1 by about 60°C and Ac 3 by about 45°C, which narrows the intercritical temperature range (ICR). At the same time, comparison of the critical points determined by the two methods shows (Table 2) that the temperature Ac 1 by the data of the calorimetric analysis is 40 – 50°C higher and Ac 3 is 25 – 40°C lower than the corresponding values of the dilatometric study. This gives different intercritical temperature ranges (ΔT ICR) determined by the two methods. It should be noted that by the results of the two studies the initial state of the steel before heating (controlled rolling and quenching) does not influence noticeably the positions of the critical points and ΔT ICR.
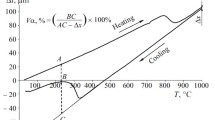
A similar kind of influence of the rate of heating and of the initial condition of the steel is observed for the temperature curves (Fig. 1 a). Increase in the rate of heating by two orders of magnitude does not change the form of the dependence q A = f (T) but shifts the curves to higher temperatures. Therefore, the content of austenite at any T h at v h = 0.3 K/sec is about 35% higher than at v h = 90 K/sec.
Dependence of the content of austenite (q A ) on the temperature of heating of steel 08G2B: a) data of dilatometric analysis [1, 2) controlled rolling + heating at a rate v h = 90 and 0.3 K/sec, respectively; 3, 4) quenching + heating at v h = 90 and 0.3 K/sec, respectively]; b) data of dilatometric analysis (DA) and of calorimetric analysis (CA) (heating with v h = 0.3 K/sec after quenching).
The differences in the critical points Ac 1 and Ac 3 and in the behaviors of the temperature curves q A = f (T) may be caused by higher sensitivity of the calorimetric method to the behavior of carbide phases and dislocations as compared to the dilatometric method [10]. It can be seen from Fig. 1 b that there is a temperature A k at which the value of q A determined by both methods is the same. In continuous heating starting with t > A k the process of precipitation of special carbides is imposed on the process of formation of austenite, and the calorimetric curve is located below the dilatometric curve. At t < A k the curves change places, which is connected with coagulation of the precipitated particles and decrease in the dislocation density.
Figure 2 presents the variation of the content of austenite in the process of α – γ transformation in the steel studied in accordance with Eq. (1) in rectified coordinates. It can be seen that independently of the initial condition and of the heating rate the reaction occurs in two stages, each of which is characterized by its own kinetic coefficient n i , and n I > n II due to deceleration of growth of the content of austenite in stage II.
The kinetic curves in rectifying coordinates with close values of coefficients n I and n II behave similarly at v h = 0.3 K/sec too. This allows us to think that the mechanism of formation of austenite in steel 08G2B in the two different initial states is the same and does not depend on the rate of heating in the range of 0.3 – 90 K/sec.
The temperature the change of transformation stages A t is independent of the initial state of the steel, but is shifted toward lower temperatures at lower heating rates (A t = 810 and 830°C at v h = 0.3 and 90 K/sec, respectively).
Microstructure
The structure of the steel formed in the production of sheets by the process of controlled rolling with accelerated cooling is represented by a heterophase mixture of superfine ferrite matrix (3 – 5 μm) and dispersed bainite-martensite crystals in an amount of about 30% [10].
Quenching from 1000°C yields a lath structure with high dislocation density, which is a result of a shear transformation (Fig. 3 a). It also contains grains of polyhedral ferrite (about 10%) surrounded with bent boundaries. The dislocation density in such grains is an order of magnitude lower than in the products of the shear transformation. Particles of second phases are virtually absent inside laths but are observable on dislocations inside ferrite grains. These particles about 30 – 50 nm in size have a round shape and most probably are carbonitrides not dissolved during the heating for quenching. A contrast in the form of continuous or discrete bands is observable over lath boundaries.
Continuous heating of quenched specimens to 650°C at a rate of 0.3 K/sec leads to preservation of lath crystals with high dislocation density, on which carbides precipitate in the form of bent particles with a length of 50 – 60 nm. The special carbides based on niobium (vanadium) precipitated during high tempering [12] have a round shape and a size an order of magnitude smaller. This allows us to infer that the particles precipitated in continuous heating without holding to 650°C are cementite ones.
After the heating to 760°C followed by cooling in water, the fraction of lath crystals decreases somewhat (to about 80%). Most of them preserve an elevated density of dislocations fixed by round nanoparticles 50 – 70 nm in size (Fig. 3 b). In some microvolumes the laths and the round subgrains 0.5 – 1 μm in size with migrating boundaries are already free of dislocations and dispersed particles, which indicates coagulation. Round-shape austenite nuclei (about 1 μm in size) are located on the boundaries of former austenite grains in the form of fine grains (“grain boundary effect” [13]).
After the heating to 850°C (q A ≈ 80%) we observe total dissolution of cementite; the density of the spherical particles decreases and their sizes grow to up to 80 nm, which may be treated as coagulation of special carbides. A special feature of such coagulation under the conditions of migrating boundaries both in the ferrite and in the newly formed austenite is the fact that the growing large particles do not have enough time for precipitation on the boundaries of crystals and are located inside them (Fig. 3 c). The untransformed ferrite continues to undergo the same process as in heating to 760°C. The formed austenite grains transform into upper bainite under subsequent cooling.
Discussion
It is reported that two or, rarely, three stages of the kinetics of formation of austenite are distinguished in differently alloyed low-carbon steels [3,4,5]. However, the processes controlling this transformation are interpreted differently. A satisfactory description of the kinetics of formation of austenite in steel 08G2B in the two initial conditions with the help of equation (1) under heating at the rates of 0.3 and 90 K/sec allows us to classify the α – γ transformation as a diffusion-controlled isokinetic reaction [8].
According to Cahn (cited from [8]), such reactions are characterized by absence of dependence of the number of nuclei (q A ) on the temperature due to the easy and fast nucleation of a new phase. Then, at any moment of time q A is determined only by the rate of growth of the phase, which is proportional to the diffusion coefficient. For such reactions a new phase nucleates on grain boundaries, which is true if the grain size remains constant in the whole of the temperature range of the transformations.
The results of the structural study presented above show that these conditions of isokinetic reactions are met satisfactorily during formation of austenite in the process of heating of steel 08G2B with the chosen rates. Austenite nuclei appear primarily on the boundaries of former austenite grains. Then stage I should be understood as individual growth of austenite regions not contacting each other, which is accompanied by diffusion to close distances, and stage II should be understood as growth under the conditions of “soft” collision of austenite regions (overlapping of their diffusion fields), which develops into actual collision. It is natural that the rate of growth of the content of austenite decreases in stage II, and the kinetic coefficients obey the inequality n I > n II.
Diffusion-controlled mechanism of the α – γ transformation under the conditions considered is also confirmed by the shift of the critical points Ac 1, Ac 3, of the temperature At, of the intercritical range, and of the curve q A = f (τ, T) as a whole toward higher temperatures upon increase in the heating rate. The coefficients of self-diffusion and of the diffusion of the substitutional atoms are proportional to the concentration of vacancies (C U ). Fast heating results in deficit of thermal vacancies, which is the higher the higher the heating rate. Vacancies are emitted (absorbed) by dislocations and grain boundaries, which is accompanied by displacement of the sources (sinks) of vacancies [14]. Form this standpoint, the appearance and the migration of the F/A interphase, which lead to formation of austenite nuclei, are representable as splitting of the interphase from the F/F grain boundary when it emits vacancies and then displaces. The more powerful is the source (the sink), the more intense is the flow of vacancies emitted (absorbed) by it. By the data of the study of the microstructure this is the reason behind the fact that the first austenite nuclei form on the boundaries of initial austenite grains or at the ends of martensite laths.
Growth of the austenite grains not yet contacting each other, just as their diffusing fields, is the main process developing in stage I under heating in the range Ac 1 – A t. By the data of both methods of study, the temperature range of stage I of the transformation (Ac 1 – A t) narrows noticeably when the heating rate is increased from 0.3 to 90 K/sec mainly due to the elevation of Ac 1, i.e., earlier formation of the first portions of austenite under the low heating rate. At the same time, stage II in the initially quenched specimens, where the proportion of the products of the shear transformation and the total density of defects are larger, occurs at lower temperatures than in the specimens after controlled rolling. These experimental data are explainable from the standpoint of the role of vacancies as a necessary element of the mechanism of diffusion phase transitions [15].
During heating in the ICR the formation of austenite is accompanied by additional decomposition in the untransformed ferrite; cementite and fine particles of carbonitride phases precipitate inside the initial laths and on their boundaries, which results in preservation of the initial lath structure until high temperatures. When the temperature A k, which is close to A t, is exceeded, the precipitation of carbide phases is replaced by dissolution of cementite and coagulation of special carbides, freeing of crystal structure defects and their intense rearrangement in the ferrite up to migration of high-angle boundaries. These processes develop chiefly in stage II of the transformation and are accompanied by diffusion of the dissolved elements to long distances in the growing and coming into contact austenite grains.
In accordance with the studies of microstructure, these processes in the austenite do not finish in heating to T h ≥ Ac 3, and the steel preserves a metastable condition with respect to such parameters as the size and shape of the austenite grains, the carbide phases, and the homogeneity of the chemical composition.
Conclusions
Studying the formation of austenite in steel 08G2B under continuous heating with the help of dilatometric analysis we determined the critical points Ac 1 = 700 and 770°C, Ac 3 = 875 and 915°C for the heating rates v h = 0.3 and 90 K/sec, respectively, which did not depend on the initial condition of the specimens (after recrystallization-free controlled rolling with accelerated cooling or after water quenching from 1000°C). At all variations of the critical temperatures the calorimetric analysis gave the values of Ac 1 ~ 50°C higher and of Ac 3 ~ 50°C lower than the dilatometric analysis. This is explainable by higher sensitivity of thermal effects to the behavior of carbide phases and crystal structure defects, which accompany the α – γ transformation.
A satisfactory description of the content of the forming austenite (q A ) in the form of function q A = f (τ) for continuous heating allowed us to classify the α – γ transformation under the conditions studied as an isokinetic reaction, to single out the stages of the transformation, and to determine the kinetic coefficients n in each stage. By the data of the study of the microstructure, austenite nuclei form rapidly over the boundaries of initial austenite grains (at the ends of laths), which is typical for isokinetic reactions. Then stage I can be treated as diffusion-controlled growth of the still not contacting austenite grains, and stage II can be classified as growth of the austenite grains undergoing “soft” collision that later transfers into actual collision.
The study of the microstructure has shown that heating of the initially quenched steel at a rate of 0.3 K/sec to the temperature of stage I and subsequent quenching preserves a lath structure with elevated density of dislocations fixed by carbide particles in the untransformed ferrite and produces austenite regions (y A < 30%) over boundaries of initial austenite grains. Heating to the temperatures of stage II of the transformation causes growth of the round austenite grains due to migration of boundaries, dissolution of cementite particles in them, and coagulation of special carbides. Subsequent quenching transforms the formed austenite into upper bainite.
References
Al. Ar. Popov, “Laws of formation of austenite,” in: Phase Transformations in Iron-Carbon Alloys [in Russian], Mashgiz, Moscow (1950), pp. 36 – 64.
Al. Ar. Popov, Phase Transformations in Metallic Alloys [in Russian], Metallurgizdat, Moscow (1963), 311 p.
S. S. D’yachenko, Formation of Austenite in Iron-Carbon Alloys [in Russian], Metallurgiya, Moscow (1982), 182 p.
V. I. Zel’dovich, I. V. Khomskaya, and O. S. Finkevich, “Formation of austenite in low-carbon iron-nickel alloys,” Fiz. Met. Metalloved., No. 3, 5 – 28 (1992).
L. Ts. Zayats, D. O. Panov, Yu. N. Simonov, et al., “Formation of austenite in initially quenched low-carbon steels of different alloying systems in the intercritical temperature interval,” Phys. Met. Metallogr., 112(5), 480 – 487 (2011).
R. Wei, M. Emonoto, R. Hadian, et al., “Growth of austenite from as-quenched martensite during intercritical annealing in an Fe – 0.1C – 3Mn – 1.5Si alloy,” Acta Mater., 61, 697 – 707 (2013).
A. N. Makovetskii, T. I. Tabatchikova, I. L. Yakovleva, et al., “Formation of structure of low-alloy pipe steel under heating in the intercritical temperature range,” Fiz. Met. Metalloved., 113(7), 744 – 755 (2012).
J. Christian, The Theory of Transformations in Metals and Alloys. Pt. 1. Thermodynamics and General Kinetic Theory [Russian translation], Mir, Moscow (1978), 803 p.
Al. Ar. Popov, The Theory of Phase Transformations in Solid State [in Russian], GOU VPO UGTU-UPI, Ekaterinburg (2004), 168 p.
B. G. Livshits, V. S. Kraposhin, Ya. L. Linetskii, Physical Properties of Metals and Alloys [in Russian], Metallurgiya, Moscow (1980).
A. B. Arabey, V. M. Farber, I. Yu. Pyshmintsev, et al., “Microstructure and fine phases of tube steels of strength class Kh80 for long-distance gas lines,” Izv. Vysh. Uchebn. Zaved., Chern. Metall., No. 1, 30 – 37 (2012).
M. I. Goldshtein and V. M. Farber, Precipitation Hardening of Steel [in Russian], Metallurgiya, Moscow (1979), 208 p.
V. D. Sadovskii, Inheritance of Structure in Steel [in Russian], Metallurgiya, Moscow (1973), 205 p.
M. A. Shtremel, Strength of Alloys. Pt. 1. Lattice Flaws [in Russian], MISiS, Moscow (1999), 384 p.
B. Pieraggi, R. A. Rapp, F. J. J. Loo, and J. P. Hirth, “Interfacial dynamics in diffusion-drive phase transformations,” Acta Metall. Mater., 38(9), 1781 – 1788 (1990).
The work has been performed with financial support of Regulation No. 211 of the Government of the Russian Federation, Contract No. 02.A03.21.0006 and within a state assignment of the Ministry of Education and Science of the Russian Federation, Project No. 11.1465.2014/K.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 11, pp. 11 – 16, November, 2016.
Rights and permissions
About this article
Cite this article
Farber, V.M., Khotinov, V.A., Selivanova, O.V. et al. Kinetics of Formation of Austenite and Effect of Heating in the Intercritical Temperature Range on the Structure of Steel 08G2B. Met Sci Heat Treat 58, 650–655 (2017). https://doi.org/10.1007/s11041-017-0073-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-017-0073-5