Abstract
Background
Biocides are frequently used as preservative, disinfectant and sterilizer against many microorganisms in hospitals, industry and home. However, the reduced susceptibility rate of Pseudomonas aeruginosa (P. aeruginosa) strains to biocides is increasing. The aim of this study was to evaluate the antimicrobial activity of four frequently used biocides against P. aeruginosa and to determine the prevalence of genes involved in biocide resistance.
Methods
A total of 76 clinical isolates of P. aeruginosa strains were used in the present study. The minimum inhibitory concentrations (MICs) of four biocides, i.e. chlorhexidine digluconate, benzalkonium chloride, triclosan and formaldehyde, against P. aeruginosa strains were determined using agar dilution method. In addition, the prevalence of biocide resistance genes was determined using the polymerase chain reaction (PCR) method.
Results
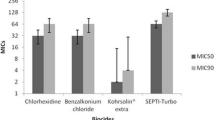
In the present study, the highest MIC90 and MIC95 (epidemiological cut-off) values were observed for benzalkonium chloride (1024 μg/mL), followed by formaldehyde (512 μg/mL), triclosan (512 μg/mL) and chlorhexidine digluconate (64 μg/mL). Furthermore, the prevalence of qacEΔ1, qacE, qacG, fabV, cepA and fabI genes were 73.7% (n = 56), 26.3% (n = 20), 11.8% (n = 9), 84.2% (n = 64), 81.5% (n = 62) and 0% (n = 0), respectively. A significant association was observed between the presence of biocide resistance genes and MICs (p < 0.05). Furthermore, there was no significant association between the presence of biocide resistance genes and antibiotic resistance (p > 0.05), except for levofloxacin and norfloxacin antibiotics and qacE and qacG genes (p < 0.05).
Conclusion
Our results revealed that chlorhexidine digluconate is the most effective biocide against P. aeruginosa isolates in Ardabil hospitals. However, we recommend continuous monitoring of the antimicrobial activity of biocides and the prevalence of biocide-associated resistance genes for a better prevention of microorganism dissemination and infection control in hospitals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas aeruginosa (P. aeruginosa) is a Gram-negative bacillus, which is frequently isolated from hospital environments, especially from medical equipments used in intensive care units (ICUs) [1,2,3]. P. aeruginosa is an opportunistic human pathogen that is associated with various community-acquired and nosocomial infections such as ventilator-associated pulmonary infections, skin and soft-tissue infections, catheter-related urinary tract infections, eye and ear infections, bloodstream infections, endocarditis and surgical site/transplantation infections [1,2,3]. Therefore, it seems that hygiene control of surfaces and medical equipments in the hospital settings in terms of P. aeruginosa contamination is important because nosocomial infections are considered as a growing global threat in terms of economical and public health [3]. The most common antiseptic and disinfectant biocides used in clinical settings are chlorhexidine digluconate (a biguanide, disrupting the cell membrane), benzalkonium chloride (a quaternary ammonium compound, disrupting the cell membrane), triclosan (a bisphenol, blocking of lipid biosynthesis) and formaldehyde (an aldehyde, alkylating agent) [4, 5]. However, several studies have reported bacterial resistance to various biocides due to the presence of resistance genes [6]. On the other hand, the risk of P. aeruginosa infections in ICU-hospitalized patients is high (up to 30%) despite applying hygiene programs [5]. Such a high prevalence can be attributed to the reduced susceptibility of P. aeruginosa strains against a variety of antiseptics and disinfectants over time [7]. Another problem is the emergence of multi-drug resistant (MDR) P. aeruginosa strains, which can lead to treatment failure [3]. Interestingly, P. aeruginosa strains have shown cross-resistance to biocides and antibiotics with probably similar mechanisms, thereby making bacterial elimination from hospital environments difficult [5, 8]. Therefore, obtaining information on the prevalence and mechanisms of bacterial resistance to antimicrobial agents and the selection of suitable biocides and antibiotics can be helpful for controlling hospital-acquired infections caused by P. aeruginosa. Several mechanisms of resistance to antibiotics and tolerance to biocides have been identified in P. aeruginosa strains including: 1) simple growth requirements and ability of biofilm formation, 2) enzymatic degradation, 3) target site modification, 4) outer membrane impermeability, and 5) presence of efflux pumps. These properties are involved in bacterial persistence in medical environments and development of nosocomial infections [2, 9,10,11,12,13,14]. Among these resistance mechanisms to biocides, efflux pumps encoded by qacEΔ1, qacE, qacG and cepA genes play an important role in P. aeruginosa resistance to benzalkonium chloride and chlorhexidine [14]. On the other hand, fabV gene confers high-level triclosan resistance. Various compounds are used in hospitals and non-hospital environments in Ardabil city but there is no exact data on the rate of biocide effectiveness against local isolates of P. aeruginosa. Moreover, the mechanisms of biocide resistance in P. aeruginosa clinical isolates of Ardabil are still unclear. Therefore, the aim of the current study was to assess the distribution of biocide resistance genes, namely qacEΔ1, qacE, qacG, fabV, cepA and fabI, and to determine the minimum inhibitory concentration (MIC) and the epidemiological cut-off (ECOFF) values of various biocides against antibiotic-resistant P. aeruginosa strains isolated from clinical samples in Ardabil.
Methods
Data on P. aeruginosa isolates
A total of 76 confirmed clinical P. aeruginosa isolates were obtained from various specimens in Ardabil hospitals and then used to assess the distribution of biocide resistance genes as well as determination of the MIC and the ECOFF of four biocides. Drug resistance characteristics of P. aeruginosa isolates was evaluated using the disk diffusion method based on the Clinical and Laboratory Standards Institute (CLSI, 2018) guideline [15]. Data on the prevalence of class I integron, harboring resistance genes to biocides and antibiotics, was used in this study in order to evaluate the association with resistance to biocides. It is noteworthy, the prevalence of antibiotic resistance along with class I integrin rate were previously determined by authors [16, 17].
Preparation of biocide solutions
Biocides used in this study were chlorhexidine digluconate (20%) (Sigma-Aldrich, USA), benzalkonium chloride (>95%) (Sigma-Aldrich, USA), triclosan (98%) (Bio Basic, Canada) and formaldehyde (37%) (Thermo Fisher Scientific, USA). Stock solutions of antimicrobial agents were prepared in distilled water or water-alcohol for water-insoluble antimicrobial agents. All antibacterial solutions were sterilized using sterile syringe filters (0.22 µm) before use.
Determination of the MICs of biocides
The MICs of antiseptics and disinfectants including chlorhexidine digluconate, benzalkonium chloride, triclosan and formaldehyde were determined by agar dilution technique and according to the CLSI guideline [15]. The CLSI and similar organizations have not been defined a standard protocol describing bacterial resistance or susceptibility against non-therapeutic antimicrobials in susceptibility tests. Therefore, estimation of the ECOFF value for each biocide was done based on the MIC distributions in vitro. For this purpose, at first a range of biocide concentrations (0.125-1024 μg/mL) was prepared in Mueller-Hinton agar medium and then a 0.5 McFarland standard concentration of P. aeruginosa isolates (1.5 × 108 CFU/mL) was prepared in normal saline. Finally, diluted bacterial inoculum (1:10) (1.5 × 107 CFU/mL) was poured onto medium containing different biocides as a spot (104 CFU per spot). The plates were incubated at 37 ˚C overnight and then checked in terms of bacterial growth. In the current study, the ECOFF value for the antibacterial susceptibility testing against four biocides was determined equal to MIC95 (95% rule). Therefore, P. aeruginosa strains with higher MIC value compared with ECOFF value were considered as non-wild-type isolates, i.e. organisms with detectable resistance and reduced susceptibility for each biocide.
Detection of biocide resistance genes
Extraction of total genomic DNA was done using a simple boiling method according to previous instructions [18]. DNA quantity and quality was controlled using NanoDropTM 2000/2000c Spectrophotometer (Thermo Fisher Scientific, USA) and then validated using sequencing of amplified genes. Extracted DNA was stored at -20 ˚C until use for detection of biocide resistance genes. The presence of qacEΔ1, qacE, qacG, fabV, cepA and fabI genes was detected by specific primers in polymerase chain reaction (PCR). According to the thermal cycling condition for amplification and the oligonucleotide primer sequences presented in Table 1, PCR was performed in a volume of 25 μL containing 20 μL of master mix (Ampliqon, Denmark), 1 μL of each primers (10 μmol/L) and 3 μL of extracted DNA. PCR products were detected by electrophoresis on 1% agarose gel in a TBE 0.5x buffer and then confirmed by sequencing technique. In addition, a fabI gene positive Acinetobacter baumannii clinical isolate was used as positive control. Also, in this study, identified positive isolates for qacEΔ1, qacE, qacG, fabV and cepA genes were used for quality control.
Data analysis
All data on the MICs of biocides against P. aeruginosa strains, the presence of resistance genes to biocides and P. aeruginosa resistance to various antibiotics were collected and their correlation analyzed by the SPSS software version 16. The Chi-square test was used to analyses and a p value of <0.05 was considered statistically significant.
Results
In the present study, among 76 P. aeruginosa clinical isolates, the prevalence of qacEΔ1, qacE, qacG, fabV, cepA and fabI genes were 73.7% (n=56), 26.3% (n=20), 11.8% (n=9), 84.2% (n=64), 81.5% (n=62) and 0% (n=0), respectively. As shown in Table 2, the MIC range for various biocides was as follows: benzalkonium chloride 256-1024 μg/mL, formaldehyde 32-512 μg/mL, triclosan 32-512 μg/mL and chlorhexidine digluconate 4-64 μg/mL. In total, the highest MIC90 was observed for benzalkonium chloride (MIC90=1024 μg/mL), followed by formaldehyde (MIC90=512 μg/mL), triclosan (MIC90=512 μg/mL) and chlorhexidine digluconate (MIC90=64 μg/mL), Therefore, it seems that chlorhexidine digluconate and benzalkonium chloride had the highest and the lowest effects, respectively, in terms of growth inhibition of P. aeruginosa isolates in this study. As shown in Table 3, a significant association was observed between the presence of biocide resistance genes and MICs (p<0.05). Additionally, biocide resistance gene profiles revealed that isolates simultaneously harboring qacEΔ1, cepA and fabV genes are more prevalent (n=35, 46%), while 8 isolates (10.5%) did not harbor any biocide resistance genes (Table 4). Moreover, strains containing biocide resistance genes had higher MIC50 and MIC90 values compared with isolates without these genes. In this study, ECOFF value for the reduced susceptibility to benzalkonium chloride, triclosan, formaldehyde and chlorhexidine digluconate were determined as 1024, 512, 512 and 64 μg/mL, respectively.
Isolates with and without biocide resistance genes were compared in terms of resistance rate to the following antibiotics: piperacillin, piperacillin-tazobactam, ticarcillin-clavulanate, ceftazidime, cefepime, aztreonam, doripenem, imipenem, meropenem, gentamicin, tobramycin, amikacin, netilmicin, ciprofloxacin, levofloxacin, norfloxacin, lomefloxacin and ofloxacin. There was no significant association between the presence of biocide resistance genes and antibiotic resistance (p>0.05), except for levofloxacin and norfloxacin antibiotics and qacE and qacG genes (p<0.05). The prevalence of integron I positive P. aeruginosa strains harboring qacEΔ1 gene was 32 out of 76 (42.1%). No significant association was observed between the presence of class I integron and biocide resistance genes (qacEΔ1, qacE, cepA and fabV), except for qacG gene (p = 0.00).
Discussion
Biocides are basic compounds to control microorganism dissemination and ensuing infections and are frequently used as preservative, disinfectant and sterilizer against various microorganisms, in particular P. aeruginosa [20, 21]. One of the most useful biocides against microbes, especially Gram-positive bacteria, is triclosan, which is widely used in toothpastes, soaps and other daily products [20]. Triclosan is an anionic and lipophilic compound, which its anti-bacterial function stems from inhibition of enoyl-acyl-carrier protein reductase (ENR), an enzyme involved in fatty acid synthesis [22,23,24]. However, P. aeruginosa strains are inherently resistant to this biocide (MIC>2000 μg/mL) [24]. ENR enzymes show diversity among different bacteria in terms of sequence and structure, and contain four isozymes including FabI (triclosan- sensitive ENR), FabL, FabV and FabK (triclosan-resistant ENRs) [22,23,24]. The FabV isozyme is involved in swimming motility, energy metabolism, protein secretion and adherence, and is responsible for P. aeruginosa resistance to triclosan biocide [24]. Genes encoding FabI and FabV enzymes are found in most bacterial chromosomes such as P. aeruginosa [22,23,24]. Bacterial resistance to triclosan is associated with mutation in the active site of fabI gene and the presence of fabV gene [20, 24]. In the current study, the prevalence fabI resistance gene among P. aeruginosa isolates was 0%. Unlike fabI gene, the frequency of fabV gene was high in the present study (84.2%). Zhu et al. showed that deletion of fabV gene confers extremely high susceptibility to triclosan (>2,000 folds) in P. aeruginosa isolates [22]. Similar result was reported by Huang et al. [24]. In this study, the MIC50 and MIC90 values of triclosan for fabV resistance gene- harboring P. aeruginosa strains was higher than fabV gene-negative strains (Table 4).
Chlorhexidine digluconate, an antiseptic, disinfectant and preservative, is a bactericidal biocide, which has higher antibacterial activity against Gram-positive compared with Gram-negative bacteria [25]. This biocide is used in oral health antiseptics, hand washes and other hygienic solutions. The antibacterial mechanism of chlorhexidine digluconate is via the bacterial cell membrane [20]. However, P. aeruginosa is intrinsically resistant to this biocide due to the presence of an outer membrane [25]. Adaptive resistance to chlorhexidine biocide is mediated by a membrane protein encoded by Acinetobacter chlorhexidine efflux gene (aceI). The AceI protein identified in Acinetobacter baumannii is involved in chlorhexidine efflux via an energy-dependent mechanism [26]. However, genes encoding this protein were not identified in P. aeruginosa strains in the current study (data not shown). The antiseptic resistance gene cepA, an efflux pump gene, is associated with chlorhexidine resistance in Gram-negative bacteria causing high chlorhexidine MICs [27, 28]. In our study, 62 (81.5%) cepA-positive strains were found, which is higher than those reported by Mendes et al. (44.5%) and Vijayakumar et al. (63.6%) [27, 28]. According to MIC results, chlorhexidine digluconate is more effective than other biocides against P. aeruginosa isolates (MIC range=4–64 μg/mL) (Table 2). In this study, the presence of cepA gene had variable effects on the MIC50 and MIC90 values of chlorhexidine (Table 4).
A major biocide resistance mechanism in Gram-negative bacteria including P. aeruginosa is the action of efflux pumps such as the small multidrug resistance family (SMR) [14, 21]. Biocide resistance genes qacEΔ1, qacE and qacG encode multidrug efflux pumps, which confer resistance to quaternary ammonium compounds like benzalkonium chloride [14, 21]. In our study, the qacEΔ1 gene was observed in 73.7% of clinical isolates of P. aeruginosa, while in studies conducted by Subedi et al., Roma˜o et al., Kücken et al., Helal et al. and Mahzounieh et al. the qacEΔ1 gene was detected in 46.1%, 48%, 10%, 48% and 91.5% of the isolates, respectively [14, 21, 29,30,31]. According to the reports of Subedi et al., Kücken et al., Helal et al. and Mahzounieh et al., 100%, 2.7%, 13.5% and 50% of P. aeruginosa strains, respectively, had the qacE gene [14, 29,30,31], while we detected this gene in 26.3% of isolates. The frequency of qacG gene in the present study was 11.8%, which is higher compared to the frequency reported by Subedi et al. (0%) [14]. The MIC50 and MIC90 values of benzalkonium chloride were significantly high for qacEΔ1-, qacE- and qacG-positive P. aeruginosa strains compared with the negative strains (Table 4).
Class I integron carries qacEΔ1 and antibiotic resistance genes in clinical isolates of P. aeruginosa [14]. Therefore, P. aeruginosa strains harboring class I integron are resistant to benzalkonium chloride and various antibiotics [29]. Comparison of our current and previous study [17] showed that the frequency of integron I-positive P. aeruginosa strains harboring qacEΔ1 gene was 32 out of 76 (42.1%). No significant association was observed between the presence of class I integron and biocide resistance genes (qacEΔ1, qacE, cepA and fabV), except for qacG gene (p = 0.00).
Formaldehyde is an organic electrophilic biocide, which its mechanism of action involves cross-linking of macromolecules (proteins, RNA and DNA) [20, 32]. Our results indicated that the MIC50 and MIC90 values of formaldehyde were high for biocide resistance genes-positive P. aeruginosa strains compared with the negative strains (Table 4).
A study by Chuanchuen et al. showed a cross-resistance between biocide and antibiotic resistance. They demonstrated a link between P. aeruginosa exposure to triclosan biocide and efflux-mediated resistance to ciprofloxacin [13]. In the present study, there was no significant association between biocide resistance genes and antibiotic resistance, except for levofloxacin and norfloxacin antibiotics and qacE and qacG genes. However, more studies are needed to substantiate the existence of biocide-antibiotic cross-resistance.
Conclusion
Our results revealed that the frequency of resistance genes to benzalkonium chloride, chlorhexidine digluconate, triclosan and formaldehyde was high in clinical isolates of P. aeruginosa. Furthermore, P. aeruginosa isolates harboring resistance genes had higher MIC values compared with those lacking these genes. On the other hand, chlorhexidine digluconate was the most effective biocide against P. aeruginosa isolates in Ardabil hospitals. We recommend continuous monitoring of the antimicrobial activity of biocides and biocide-associated resistance genes in order to prevent microorganism dissemination and infection control in hospitals.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Abbreviations
- MIC:
-
Minimum inhibitory concentration
- PCR:
-
Polymerase chain reaction
- P. aeruginosa :
-
Pseudomonas aeruginosa
- ICU:
-
Intensive care unit
- MDR:
-
Multi-drug resistant
- CLSI:
-
Clinical and Laboratory Standards Institute
- ECOFF:
-
Epidemiological cut-off value
- ENR:
-
Enoyl-acyl-carrier protein reductase
References
Driscoll JA, Brody SL, Kollef MH (2007) The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 67(3):351–368
Vaez H, Salehi-Abargouei A, Khademi F (2017) Systematic review and meta-analysis of imipenem-resistant Pseudomonas aeruginosa prevalence in Iran. Germs 7(2):86–97
Moradali MF, Ghods S, Rehm BH (2017) Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol 7:39
Ignak S, Nakipoglu Y, Gurler B (2017) Frequency of antiseptic resistance genes in clinical staphycocci and enterococci isolates in Turkey. Antimicrob Resist Infect Control 6(1):1–7
Vásquez-Giraldo DF, Libreros-Zúñiga GA, Crespo-Ortiz MD (2017) Effects of biocide exposure on P. aeruginosa, E. coli and A. baumannii complex isolates from hospital and household environments. Infection 21(4):243–50
Babenko D, Turmuhambetova A, Sandle T, Pestrea SA, Moraru D, CHEŞCĂ A (2017) In silico comparison of different types of MLVA with PFGE based on Pseudomonas aeruginosa genomes. Acta Medica 33:347–352
Wesgate R, Grasha P, Maillard J-Y (2016) Use of a predictive protocol to measure the antimicrobial resistance risks associated with biocidal product usage. Am J Infect Control 44(4):458–464
Ortega-Morente E, Fernández-Fuentes MA, Grande-Burgos MJ, Abriouel H, Pérez-Pulido R, Gálvez A (2013) Biocide tolerance in bacteria. Int J Food Microbiol 162(1):13–25
Murray PR, Rosenthal KS, Pfaller MA (2015) Medical microbiology, 8th edn. Elsevier Health Sciences, UK, pp 272–277
Bridier A, Dubois-Brissonnet F, Greub G, Thomas V, Briandet R (2011) Dynamics of the action of biocides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 55(6):2648–2654
Mima T, Joshi S, Gomez-Escalada M, Schweizer HP (2007) Identification and characterization of TriABC-OpmH, a triclosan efflux pump of Pseudomonas aeruginosa requiring two membrane fusion proteins. J Bacteriol 189(21):7600–7609
Vikram A, Bomberger JM, Bibby KJ (2015) Efflux as a glutaraldehyde resistance mechanism in Pseudomonas fluorescens and Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 59(6):3433–3440
Chuanchuen R, Beinlich K, Hoang TT, Becher A, Karkhoff-Schweizer RR, Schweizer HP (2001) Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob Agents Chemother 45(2):428–432
Subedi D, Vijay AK, Willcox M (2018) Study of disinfectant resistance genes in ocular isolates of Pseudomonas aeruginosa. Antibiotics 7(4):88
CLSI, 2018. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Eighth Informational Supplement. CLSI Document M100. Clinical and Laboratory Standards Institute, Wayne, Pennsylvania, USA.
Bazghandi SA, Safarirad S, Arzanlou M, Peeri-Dogaheh H, AliMohammadi H, Khademi F (2021) Prevalence of multidrug-resistant Pseudomonas aeruginosa strains in Ardabil. J Ardabil Univ Med Sci 20(2):280–286
Khademi F, Ashrafi SS, Neyestani Z, Vaez H, Sahebkar A (2021) Prevalence of class I, II and III integrons in multidrug-resistant and carbapenem-resistant Pseudomonas aeruginosa clinical isolates. Gene Rep 25:101407
Delarampour A, Ghalehnoo ZR, Khademi F, Delarampour M, Vaez H (2019) Molecular detection of carbapenem-resistant genes in clinical isolates of Klebsiella pneumoniae. Ann Ig 31(4):349–355
Rizzotti L, Rossi F, Torriani S (2016) Biocide and antibiotic resistance of Enterococcus faecalis and Enterococcus faecium isolated from the swine meat chain. Food Microbiol 60:160–164
Chapman JS (2003) Biocide resistance mechanisms. Int Biodeterior. Biodegradation 51(2):133–138
Romão C, Miranda CA, Silva J, Clementino MM, de Filippis I, Asensi M (2011) Presence of qacEΔ1 gene and susceptibility to a hospital biocide in clinical isolates of Pseudomonas aeruginosa resistant to antibiotics. Curr Microbiol 63(1):16–21
Zhu L, Lin J, Ma J, Cronan JE, Wang H (2010) Triclosan resistance of Pseudomonas aeruginosa PAO1 is due to FabV, a triclosan-resistant enoyl-acyl carrier protein reductase. Antimicrob Agents Chemother 54(2):689–698
Heath RJ, Rock CO (2000) A triclosan-resistant bacterial enzyme. Nature 406(6792):145–146
Huang YH, Lin JS, Ma JC, Wang HH (2016) Functional characterization of triclosan-resistant enoyl-acyl-carrier protein reductase (FabV) in Pseudomonas aeruginosa. Front Microbiol 7:1903
Thomas L, Maillard JY, Lambert RJ, Russell AD (2000) Development of resistance to chlorhexidine diacetate in Pseudomonas aeruginosa and the effect of a “residual” concentration. J Hosp Infect 46(4):297–303
Hassan KA, Liu Q, Henderson PJ, Paulsen IT (2015) Homologs of the Acinetobacter baumannii AceI transporter represent a new family of bacterial multidrug efflux systems. MBio 6(1):e01982-e2014
Mendes ET, Ranzani OT, Marchi AP, da Silva MT, Amigo Filho JU, Alves T, Guimarães T, Levin AS, Costa SF (2016) Chlorhexidine bathing for the prevention of colonization and infection with multidrug-resistant microorganisms in a hematopoietic stem cell transplantation unit over a 9-year period: Impact on chlorhexidine susceptibility. Medicine 95(46):1–8
Vijayakumar R, Sandle T, Al-Aboody MS, Alfonaisan MK, Alturaiki W, Mickymaray S, Premanathan M, Alsagaby SA (2018) Distribution of biocide resistant genes and biocides susceptibility in multidrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii—A first report from the Kingdom of Saudi Arabia. J Infect Public Health 11(6):812–816
Kücken D, Feucht HH, Kaulfers PM (2000) Association of qacE and qacEΔ1 with multiple resistance to antibiotics and antiseptics in clinical isolates of Gram-negative bacteria. FEMS Microbiol Lett 183(1):95–98
Helal ZH, Khan MI (2015) QacE and QacEΔ1 Genes and Their Correlation to Antibiotics and Biocides Resistance Pseudomonas aeruginosa. Am J Biomed Sci 7(2):52–62
Mahzounieh M, Khoshnood S, Ebrahimi A, Habibian S, Yaghoubian M (2014) Detection of antiseptic-resistance genes in Pseudomonas and Acinetobacter spp. isolated from burn patients. Jundishapur J Nat Pharm Prod 9(2):e15402
McDonnell G, Russell AD (1999) Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12(1):147–179
Acknowledgements
The authors would like to acknowledge the Vice Chancellor for Research and Technology, Ardabil University of Medical Sciences, Ardabil, Iran, due to financial support.
Funding
This research was supported by Ardabil University of Medical Sciences, Iran (Grant Number: 1005761).
Author information
Authors and Affiliations
Contributions
MN, SS and SAB collected the data. FK and HV analyzed the data and led the writing of the manuscript. SH, MA and AS revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This research was approved by the Research Ethics Committee of Ardabil University of Medical Sciences.
Consent to participate
Informed written consent was given to subjects from whom the samples were obtained for this study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Namaki, M., Habibzadeh, S., Vaez, H. et al. Prevalence of resistance genes to biocides in antibiotic-resistant Pseudomonas aeruginosa clinical isolates. Mol Biol Rep 49, 2149–2155 (2022). https://doi.org/10.1007/s11033-021-07032-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-07032-2