Abstract
Objectives
Acute myeloid leukemia (AML) is still challenging in predicting the prognosis due to its high heterogeneity. Molecular aberrations and abnormalities play a significant prognostic role in AML patients. Our aim of the study was to investigate the prognostic role of TNFRSF4 gene expression in AML patients and its potential effect on treatment protocols.
Methods
Bone marrow mononuclear cells were analyzed for TNFRSF4 expression by real-time quantitative PCR as well as of FLT3/ITD and NPM1 mutations in 80 newly diagnosed AML patients and 80 control subjects.
Results
TNFRSF4 was significantly overexpressed in the AML patients (p < 0.001). TNFRSF4 expression was associated with unfavorable clinical outcomes including treatment response, relapse free survival, and overall survival. On multivariate testing, TNFRSF4 high expression proved to be an independent prognostic marker for clinical remission and relapse free survival but not overall survival.
Conclusion
TNFRSF4 expression was revealed as an unfavorable prognostic marker and might be a target for immunotherapy in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is a group of heterogeneous malignancy of the hematopoietic stem cells in the bone marrow characterized by uncontrolled proliferation of clonal myeloid blast cells [1]. These blasts replace the normal hematopoietic cells leading to cytopenias [2, 3].
Although recognition of the molecular pattern is of utmost importance for the prognosis and treatment of AML, it is still extremely difficult and challenging to classify AML due to the high heterogeneity of the disease [1]. Depending only on the cytogenetic analysis is not satisfactory, because there is almost 50% of AML patients who have normal cytogenetic testing with variable outcomes [1, 4, 5]. Therefore, studying the molecular background for the occurrence and progression of AML has become essential for early diagnosis to avoid treatment delay and to improve the overall prognosis [3, 6, 7].
Mutations in nucleophosmin (NPM1), TP53 and FMS-like tyrosine kinase 3/internal tandem duplication (FLT3/ITD) genes are the most common molecular abnormalities in AML [1, 8, 9]. These mutations may lead to disturbance of the transcription factors and faulty gene expressions in AML pathogenesis [1, 10].
NPM1 is mainly present in the nucleolus where it interacts with proteins involved in ribosomal synthesis and transport of ribosomal proteins through the membrane of the nucleus [11,12,13]. NPM1 binds to R-motifs which are proteins that contain multiple copies of the amino acid arginine [14]. It has been proved that NPM1 mutations occur in high frequencies in AML with normal karyotypes disrupting the cytoplasmic NPM1 functions as an interacting partner and transporter protein leading to the assumption that NPM1 mutation might be an early risk for leukemogenesis [13]. An important role for NPM1 in leukemias and lymphomas has been investigated in previous studies [13].
FLT3 belongs to the receptor tyrosine kinase class III (RTK) family [15, 16]. In normal hematopoiesis, FLT3 gene expression is confined to myeloid and lymphoid progenitors [15, 17]. In hematologic malignancies, FLT3 mutations are highly expressed in 70% to 100% of the AML blasts [15, 18]. There are two major types of FLT3 mutations in AML: internal-tandem duplication mutation in the juxtamembrane domain (ITDs) and point mutations in the tyrosine kinase domain (TKD) [15]. Mutations of FLT3 gene occur in up to 35% of AML patients, and specifically, the internal tandem duplication (ITD) which is the most common type that accounts for about 25% of all AML cases [19]. FLT3-ITD is considered a negative risk factor in patients with AML [19].
The tumor necrosis factor ligand superfamily number 4 (TNFSF4) is present on cell surfaces of dendritic cells, endothelial cells, and B lymphocytes, with its receptor TNFRSF4, also known as OX40 or CD134, being expressed on activated T lymphocytes [20]. TNFSF4/TNFRSF4 pathway is one of the main positive costimulatory signal pathways for immune cell stimulation [20, 21]. It induces T helper two differentiation, stimulates CD8 + T cells and promotes cytokine synthesis [20, 22]. It can also activate NF-kappa B pathway via TRAF2 and TRAF5 [3, 23]. Studies on TNFRSF4 gene expression suggest its role in T cell-APC interaction leading to immunologic activation producing proinflammatory cytokines resulting in various active diseases as SLE and atherosclerosis [20, 24, 25]. Some recent research has studied TNFRSF4's role in the immunotherapy of some tumors [3, 26, 27] .
Consequently, and in the context of TNFRSF4 gene expression on CD8+ T cells and T regulatory cells, various studies proved TNFRSF4 gene expression to be significantly increased in relapsed AML [3, 28]. Therefore, we decided to investigate the expression profile of TNFRSF4 in AML, to explore its potential prognostic value among AML patients and its potential benefit in future therapies.
Materials and methods
Our research was carried out on 80 newly diagnosed non-M3 AML patients with normal cytogenetic analysis at Alexandria University Hospital between August 2018 and August 2020. A total of 80 age- and sex-matched subjects with normal bone marrow, and without malignancy who were examined at the hospital at the same time were enrolled as the control group. The sample size was calculated using the G power version 3.1 statistical software program with a 0.05 level of significance and 80% power of the study. Written informed consent was signed by each participant in the study. This study was approved by the Ethics Committee of Alexandria Faculty of Medicine.
All our AML patients were subjected to laboratory investigations that included complete blood count (CBC), BM aspirate and/or biopsy, blood film morphology, flow cytometry, and cytogenetic analysis at the time of diagnosis. AML was diagnosed by the French-American-British classification (FAB) classification and the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues [2]. 20% was the cut-off of the blast count for the diagnosis of AML. The exclusion criteria included M3-AML, abnormal cytogenetic analysis, or previously diagnosed AML on treatment. Details of the study groups are listed in Table 1.
All AML patients received induction chemotherapy with 3 and 7 regimen including daunorubicin 45–90 mg/m2 intravenously for three days and infusion of cytosine arabinoside 100 mg/m2 over 24 h daily for one week [29]. Response to induction therapy was assessed using BM examination on day 28 after the induction therapy. All patients were then re-evaluated quarterly for relapse by BM aspirate examination. By the end of the induction phase, patients were said to be in complete remission (CR) if they had < 5% blast cells in the BM aspirate with no Auer rods, neutrophil count of ≥ 1.5 × 109/L, platelet count of > 100 × 109/ L, as well as bone marrow cellularity of at least 20%. Partial response (PR) was diagnosed if they had from 5 to 25% blasts in BM aspirate examination. Resistance to therapy was defined as persistence of over 25% blasts in the bone marrow, persistence of blast cells in the peripheral blood, and/ or blasts found at an extramedullary site at the end of the induction therapy [30].
DNA and RNA extraction
Diagnostic bone marrow (BM) specimens were aspirated at the Department of Clinical Pathology, University of Alexandria, Egypt at the initial diagnosis. DNA and RNA were isolated from BM mononuclear cells (BMNCs) according to the manufacturer’s instructions using the QIAamp DNA and RNA blood mini kits (Qiagen, USA), respectively. The quantity and quality of the extracted DNA and RNA were determined using NanoDrop 2000 spectrophotometer (NanoDrop Technologies, USA). A ratio value of 1.8 was considered to indicate DNA purity. Single-stranded cDNA was synthesized from purified RNA sample using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA) for RNA reverse transcription and the PCR amplification was carried out on the SimpliAmp Thermal Cycler (Applied Biosystems, USA).
FLT3/ITD and NPM1 mutations detection
Exons 14 and 15 of the FLT3 gene were amplified using MyTaq Red Mix (Bioline, London, United Kingdom), 14F, and 15R primers. The master mix was composed of dNTPs (dATP, dCTP, dGTP, dTTP) 0.4 mM each and 4 mM magnesium chloride and 0.05 units/ml of Taq polymerase in a reaction buffer. The following primers were used to amplify exons 14 and 15 of the FLT3 gene. The 14 Forward primer sequence was 5’ GCAATTTAG-GTATGAAAGCCAGC-3’. The 15 Reverse primer sequence was 5’ CTTTCAGCATTTTGACGGCAACC-3’. PCR conditions were as follows: initial denaturation step at 95 °C for 7 min followed by 35 cycles that included 1 min 94 °C for denaturation, 30 s 60 °C for annealing and 90 s 72 °C for extension, followed by a final extension of 7 min at 72 °C. FLT3/ITD mutations were detected using 2% agarose gel electrophoresis, stained with ethidium bromide. Then visualization of the DNA bands was done using a 302 nm ultraviolet transilluminator. The normal FLT3 gene showed a fragment length of 328 bp produced from the wild-type allele. While the FLT3/ITD mutation produced a larger fragment than the wild type.
For NPM1 mutation detection, the allele-specific oligonucleotide reverse transcriptase PCR (ASO-RTPCR) strategy was used to analyze the NPM1 exon 12 mutation. 5´CCA AGA GGC TAT TCA AGA TCT CTC TC-3´ was used as a forward primer and 5´ACC ATT TCC ATG TCT GAG CAC C-3´ was used as a reverse primer to amplify the cDNA. 50 ng cDNA was amplified using 20 pmol of each primer in a 25 μl reaction mix. The PCR master mix was composed of buffer containing dNTPs (dATP, dCTP, dGTP, dTTP) 0.4 mM each, 1.5 mM MgCl2 and 2.5 units/ml of Taq polymerase. PCR conditions were as follows: initial denaturation at 95 ºC for 15 min followed by 42 cycles of PCR (each cycle consisted of 95 ºC for 30 s, 67 ºC for 30 s, 60 ºC for 20 s), and final extension step of 5 min at 72 ºC. Amplification of a 258 bp fragment of the Abelson (ABL) gene was used as an internal quality control. The Amplified products were then electrophoresed using 2% agarose gels and examined under UV light with ethidium bromide stain. A 320-bp fragment was detected in NPM1 mutation status, while the unmutated samples did not show any bands.
Reverse transcription-quantitative PCR for gene expression
Quantitative analysis of TNFRSF4 gene expression level in bone marrow cells was carried out on Stratagene Mx3000P Real-time PCR system (Agilent, Germany). qPCR was conducted using the presynthesized cDNA, Maxima SYBR Green qPCR Master Mix (Thermo Scientific, USA), and sequence-specific primers. The primers sequences were shown as follows: forward, 5′-ACA ACG ACG TGG TCA GCT CCAA-3′ and reverse, 5′-CAG CGG CAG ACT GTG TCC TGT- 3′. The PCR conditions were composed of pre-denaturation at 95 °C for 3 min, 40 cycles of denaturation at 95 °C for 10 s, and annealing and extension at 60 °C for 34 s. GADPH and 18sRNA housekeeping genes were used as endogenous controls; their expression was constant in all samples. The 2−ΔΔCT CT method was used to analyse the relative mRNA expression levels [31].
Statistical analysis
SPSS statistical software v20.0 was used for statistical calculations. Statistical analysis was performed using the Chi-square test and Fisher’s Exact test for categorical data. Quantitative data were presented as mean and standard deviation (SD). Student’s t-test was used to compare significant differences between two groups for normally distributed quantitative variables. For abnormally distributed data, the Mann–Whitney test was used. Multivariate logistic regression model was adjusted for multiple comparisons. Kaplan– Meier curve was made to assess the relationship between genetic mutations or target gene expression levels and CR, RFS, or OS. Survival studies were tested using Cox proportional hazards models. Statistical significance was made when the p value < 0.05.
Results
Characteristics of healthy controls and AML patients
In the control group, the mean age was 59.4 years, with 61 (76.3%) males and 19 (23.8%) females. The median WBC count was 6.5 with a range of 4 to 10.2 X 109 cells/l. In the group of patients with AML, the mean age was 44 years, with 48 (60%) males and 32 (40%) females. The median WBC count was 13.5 with a range of 0.6 to 302 X109/l in the patients group. Regarding the FAB classification, 11 (13.8%) of the AML patients were classified as M0, 14 (17.5%) as M1, 18 (22.5%) as M2, 19 (23.8%) as M4, 14 (17.5%) as M5, 1 (1.3%) as M6 and 3 (3.8%) as M7. In terms of molecular analysis, 38 (47.5%) AML patients showed internal tandem duplications in FMS-like tyrosine kinase 3 (FLT3-ITD) gene, and 27 (33.8%) AML patients exhibited mutations in nucleophosmin 1 (NPM1) gene (Table 1).
Association of TNFRSF4 expression with AML risk
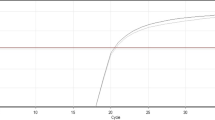
The relative expression of TNFRSF4 was significantly increased in AML patients compared with the control group (p < 0.001; Fig. 1), with a mean of 1 in the patients group compared to a mean of 0.6 in the control group (Table 1). ROC curve analysis showed that TNFRSF4 was able to differentiate AML patients from healthy controls (area under the curve (AUC) = 0.699, 95% CI = 0.614–0.783), with a sensitivity of 71.25% and a specificity of 61.25% at the best cut-off point (Table 2).
Association of TNFRSF4 expression with FAB classification and molecular genetics
TNFRSF4 expression was associated with both elevated NPM1 mutation (p = 0.022) and elevated FLT3-ITD gene mutation (p = 0.045). In addition, there was a significant statistical relationship with FAB classification (p = 0.023) (Table 3).
Predictive value of TNFRSF4 for treatment response
In the TNFRSF4-low expression group, 85.4% of cases achieved CR, while 14.6% did not. In the TNFRSF4-high expression group, 41% of cases achieved CR, while 59% did not (Table 3). Further analysis suggested that the CR rate was significantly lower in the TNFRSF4-high expression group than in the low-expression group (p < 0.001) (Table 3). Furthermore, the intensity of consolidation showed statistical significance with TNFRSF4 expression, with higher intensity of consolidation in the TNFRSF4-high expression group (p = 0.014) (Table 3).
Predictive value of TNFRSF4 for AML relapse rate (RFS) and OS
The number of patients who showed RFS was 46.2% in the TNFRSF4-high expression group compared with 87.8% in the TNFRSF4-low expression group. Similarly, high TNFRSF4 expression levels were associated with higher AML relapse rates compared with low TNFRSF4 expression levels (53.8% vs 12.2% respectively, p < 0.001). Regarding the OS, the number of patients who showed OS was lower in the TNFRSF4-high expression group (46.2%) compared with that in the TNFRSF4-low expression group (78%) (p = 0.003; Table 3).
Furthermore, survival analysis was tested using the Kaplan–Meier analysis. Firstly, regarding gene mutations, NPM1 mutations were associated with favorable OS and RFS (p < 0.001 for OS and p = 0.040 for RFS), while FLT3/ITD mutations were associated with poor OS and RFS (p < 0.001 for OS and p < 0.001 for RFS). Secondly, regarding target gene expressions, high TNFRSF4 levels were associated with poor OS and RFS (p = 0.022 for OS and p < 0.001 for RFS) (Fig. 2a-f).
a Kaplan–Meier survival curve for Overall Survival in AML group according to NPM1. b Kaplan–Meier survival curve for Relapse Free Survival in AML group according to NPM1. c Kaplan–Meier survival curve for Overall Survival in AML group according to FLT3/ITD. d Kaplan–Meier survival curve for Relapse Free Survival in AML group according to FLT3/ITD. e Kaplan–Meier survival curve for Overall Survival in AML group according to TNFSF4. f Kaplan–Meier survival curve for Relapse Free Survival in AML group according to TNFSF4
Associations with genetic mutations
The CR rate showed statistical significance with both NPM1 mutations and FLT3-ITD gene mutations (p = 0.038 for NPM1 and p = 0.004 for FLT3-ITD; Table 4). On the other hand, the intensity of consolidation showed statistical significance only with FLT3-ITD mutations (p = 0.028). Furthermore, the relapse rate was statistically significant in FLT3/ITD mutation (52.6% in the mutated state vs 14.3% in the non-mutated state, p < 0.001), while the group with NPM1 mutation showed significantly decreased relapse rates in comparison with the unmutated group (48.1% vs 24.5% respectively, p = 0.033) (Table 4). Regarding the OS, it was statistically significant with both NPM1 and FLT3-ITD genetic mutations. The percentage of patients who survived in the NPM1 mutated group was 81.5% compared with 52.8% in the non-mutated NPM1 mutated group, and 42.1% survived in the FLT3-ITD mutated group compared with 81% in the non-mutated FLT3-ITD group (p = 0.012 for NPM1 mutation and p < 0.001 for FLT3-ITD mutation) (Table 4).
Prognostic factors for CR
Univariate Cox regression analysis demonstrated that TNFRSF4 high expression (p < 0.001, HR = 0.041–0.350, 95% CI = 0.119), high intensity of consolidation (p = 0.003, HR = 0.088–0.610, 95% CI = 0.231), NPM1 mutation (p = 0.041, HR = 0.140–0.960, 95% CI = 0.367) and FLT3-ITD mutation (p = 0.005, HR = 0.093–0.650, 95% CI = 0.245) were predictors of lower CR in AML patients (Table 5). Subsequent multivariate analysis adjusted for these variables demonstrated that high expression of TNFRSF4 (p = 0.003, HR = 0.054–0.550, 95% CI = 0.172) and FLTS-ITD mutation (p = 0.027, HR = 0.081–0.860, 95% CI = 0.264) were considered as independent risk factors for poor CR in AML patients (Table 5).
Prognostic factors for RFS
Univariate Cox regression analysis showed that high TNFRSF4 expression (p = 0.001, HR = 2.347–26.65; 95% CI = 7.908), FLT3/ITD (p = 0.001, HR = 1.937–12.28; 95% CI = 4.878), and WBC counts (p = 0.020, HR = 1.001–1.013, 95% CI = 1.007) were predictors of unfavourable RFS in AML patients (Table 5). Subsequently, multivariate analysis was performed for those variables and revealed that both high TNFRSF4 expression (p = 0.003, HR = 1.926–22.03, 95% CI = 6.513) and FLT3-ITD gene mutation (p = 0.009, HR = 1.388–9.660, 95% CI = 3.661) were independent risk factors for poor RFS in AML patients (Table 5).
Prognostic factors for OS
Univariate Cox regression analysis revealed that TNFRSF4 high expression (p = 0.030, HR = 1.087–5.230, 95% CI = 2.385), high intensity of consolidation (p = 0.046, HR = 0.015–4.327, 95% CI = 2.095), NPM1 mutation (p = 0.001, HR = 0.071–0.507, 95% CI = 0.190) and FLT3-ITD mutation (p = 0.001, HR = 1.687–8.554, 95% CI = 3.798) were predictors of poor OS in AML patients. Subsequent multivariate analysis adjusted for the previous variables demonstrated that high consolidation intensity (p = 0.007, HR = 1.365–6.912, 95% CI = 3.071), NPM1 mutation (p < 0.001, HR = 0.035–0.338, 95% CI = 0.109) and FLT3-ITD mutation (p = 0.001, HR = 1.856–10.33, 95% CI = 4.380) were independent prognostic factors for shorter OS in AML patients (Table 5).
Discussion
Acute myeloid leukemia (AML) is a highly heterogeneous stem cell disorder that is caused by the uncontrolled proliferation of hemopoietic cells in the bone marrow [32]. Numerous genetic alterations and mutated genes expression of oncogenes and tumor suppressor genes have been detected in the pathogenesis of AML affecting the prognosis [1].
With the advanced technologies, many new molecular markers have been discovered, which in turn, has led to more precise and sophisticated AML classification affecting the prognosis and treatment modalities [1]. In our study, we chose a new molecular marker, TNFRSF4 gene expression, which might have a role in the carcinogenesis and tumorigenesis environment of different cancers including AML, and could be a target for immunotherapy in the future.
Previous studies have shown that TNFRSF4 is implicated in diverse malignant tumors. For example, Puntigam et al. detected significantly increased TNFRSF4 levels on circulating T regulatory cells of HPV + patients suffering from head and neck squamous cell carcinoma, presuming that antibody agonists of TNFRSF4 could provide a therapeutic tool through inhibition of T regulatory cells and enhancing anti-tumor activity [33]. Similarly, Kumar et al. previously reported that TNFRSF4 expression was high in cancers including melanoma, colon carcinoma, breast cancer, and B-cell lymphoma [26, 34]. In that study, they revealed that TNFSRF4 agonistic antibodies could be used for treatment protocols in melanoma, fibrosarcoma, colon cancer, and glioma, through inducing anti-tumor response in preclinical tumour models, mentioning that anti-TNFRSF4 antibodies are still in clinical trials, together with radiation and chemotherapy for many cancer subtypes [34, 35]. Moreover, TNFRSF4 signaling has been studied and proved in many autoimmune diseases. For instance, in systemic lupus erythematosus (SLE), TNFRSF4 has been located in multiple genetic loci, indicating that it might have a role in disease occurrence [34, 36]. In addition, TNFRSF4 interaction with its receptor has been reported in patients with collagen induced arthritis at the inflamed joints, with improvement of the inflammatory process after disrupting the TNFRSF4 mechanism [34, 37]. In another study, Zhou et al. studied TNFRSF4 and miR-744 in cardiac transplant patients and demonstrated that miR-744 negatively regulated TNFRSF4 expression by binding of the 3'UTR of TNRSF4 mRNA and that this TNFRSF4 inhibition reduced the rate of rejection of heart transplantation [38]. Daniel He et al., on the other hand, studied gene profiling of 730 immune-related genes in asthmatic patients and found out that there was an up-regulation of the TNFRSF4 protein expression and was considered as a potential therapeutic target in patients suffering from asthma [39]. Lastly, Gao et al. investigated various TNF genes associated with scleritis in Chinese people and reported that the TNFRSF4 GT haplotype was a risk factor for scleritis in his study population together with other TNF genes [40]. With that being said, we can say that many studies have been carried out to investigate TNFRSF4 gene expression in various cancers and autoimmune diseases, but studies addressing the direct role of TNFRSF4 in AML are still very few. Therefore, further research is required to demonstrate the potential effect of TNFRSF4 on AML leukemogenesis.
In our present study, we analyzed the clinicopathological implications of TNFRSF4 expression in AML and its possible relationship with various characteristics of the disease to improve the efficacy of the evaluation of AML. Our study revealed that TNFRSF4 was elevated in AML compared to healthy controls. We showed that higher TNFRSF4 gene expression was significantly related to and can predict poorer overall survival of AML patients. High TNFRSF4 was associated with mutated FLT3-ITD, mutated NPM1 genes, lower CR rate after induction therapy, high intensity of consolidation, and higher relapse rate. Considering our results, we hypothesized that TNFRSF4 high expression may be a part of an aggressive course of AML. Although very few studies investigated TNFRSF4 expression in AML, one study carried out by Gu et al. in 2020 has highlighted the prognostic role of TNFRFS4 expression in AML[3]. Our results coincide with their results that demonstrated clearly that higher TNFRSF4 expression was associated with NPM1 and FLT3 mutations and was positively correlated with bone marrow blast percentage, besides, they proved that TNFRSF4 high expression contributed to the poor clinical outcome of patients regarding the CR, OS, and relapse free survival (RFS). Another research conducted by Neubling et al. showed that TNFRSF4 is detected on AML blasts and that the TNFRSF4 can, after using agonistic TNFRSF4 antibodies, induce proliferation and secretion of cytokines that mediate growth and survival of leukemic cells. They also demonstrated that disruption of TNFSF4/TNFRSF4 mechanism of action impaired Natural Killer cells reactivity against primary AML cells, strongly suggesting that TNFRSF4 is implicated in disease pathophysiology of AML and that developing TNFRSF4-targeted approaches can be successful in cancer immunotherapy. Moreover, another research found that TNFRSF4 co-stimulation in vitro could reverse the CD8 T cell dysfunction partly in patients with AML [41], presuming that TNFRSF4 could play a role in the treatment of AML. In addition, one more study that was carried out on mice has revealed that direct ligation of TNFRSF4 on CD8 T cells increased tumor specific cytotoxicity and that concomitant treatment with agonistic antibodies to TNFRSF4 raises the chances of disease control after surgical or radiation therapy [42]. Our results proved that TNFRSF4 high expression negatively impacts AML prognosis. Thus, our results together with the previous studies results suggested clearly that it is very likely that TNFRSF4 expression plays an important role in AML and has a crucial role in determining the clinical outcome and treatment response of the disease, and that immunotherapy targeting TNFRSF4 receptors could be beneficial in treating patients with AML.
Furthermore, the current study showed that high TNFRSF4 expression was strongly related to worse AML disease progression, lower CR, higher intensity of consolidation, higher relapse rate, and shorter OS, which helped in clear differentiation between AML cases according to their TNFRSF4 gene expression level. High TNFRSF4 expression was an independent unfavorable prognostic marker for CR and RFS in AML patients. On contrary, OS was not affected by TNFRSF4 in the multivariate analysis. However, TNFRSF4 expression was significantly related to lower CR, shorter RFS, and OS by univariate analysis, suggesting that TNFRSF4 is at least playing a part in the prognostic evaluation of AML that could be used to predict clinical outcome and survival in AML patients more precisely.
A limitation of our study to be noted was that the TNFRSF4 expression was measured only at baseline, while changes in the expression levels after receiving treatment were not measured, therefore, further investigations would be recommended in AML patients to explore whether there will be changes in the expression later in the course of the disease. In addition, our study was observational, it could not differentiate the possible mechanisms behind our findings. Thus, functional studies are recommended to understand the research topic in depth.
In conclusion, our findings from this study indicate that TNFRSF4 high expression worsens the prognosis in AML patients with worse clinical outcome than in patients with low expression. Therefore, TNFRSF4 expression might be used to assess the prognosis and might have a role in treatment guidance in AML patients. Because agonistic antibodies to TNFRSF4 are proved effective in improving the prognosis of various cancers and autoimmune diseases, they are considered promising alternative treatment options for patients with AML. Based on our findings, we suggest that clinical trials evaluating the use of agonistic antibodies to TNFRSF4 should be considered for patients with AML showing high TNFRSF4 gene expression.
References
Abo Elwafa R, Gamaleldin M, Ghallab O (2019) The clinical and prognostic significance of FIS1, SPI1, PDCD7, and Ang2 expression levels in acute myeloid leukemia. Cancer Genet 234:84–95
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Vardiman JW (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20):2391–2405. https://doi.org/10.1182/blood-2016-03-643544
Gu S, Zi J, Han Q, Song C, Ge Z (2020) Elevated TNFRSF4 gene expression is a predictor of poor prognosis in non-M3 acute myeloid leukemia. Cancer Cell Int 20(146):020–01213
Bhatnagar B, Garzon R (2014) The use of molecular genetics to refine prognosis in acute myeloid leukemia. Curr Hematol Malig Rep 9(2):148–157
Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Bloomfield CD (2002) Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood 100(13):4325–4336. https://doi.org/10.1182/blood-2002-03-0772
Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, Eley G (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368(22):2059–2074. https://doi.org/10.1056/NEJMoa1301689
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, Campbell PJ (2016) Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374(23):2209–2221. https://doi.org/10.1056/NEJMoa1516192
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Bloomfield CD (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115(3):453–474. https://doi.org/10.1182/blood-2009-07-235358
O’Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, Ogba N (2017) Acute Myeloid Leukemia, Version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15(7):926–957
Zhu YM, Wang PP, Huang JY, Chen YS, Chen B, Dai YJ, Shen Y (2017) Gene mutational pattern and expression level in 560 acute myeloid leukemia patients and their clinical relevance. J Transl Med 15(1):017–1279
Borer RA, Lehner CF, Eppenberger HM, Nigg EA (1989) Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 56(3):379–390
Dumbar TS, Gentry GA, Olson MOJ (1989) Interaction of nucleolar phosphoprotein B23 with nucleic acids. Biochemistry 28(24):9495–9501. https://doi.org/10.1021/bi00450a037
Verhaak RG, Goudswaard CS, van Putten W, Bijl MA, Sanders MA, Hugens W, Valk PJ (2005) Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood 106(12):3747–3754
Mitrea DM, Cika JA, Guy CS, Ban D, Banerjee PR, Stanley CB, Kriwacki RW (2016) Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. Elife 2(5):13571
Kindler T, Lipka DB, Fischer T (2010) FLT3 as a therapeutic target in AML: still challenging after all these years. Blood 116(24):5089–5102. https://doi.org/10.1182/blood-2010-04-261867
Stirewalt DL, Radich JP (2003) The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 3(9):650–665. https://doi.org/10.1038/nrc1169
Rosnet O, Bühring HJ, deLapeyrière O, Beslu N, Lavagna C, Marchetto S, Birnbaum D (1996) Expression and signal transduction of the FLT3 tyrosine kinase receptor. Acta Haematol 95(3–4):218–223
Carow CE, Levenstein M, Kaufmann SH, Chen J, Amin S, Rockwell P, Small D (1996) Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood 87(3):1089–1096
Daver N, Schlenk RF, Russell NH, Levis MJ (2019) Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia 33(2):299–312
Jindra PT, Conway SE, Ricklefs SM, Porcella SF, Anzick SL, Haagenson M, Abdi R (2016) Analysis of a genetic polymorphism in the costimulatory molecule TNFSF4 with hematopoietic stem cell transplant outcomes. Biol Blood Marrow Transplant 22(1):27–36
Hori T (2006) Roles of OX40 in the pathogenesis and the control of diseases. Int J Hematol 83(1):17–22
Wythe SE, Dodd JS, Openshaw PJ, Schwarze J (2012) OX40 ligand and programmed cell death 1 ligand 2 expression on inflammatory dendritic cells regulates CD4 T cell cytokine production in the lung during viral disease. J Immunol 188(4):1647–1655
Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T (1998) Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J Biol Chem 273(10):5808–5814
Bossini-Castillo L, Broen JC, Simeon CP, Beretta L, Vonk MC, Ortego-Centeno N, Rueda B (2011) A replication study confirms the association of TNFSF4 (OX40L) polymorphisms with systemic sclerosis in a large European cohort. Ann Rheum Dis 70(4):638–641
Ria M, Lagercrantz J, Samnegård A, Boquist S, Hamsten A, Eriksson P (2011) A common polymorphism in the promoter region of the TNFSF4 gene is associated with lower allele-specific expression and risk of myocardial infarction. PLoS ONE 6(3):0017652
Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A (2016) Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer 52:50–66
Linch SN, McNamara MJ, Redmond WL (2015) OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. https://doi.org/10.3389/fonc.2015.00034
Williams P, Basu S, Garcia-Manero G, Hourigan CS, Oetjen KA, Cortes JE, Daver NG (2019) The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer 125(9):1470–1481
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Bloomfield CD (2003) Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21(24):4642–4649
Sheikhha MH, Awan A, Tobal K, Liu Yin JA (2003) Prognostic significance of FLT3 ITD and D835 mutations in AML patients. Hematol J 4(1):41–46
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Wang X, Li W, Chen Y, Zhou L (2021) Long non-coding RNA SNHG14 affects the proliferation and apoptosis of childhood acute myeloid leukaemia cells by modulating the miR-193b-3p/MCL1 axis. Mol Med Rep. https://doi.org/10.3892/mmr.2020.11729
Puntigam LK, Jeske SS, Götz M, Greiner J, Laban S, Theodoraki MN, Schuler PJ (2020) Immune checkpoint expression on immune cells of HNSCC patients and modulation by chemo- and immunotherapy. Int J Mol Sci 21(15):5181. https://doi.org/10.3390/ijms21155181
Kumar P, Bhattacharya P, Prabhakar BS (2018) A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun 95:77–99. https://doi.org/10.1016/j.jaut.2018.08.007
Schaer DA, Hirschhorn-Cymerman D, Wolchok JD (2014) Targeting tumor-necrosis factor receptor pathways for tumor immunotherapy. J Immunother Cancer 2:7. https://doi.org/10.1186/2051-1426-2-7
Manku H, Langefeld CD, Guerra SG, Malik TH, Alarcon-Riquelme M, Anaya JM, Vyse TJ (2013) Trans-ancestral studies fine map the SLE-susceptibility locus TNFSF4. PLoS Genet 9(7):e1003554. https://doi.org/10.1371/journal.pgen.1003554
Gwyer Findlay E, Danks L, Madden J, Cavanagh MM, McNamee K, McCann F, Hussell T (2014) OX40L blockade is therapeutic in arthritis, despite promoting osteoclastogenesis. Proc Natl Acad Sci U S A 111(6):2289–2294. https://doi.org/10.1073/pnas.1321071111
Zhou B, Mei F, Wu C, Liu Z, Xu H, Cui Y (2020) Effect of miR-744 on ameliorating heart allograft rejection in BALB/c mice via regulation of TNFRSF4 expression in regulatory T cells. Transplant Proc 52(1):398–405. https://doi.org/10.1016/j.transproceed.2019.10.014
He D, Yang CX, Sahin B, Singh A, Shannon CP, Oliveria JP, Tebbutt SJ (2019) Whole blood vs PBMC: compartmental differences in gene expression profiling exemplified in asthma. Allergy Asthma Clin Immunol 15:67. https://doi.org/10.1186/s13223-019-0382-x
Gao Y, Du L, Li F, Ding J, Li G, Cao Q, Yang P (2020) The haplotypes of various TNF related genes associated with scleritis in Chinese Han. Hum Genomics 14(1):46. https://doi.org/10.1186/s40246-020-00296-y
Nuebling T, Schumacher CE, Hofmann M, Hagelstein I, Schmiedel BJ, Maurer S, Salih HR (2018) The immune checkpoint modulator OX40 and its ligand OX40L in NK-cell immunosurveillance and acute myeloid leukemia. Cancer Immunol Res 6(2):209–221. https://doi.org/10.1158/2326-6066.Cir-17-0212
Knaus HA, Berglund S, Hackl H, Blackford AL, Zeidner JF, Montiel-Esparza R, Gojo I (2018) Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight. https://doi.org/10.1172/jci.insight.120974
Acknowledgements
The authors would like to thank all the patients and their families for participating in this project.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the current work. M.G. and S.I. had contributed substantially to the conception and design of the study, acquisition of data analyzed and interpreted the data, had performed laboratory investigations of the participants, analysis, and interpretation of the data. All the authors approved the final version submitted for publication and take responsibility for the statements made in the published article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent to participate
Written informed consent was signed by each participant in the study. This study was approved by the Ethics Committee of Alexandria Faculty of Medicine.
Consent for publication
Consent was obtained from participants to publish the results with keeping the confidentiality of their personal information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gamaleldin, M.A., Imbaby, S.A.E. The role of tumor necrosis factor receptor superfamily member 4 (TNFRSF4) gene expression in diagnosis and prognosis of acute myeloid leukemia. Mol Biol Rep 48, 6831–6843 (2021). https://doi.org/10.1007/s11033-021-06682-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06682-6