This article presents results from studies of the thermochemical treatment of steel parts based on their nitriding in the plasma of a hydrostatic glow-spark discharge (GSD). The article examines the nitriding of carbon and alloy steels in a GSD plasma (hydroplasma nitriding), as well as combination methods of treating carbon steels. One of the combination methods discussed is diffusion saturation of the steel by a metal, nitrogen, and carbon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An analysis of the current state of progress in improving the reliability and durability of machine and instrument parts shows that thermochemical treatment (TCT) remains an effective method for the surface-hardening of steels and alloys and has good prospects for further development [1]. The large number of types and variants of TCT make it possible to select the method of hardening that is optimum based on economic and operational factors. Among the many TCT methods that are available, nitriding has been widely used for over 60 years to strengthen various steels and alloys [2 – 4]. One important advantage of nitriding is that it allows control over the composition and structure of the diffusion layer through control of the saturating atmosphere. Both furnace nitriding and ion nitriding (nitriding in a glow discharge) are actively used in industry. Given all of the advantages of these two technologies, they still share one shortcoming — the lengthy duration of the saturation process (tens of hours). The nitriding process in these methods needs to be carried out at higher rates.
Thus, such TCT technologies need to be developed with the goals of ensuring that the surface layer of the product has the prescribed service properties, significantly shortening the time required to form diffusion layers of the specified thickness, and conserving scarce metals by replacing alloy steels with surface-hardened carbon steels. These objectives can be met by developing and using processes that entail the saturation of steel products by nitrogen and other elements in plasmas formed by glow-spark discharges [5]. The goal of the investigation being discussed in this article was to study two types of TCTs: nitriding in the plasma of a nitrogen-bearing liquid electrolyte (hydroplasma nitriding — HPN); combination processes that entail saturation by nitrogen, a metal, and carbon in a plasma (metallic carbon nitriding).
Methods of study
Different types of specimens were saturated in the plasma of a hydrostatic discharge (HSD): Armco iron; carbon steels containing from 0.15 to 0.8% C (steels St3, 20, 40, and U8); alloy steels of the pearlitic, martensitic, and austenitic classes (steels 40Kh, 20Kh13, 40Kh13, and 40Kh12N8G8MF).
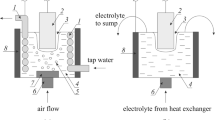
The saturation processes were carried out on a specially built laboratory unit that includes a reactor filled with the electrolyte — an aqueous solution of ammonium chloride (NH4Cl) — an ac transformer, rectifier, stabilizer, and monitoring instruments (an ammeter and a voltmeter). The main parameters of the production process are voltage, current, and saturation time. In hydroplasma nitriding, a voltage within the range 170 – 200 V is optimum from the standpoint of maintaining stable combustion of the low-temperature plasma and preventing the occurrence of anomalous arc discharges. The specific voltage that is optimum for any given case will depend on the size of the part being nitrided. The current is maximal (I max= 1.0 – 1.5 A) up to the moment that the gas-vapor envelope is formed, while it decreases to I min= 0.10 – 0.12 A during the stable combustion of the plasma. Total nitriding time is 1.5 – 3 min.
In combination TCT (metallic carbon nitriding), the hydroplasma nitriding operation was preceded by coating of the specimens with a slip containing powders of alloying elements (W, V, Cr, Co, Ti, Nb, Mo, and Al or mixtures of these elements.). The slip (suspension) is a mixture of 50% graphite + 30% powdered alloying element + 20% ammonium chloride. Nitrocellulose varnish is used as the binder. The process parameters that are optimum from the standpoint of ensuring efficient heating of the part without fusion are: voltage U = 35 – 40 V; current I = 13 – 15 A; duration of the process 3 min.
After the treatment, we studied the structure and phase composition of the surface layer of the specimens and determined the layer’s thickness, microhardness, and wear resistance. Metallographic studies were performed on an AXIOVERT 40MAT microscope, the fine structure was studied with an HITACHI S-800 scanning electron microscope, and phase composition was examined by performing an x-ray structural analysis on a DRON-3 diffractometer. Microhardness was measured on a PMT-3 microhardness tester.
Results and discussion
Essence and Mechanism of TCT in the Plasma of a Hydrostatic Discharge
The thermochemical treatment of products in a hydrostatic glow-spark discharge (low-temperature plasma) is carried out in an electrolyte (an aqueous solution of ammonium chloride) inside an open reactor (at atmospheric pressure). Rectified voltage is supplied to the part being treated (the cathode) while it is submerged in the electrolyte and to a hard electrode of chromium-nickel steel (the anode). A gas-vapor envelope of vapors of the electrolyte is formed near the surface of the part. The envelope is approximately 50 – 120 μm thick, and water and salts react to form nitrogen ions inside it. The envelope is a low-temperature plasma and is an active saturating atmosphere for nitriding. Micro-discharges take place in the hydrostatic plasma as it interacts with the surface of the part, and the conditions that are created are such as to make it possible to sustain a steady electrical current. For efficient combustion of the plasma, the area of the cathode should be five times smaller than the area of the hard anode.
A hydrostatic discharge develops in several stages. The salt undergoes electrolysis with a smooth increase in the applied direct voltage. This stage of the process is characterized by a proportional increase in current with an increase in voltage in accordance with Ohm’s law and corresponding increases in the temperature of the electrolyte.
When voltage reaches a certain value (100 – 180 V) on the surface of the part-cathode, the electrolyte on the electrode begins to boil and nucleate boiling develops. The temperature of the part is close to the temperature of the electrolyte while nucleate boiling takes place. A further increase in voltage initiates film boiling, which is characterized by a sharp reduction in current due to the fact that the gas-vapor envelope which is formed has a higher resistance than the liquid electrolyte. The formation of the envelope and the passage of electrical current through it result in the formation of a low-temperature plasma that creates a blue glow around the part. A further increase in voltage results in the occurrence of an anomalous discharge.
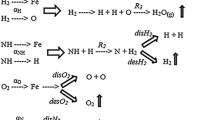
Figure 1 schematically depicts the process by which an iron matrix is saturated by nitrogen in an HSD plasma. During combustion, the vapor-gas envelope forms a low-temperature HSD plasma that contains nitrogen ions. When those ions collide with the surface of a steel part, they interact with the ions of the metal and penetrate deeply into the part. In light of the fact that the gas-vapor envelope has a lower thermal conductivity than the liquid electrolyte, the temperature of the part increases sharply because of the reduced heat-transfer coefficient in the liquid. This in turn accelerates the diffusion of nitrogen in the metal and the formation of the diffusion layer in accordance with the laws of TCT.
When the voltage is cut off, the gas-vapor envelope collapses due to the end of the ion bombardment, the part cools as a result of heat exchange with the surrounding medium, and nitrogen quenching takes place.
Diffusion saturation of a part with metal from an applied slip requires a higher temperature than the nitriding operation [6]. The temperature reached in TCT in an HSD plasma can be increased by decreasing the amount of heat removed from the surface of the part and increasing the current. The thermal conductivity of the electrolyte is reduced by making it thicker with the addition of graphite. Having a high thermal conductivity, graphite also makes it possible to increase the current. In addition, the carbon prevents oxidation of the surface of the specimen. Combined metal plating and nitriding is made possible by the simultaneous presence of nitrogen and chlorine ions in the electrolyte and the slip. The nitrogen ions provide for diffusion saturation with nitrogen, while the chlorine ions help transport the alloying element (the metal) to the surface of the part being treated.
The mechanism by which the formation of a diffusion layer in steel is sped up during TCT in an HSD plasma entails the acceleration of the elementary processes that take place: dissociation, which leads to the rapid formation of active ions of nitrogen; high-rate adsorption, which occurs as a result of ion bombardment of the surface; the diffusion of the saturating elements in the metal, which takes place due to a local increase in temperature.
Determination of the Temperature Conditions for TCT in an HSD Plasma By Mathematical Modeling
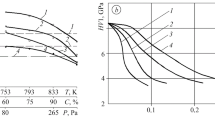
One feature of thermochemical treatment in an HSD plasma is the existence of a substantial difference between the temperature of the part and the temperature of the gas-vapor envelope. This difference makes it difficult to reliably determine the temperature conditions during the saturation process. We constructed a mathematical model of these conditions in order to determine the temperature of the part.
The following main factors affecting the temperature to which the part is heated were chosen as the initial parameters for the calculation: voltage U; cathode radius R (the cross section of the cylindrical part); depth of immersion of the part in the electrolyte h.
The below formula for calculating temperature was obtained by analyzing the quantitative relationships between these parameters [7]:
where χ is the resistivity of the gas-vapor envelope; d is the thickness of the envelope; α is the heat-transfer coefficient for the transfer of heat from the metal into the surrounding medium; λ is the thermal conductivity of the material of the part; k is a coefficient that accounts for the fraction of energy which is transferred from the envelope to the electrode.
A factorial experiment was performed under laboratory conditions to check the accuracy of this equation. The levels of variation of the input parameters in the experiment were determined by the regimes of the process and the capabilities of the laboratory equipment. Only the linear terms turned out to be significant in the ranking of the factors. The pairwise interactions were found to be insignificant, the confidence level associated with this determination being 0.95. This makes it possible to study the dependence of the temperature of the part on the geometric parameters by varying them one by one. The calculations showed that temperature increases with an increase in the depth of immersion of the specimen in the electrolyte, an increase in voltage, and a decrease in the radius of the cylindrical part-cathode (Fig. 2). These findings were corroborated by the experimental measurements.
Structure and Properties of Modified Layers
During the saturation of Armco iron from a nitrogen-bearing plasma over a period of 1.5 min, a nitrided layer consisting of a zone occupied by chemical compounds (the ε-phase) and an internal nitriding zone with γ'-phase precipitates are formed in the specimen. The first zone is 30 μm thick, while the second zone is 150 μm thick.
Differences were found in the structure of the nitrided layer in carbon and alloy steels. For example, a dense ε-phase zone 20–30 μm thick was observed in steel U8 after nitriding for 2 min. Located under this zone was a 20-μm-thick zone of nitrogen-bearing martensite formed as a result of nitrogen quenching (Fig. 3a ).
An ε-phase layer 15 μm thick is formed on the surface of chromium steel 20Kh13 above an internal nitriding zone which has a thickness of approximately 100 μm and contains precipitates of nitrides and carbides of chromium (Fig. 3b ). The chromium carbides are coarser and are located both inside the grain and along its boundaries, while the chromium nitrides are significantly smaller and have a maximum size of 100 nm (Fig. 4).
After hydroplasma nitriding, the hardness of the different steels studied here increased (to 13 – 16 GPa) compared to the hardness of the steels in the untreated state. The wear resistance of the high-chromium steels tripled after HPN. The wear resistance of the nitrided austenitic chromium-nickel steels (40Kh12R8G8MFB, Kh12N22T3MR) increased while corrosion resistance remained at a satisfactory level [4].
Slip-mediated plating of the carbon steels by transition-group metals (W, V, Cr, Co, Ti, Nb, Mo) in a nitrogen-bearing HSD in the presence of graphite results in saturation of the surface of the product by metal, carbon, and nitrogen and the formation of diffusion layers of complex composition. The diffusion layers have thicknesses within the range from 40 to 90 /m, the exact thickness of a specific layer depending on the type of alloying element (see Table 1).
Such a combination treatment is can be classified as metallic carbon nitriding, and the diffusion layer that is formed is a second-order internal nitriding zone. The modified layer usually contains nitrides, carbides (carbonitrides), an alloying element, intermetallide phases, and in some cases nitrides (carbonitrides) of iron precipitated in a solid solution doped with nitrogen and metal: Fe x Me y Me x (C, N) + α(Me, N). Mainly nitride phases are formed by surface alloying with strong nitride-forming elements such as titanium.
The modified layers are distinguished by high micro-hardness (Fig. 5), the level of which depends on the volume fraction and dimensional characteristics of the nitrides (carbonitrides) of the alloying elements. The volume fraction and dimensional characteristics of the nitrides are in turn determined by the thermodynamic affinity of the alloying elements for nitrogen (carbon) and the concentrations of those elements and nitrogen in the solid solution.
Studies showed that there are certain distinctive features to the structure of the modified layer in steel 40 that is obtained when slip-mediated plating with aluminum is combined with nitriding in an HSD plasma (Fig. 6a ). A 25-μm-thick film of the oxide Al2O3 is seen on the surface and is followed by a transitional diffusion layer that has a thickness of roughly 70 μm and contains high concentrations of aluminum, carbon, and nitrogen and precipitates of the aluminum-alloyed γ'-phase. Below the transitional layer is a characteristic hypereutectoid structure with a carbide network along its grain boundaries and martensite needles inside the grains. The total thickness of the hardened layer is about 150μ/m.
The curve that describes the distribution of microhardness in the alumo-nitrided layer contains several sections corresponding to specific features of the layer’s phase composition (Fig. 6b ). The maximum hardness of 15000 MPa is seen on the surface in the region where aluminum oxide forms. Hardness then decreases sharply to 8000 MPa in the transitional zone, this being followed by a smooth decrease in hardness (to 2000 MPa) in the direction of the core over a distance of roughly 100 μm. An abrupt decrease in hardness at a depth of 20 – 40 μm and the presence of a cementite network are prerequisites for an increase in the brittleness of the layer.
Brittleness can be avoided by ensuring that microhardness changes smoothly through the entire thickness of the layer. Experiments showed that it is possible to achieve sufficiently high hardness, have it change smoothly through the thickness of the layer, and still obtain the anti-corrosion properties realized with alumo-nitriding by resorting to multi-component saturation. The combination of properties just mentioned is obtained when slip-mediated plating with aluminum and titanium is combined with nitriding in an HSD plasma. The hardness of such a coating (7000 MPa) is higher than that of the coating obtained by alloying with titanium alone but is lower than that of the coating obtained through saturation with just aluminum. However, the micro-hardness is evenly distributed over the layer, which accounts for the favorable nature of the internal stresses that are created. These stresses help reduce brittleness and increase wear resistance.
Thermochemical treatment in an HSD plasma is being used to improve the service properties of products employed in the fabrication of farm machinery that is subjected to rapid wear in aggressive media: moisture, animal fats, acids, and sand. The treatment of wire-based steel U8 products in an HSD plasma leads to the formation of a structure composed of a surface ε-phase that is responsible for corrosion resistance and a nitrogen-bearing martensite layer that is as much as 16 GPa harder than quenched steel.
The technology of hydroplasma nitriding has been tested for the surface-hardening of steel fasteners used in well-logging modules employed in the oil and gas industry. During service, parts of the module (shafts, screws, nuts, pins, jaws, sleeves, and clamps made of alloy steels) come into direct contact with drilling fluid and the wall of the borehole. In the process, they are subject to hydroabrasive wear and corrosion in an aggressive medium that contains water, oil, salt solutions, natural gas (methane), hydrogen, acids and alkalis, and hydrogen sulfide. The parts of the module are simultaneously subjected to significant gradients of hydrostatic pressure (0.1 – 150 MPa) and temperature (from – 45 to + 450°C). The tests showed that the service properties of nitrided chromium steels under such conditions are appreciably superior to the properties of untreated steels and steels hardened by conventional TCT methods. The hardness of the modified layer of the various steels that were tested was 4.7 – 5.7 times greater than the hardness of the same steels without treatment and 1.4 – 3 times greater than the hardness of the diffusion layers obtained by classical nitriding methods. The service life of different parts under conditions that exposes them to wear and dynamic loads was shown to be increased by a factor in the range 3 – 7.5 through the use of HPN.
Conclusions
Thermochemical treatment in a low-temperature HSD plasma — a process referred to as hydroplasma nitriding — has been proven effective for increasing the hardness and wear resistance of carbon and alloy steels. One variant of this technology entails the combined saturation of steels with nitrogen, carbon, and an alloying metal (W, V, Cr, Mo, Ti, Al) from an HSD plasma. This variant makes it possible to regulate the level of hardening and the character of the hardness distribution through the thickness of the hardened layer. One advantage of thermochemical treatment in an HSD plasma is the high rate of diffusion saturation of steels by nitrogen and other elements.
The process has been tested for practical use with the objective of increasing the service life of small parts used in machines and equipment employed in agriculture and the oil and gas industry.
References
L. G. Petrova, “Development of the theory and practice of thermochemical treatment for metals: studies of the Department of Metallurgy and Heat Treatment at MADI,” in: Prospects for the Thermochemical Treatment of Steels: Theory – Experiment – Technologies [in Russian], MADI, Moscow (2011), pp.5–17.
Yu. M. Lakhtin and Ya. D. Kogan, Nitriding of Steel [in Russian], Mashinostroenie, Moscow (1976).
Yu. M. Lakhtin, “Diffusional principles of the nitriding process,” Metalloved. Term. Obrab. Met., No. 7, 14 – 17 (1995).
L. G. Petrova, “Current trends in the development of the theory and practice of steel nitriding processes,” in: Proc. Third Scientific-Technical Conference on Heat Treatment (April 13 – 15, 2011, Tolyatti) “New Steels for Machine Construction and Their Heat Treatment” [in Russian], Izd-vo OAO “AVTOVAZ,” Tolyatti (2011), pp.7–10.
D. I. Slovetskii, S. D. Terent’ev, and V. G. Plekhanov, “Mechanism of the plasma-electrolytic heating of metals,” Teplofiz. Vys. Temp., 24(2), 353 – 363 (1986).
L. G. Petrova and V. A. Aleksandrov, “Strengthening of parts made of structural steels by combined TCT technologies based on the slip method,” in: Modern Methods of Fabricating and Studying Nanostructured Materials and Coatings [in Russian], MADI (GTU), Moscow (2009), pp. 47 – 59.
L. G. Petrova, V. A. Aleksandrov, and P. E. Demin, “Effect of the dimensions of a part on its temperature during heating in a hydro-electrolytic plasma,” Ibid., pp. 73 – 77.
Author information
Authors and Affiliations
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 6, pp. 41 – 46, June, 2012.
Rights and permissions
About this article
Cite this article
Petrova, L.G., Aleksandrov, V.A. & Demin, P.E. Thermochemical treatment of steels in the plasma of a hydrostatic glow-spark discharge. Met Sci Heat Treat 54, 309–314 (2012). https://doi.org/10.1007/s11041-012-9503-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-012-9503-6