Abstract
Our previous studies have shown that miR-125b-5p was highly expressed and significantly upregulated during abdominal fat deposition in chickens. However, the role of miR-125b in the regulation of adipogenesis is not clear in chickens. Therefore, we evaluated the effects of miR-125b-5p on preadipocyte proliferation and differentiation and the interaction between miR-125b-5p and the acyl-CoA synthetase bubblegum family member 2 (ACSBG2) gene in adipogenesis in chicken abdominal adipose tissue. Here, transfection tests of miR-125b-5p mimic/inhibitor were performed in preadipocytes, and the effects of miR-125b-5p on preadipocytes proliferation and differentiation were analyzed. The target site of miR-125b-5p in the 3′UTR (untranslated region) of ACSBG2 were verified by a luciferase reporter assay. Our results showed that miR-125b-5p overexpression inhibited proliferation and reduced the number of cells in S phase and G2/M phase in preadipocytes; conversely, miR-125b-5p inhibition promoted the proliferation and increased the number of cells in S phase and G2/M phase. In adipocytes after induction, miR-125b-5p overexpression led to a notable increase in the accumulation of lipid droplets as well as in the concentration of triglycerides, while miR-125b-5p inhibition had the opposite effect. Furthermore, miR-125b-5p could directly bind to the 3'UTR of ACSBG2, and its overexpression could significantly repress the mRNA and protein expression of ACSBG2. These results indicate that miR-125b-5p can inhibit preadipocyte proliferation and can promote preadipocyte differentiation to affect adipogenesis in chicken abdominal adipose tissues, at least partially by downregulating ACSBG2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipose tissue is closely related to economic trait in animals. Adipogenesis involving preadipocyte proliferation and adipocyte differentiation events is a major mechanism in adipose tissue formation. Adipocytes are derived from mesenchymal stem cells that originated in the mesoderm through gradual differentiation and development. The increase in the number of intermediate pre-adipocytes ultimately lays the foundation for differentiation into mature adipocytes and lipid accumulation [1]. In vitro studies on the development of preadipocytes prepared from abdominal adipose tissue may help us to explore the molecular mechanism of abdominal adipose accumulation, during which many genes, transcription factors and non-coding RNAs are expressed in a tissue- or cell-specific manner [2]. Thus, understanding the precise mechanisms of adipogenesis regulation in animals is essential. Increasing evidence has demonstrated that microRNAs (miRNAs) are involved in the regulation of adipogenesis. For instance, miR-143, miR-146b, and miR-375 have been reported to promote adipogenesis [3,4,5]. In contrast, miR-302a, miR-27b, and miR-215 have been shown to inhibit adipogenesis [6,7,8]. Therefore, miRNAs have become an important research point in understanding the posttranscriptional regulation mechanism of adipogenesis.
Chickens (Gallus gallus) is a significant agricultural economic animal. The features of chicken abdominal adipose tissue are closely related to economic traits, and excessive accumulation of abdominal fat often has adverse effects on meat quality [9, 10]. Therefore, elucidating the miRNA regulation mechanism of adipogenesis in the abdominal adipose tissue of chickens has important significance for poultry genetic breeding and production. At present, there are some reports on miRNA profiles about the chicken abdominal adipose and preadipocytes. For example, 230 known miRNAs and 83 potential new miRNAs were identified in abdominal adipose tissue in two different breeds of chicken [11]. Two other studies showed that 159 known miRNAs and 33 differentially expressed miRNAs were detected in preadipocytes obtained from chicken abdominal adipose tissues [12, 13]. In addition, a recent study also identified 80 differentially expressed miRNAs during abdominal preadipocyte differentiation in chickens [14]. These studies suggest that many miRNAs may be involved in adipogenesis in chicken abdominal adipose tissue. However, there are few studies on the individual functions of miRNAs in the formation of abdominal adipose tissue.
miR-125b is a highly conserved miRNA found in a wide range of species ranging from nematodes to humans [15]. Previous studies have indicated that miR-125b plays a pivotal role in cell differentiation, proliferation and apoptosis [16, 17]. Notably, miR-125b has also implicated in the regulation of adipogenesis [18,19,20,21]. For example, miR-125b can reduce triglyceride (TG) concentration and lipid droplet accumulation, and can diminish the monounsaturated fatty acid (MUFA) composition in the porcine adipocytes [18]. Rockstroh et al. demonstrated that miR-125b-5p can regulate human adipogenesis by targeting MMP11 [19]. Ouyang et al. showed that overexpression of miR-125b-5p can promote 3T3-L1 preadipocyte differentiation and can inhibit 3T3-L1 preadipocyte proliferation [21]. These studies suggest that miR-125b is a typical regulatory factor that can regulate adipogenesis. However, it is not clear whether miR-125b plays a role in the regulation of adipogenesis in chickens.
In chickens, mir-125b consists of the homologous genes mir-125b-1 and mir-125b-2, which are transcribed from two different sites on chromosome 24 and chromosome 1, respectively, and encodes the same mature miRNA miR-125b-5p. At present, little information regarding chicken miR-125b function is available [22, 23], and the role of miR-125b in the adipogenesis process has not been reported in chickens. In our previous studies, miR-125b-5p was highly expressed and significantly upregulated during the development of abdominal adipose tissue in Gushi chickens (a local Chinese breed) [24]. This suggests that miR-125b may be involved in adipogenesis in chickens. The acyl-CoA synthetase bubblegum family member 2 (ACSBG2) gene plays an important role in activating C16 to C24 fatty acids [25, 26], and it is also a component of several lipid metabolic pathways such as FA biosynthesis, FA degradation, adipocytokine signaling pathway and PPAR signaling pathway. Interestingly, a potential regulatory relationship between miR-125b-5p and ACSBG2 was found through correlation analysis of mRNA and miRNA transcriptome data of abdominal adipose tissues from Gushi chickens. Based on these data, we hypothesized that miR-125b-5p may regulate adipogenesis by targeting ACSBG2 gene in chicken abdominal adipose tissue. In this study, we verified a role of miR-125b-5p and the targeted relationship between miR-125b-5p and ACSBG2 in chicken preadipocytes. These results will contribute to a better understanding of the molecular mechanisms of adipogenesis in chicken abdominal adipose tissues.
Materials and Methods
Ethics Statement
All animal experiments were performed according to the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, 2004). The protocols and guidelines were approved by the Institutional Animal Care and Use Committee of Henan Agricultural University, China.
Experimental Animals and Total RNA Extraction
Gushi chicken was selected as the experimental model in this study. All experimental chickens were raised from the Avian Farm of Henan Agriculture University (Zhengzhou, China). Six healthy female individuals were selected randomly, and abdominal adipose tissue were taken for temporal expression analysis of miR-125b-5p at 6, 14, 22 and 30 weeks of age. Total RNA was extracted from abdominal adipose tissue using TRIzol Reagent (TaKaRa, Japan) according to the manufacturer’s instructions. The concentration of RNA was determined by NanoDrop 2000 spectrophotometry (Thermo Scientific, Wilmington, DE, USA) at a 260/280 nm ratio (with OD260/OD280≥1.8). The integrity was examined by 1% agarose gel electrophoresis.
Primary Preadipocytes Isolation and Culture
Preadipocytes were isolated and cultured from Gushi chicken abdominal adipose tissue. The isolated adipose tissue was washed three times in PBS with 0.8% streptomycin/penicillin. The adipose tissue was then minced and digested in 2 mg/mL collagenase type I (Gibco, USA) with shaking at 37 °C for 65 min. Subsequently, the cells were maintained with erythrocyte lysis buffer (10 mM KHCO3, 0.1 mM EDTA and 0.154M NH4Cl) for 10 min at room temperature to separate the stromal-vascular fractions and mature adipocytes. The preadipocytes were collected and centrifuged at 1360 g for 10 min and then resuspended and cultured in DMEM/F12 (HyClone, USA) supplemented with 15% fetal bovine serum (FBS) (Gibco, USA), 100 mg/mL streptomycin (Life Technologies, USA) and 100 U/mL penicillin (Invitrogen, USA). To induce differentiation, primary preadipocytes were inoculated in 6-well plates until cell fusion. Subsequently, the cells were cultured in medium containing 1 μM dexamethasone (Sigma, USA), 0.5 mM IBMX (Sigma, USA), 50 μg/mL insulin (Sigma, USA) and 300 μM oleate (Sigma, USA) for 2 days. The medium was then changed to DMEM/F12 with 10% FBS (Gibco, USA) and 5 μg/mL, 100 mg/mL streptomycin (Life Technologies, USA) and 100 U/mL penicillin (Invitrogen, USA) and insulin. After 2 days, the medium was changed to normal medium until maturation.
Transfection of miR-125b-5p Mimic and Inhibitor
To evaluate the effect of miR-125b-5p on the proliferation and differentiation of chicken preadipocytes, a transfection test was performed in cultured preadipocytes using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. The gga-miR-125b-5p mimic, mimic negative control (mNC), inhibitor, and inhibitor negative control (iNC) were synthesized by RiboBio (RiboBio, Guangzhou, China). 50 nmol of gga-miR-125b-5p mimic or inhibitor was transfected into the cells; mNC and iNC were used as negative control for the gga-miR-125b mimic and gga-miR-125b inhibitor, respectively. For proliferation, transfection tests were performed when the cells reached 50–60% confluence. For differentiation, transfection tests were performed when preadipocytes grew to 80% confluence, and preadipocytes were induced to differentiate after 6 h of transfection. Subsequently, cells were harvested at the indicated time points postinduction.
Cell Counting Kit-8 (CCK-8) and 5-Ethynyl-2′-Deoxyuridine (EdU) Assays
CCK-8 and EdU detection kits are widely used in cell proliferation. Chicken preadipocytes seeded in 96-well plates were transfected with the gga-miR-125b-5p mimic/inhibitor and mNC/iNC. Cell proliferation was measured at 12, 24, 36, and 48 h after transfection using a CCK-8 (Dojindo, Shanghai) following the manufacturer’s instructions. The working principle of CCK8 is that WST-8 can be reduced by dehydrogenase in the mitochondria to produce a highly water-soluble orange-yellow formazan product, the color of which is proportional to the proliferation of the number of surviving cells. Use the Fluoroskan Ascent FL instrument (Thermo Fisher Science, USA) microplate reader to measure the optical density (OD) after 2 h of incubation at 450 nm. The OD value can indirectly reflect the number of living cells.
EdU is a thymidine analogue that can replace thymine (T) into the DNA molecule being synthesized during the period of DNA replication. Based on the specific reaction between Apollo© fluorescent dye and EdU, DNA replication activity can be directly and accurately detected. For EdU analysis, chicken preadipocytes seeded in 24-well plates were transfected with the gga-miR-125b-5p mimic/inhibitor and mNC/iNC. After 36 h of transfection, the cells were treated with 10 μM EdU (RibiBio, Guangzhou, China) for 6 h at 37 °C. These treated cells were then fixed with 4% paraformaldehyde for 40 min at room temperature. Subsequently, the fixed cells were stained using a Cell-Light™ EdU Apollo 567 In Vitro Kit (RiboBio, Guangzhou, China) following the protocol. The EdU-stained cells were imaged by a fluorescence microscope (Olympus, Tokyo, Japan). The proliferation rate of preadipocytes was measured by normalizing the number of EdU-stained cells (red) to the number of Hoechst 33342-stained cells (blue).
The Cell Cycle Assay by Flow Cytometry Analysis
Chicken preadipocytes were inoculated into 6-well plates, cultured to 80% confluence and then transfected with the gga-miR-125b-5p mimic/inhibitor and mNC/iNC. After 48 h of transfection, the collected cells were fixed overnight with 75% ethanol at −20 °C. Then, the fixed cells were washed with PBS and stained with 50 μg/mL propidium iodide (Sigma, USA) containing 0.2% Triton X-100 (Sigma, USA) and 10 μg/mL RNase A (TaKaRa, Japan) for 15 min in the dark. The strained cells were analyzed on a BD FACSCelesta Flow Cytometer (BD Biosciences, USA), and the data were processed using FlowJo 7.6 software.
Quantitative Real-Time PCR (qRT-PCR) Analysis
For miRNA quantification, the customized BuLge-Loop™ miRNA qRT-PCR Primer kit (RiboBio, Guangzhou, China) specific for gga-miR-125b-5p and U6 was used for the qRT-PCR analysis. For mRNA quantification, synthesis of cDNA was carried out by the Prime Script™ RT Reagent Kit (TaKaRa, Dalian, China), and the primer sequences are listed in Table S1. The qRT-PCR analysis was carried out using the SYBR® Premix Ex Taq™ II Kit (TaKaRa, Dalian, China) on an Applied Biosystems™ QuantStudio™ 3 (Thermo Fisher Scientific, USA). The relative expression levels were calculated using the 2−ΔΔCt method. U6 was used as an endogenous control for miRNA, and GAPDH and β-actin were used as internal controls for mRNA.
Western Blot Analysis
We lysed the cells in lysis buffer (Sigma, Louis, MO, USA) containing phenyl methyl sulfonyl fluoride (Beyotime Biotech, Shanghai China). Total cellular protein was separated using 12% SDS-PAGE and then transferred to PVDF membranes (ISEQ00010, Millipore, Danvers, MA, USA). The membranes were blocked with 5% skim milk in Tris-buffered saline plus 0.5% Tween 20 and placed on a decolorizing shaker for 1 h. Next, the membranes were incubated with anti-PPAR γ (Novus Biologicals, Littleton, CO, United States), anti-C/EBP α (Fitzgerald, United States), or anti-ACSBG2 (Lifespan, United States) antibodies at 4 °C overnight and then incubated using a second antibody conjugated to HRP (Servicebio, China) for 1 h at room temperature. ECL Plus (Solarbio) was used to enhance the signals. Image capture and analysis were carried out using Photoshop cs 6 and AlphaView 3.0 (Alpha Innotech, USA). GAPDH (Servicebio, China) was used to normalize protein expression.
Oil Red O Staining, TG Analysis and Free Fatty Acid (FFA) Analysis
For Oil red O staining, the cells were washed three times with PBS after being treated for 2 days and fixed in 4% paraformaldehyde solution for 60 min. Subsequently, the fixed cells were washed using PBS and stained with 5% Oil red O solution for 1 h. The cells were washed twice with PBS and photographed with an Olympus microscope. Oil red O dye in the strained cells was eluted with 100% isopropanol and then quantified by Fluoroskan Ascent FL instrument s (Thermo Fisher Scientific, US) at 500 nm.
For the triglycerides (TG) assay, the TG concentrations in cell lysates were determined by a Food Triglyceride Assay Kit (APLLYGEN, Beijing, China). The TG concentrations were calibrated to the protein content by the BCA Protein Detection Kit (Bioteke Corporation, Beijing, China).
For fatty acid (FA) composition analysis, 107 cells were collected and lysed by ultrasound, and the FA content was extracted using sulfuric acid/methanol (1:40, vol/vol), followed by incubation in an 80 °C water bath for 60 min. After a methylation reaction for 30 min, the total cell lipids were extracted using 2 mL 0.1M HCl and 1 mL N-hexane. All samples were analyzed using an Agilent HP-Innowax capillary column (30 m × 0.25 mm ID × 0.25 × m) on an Agilent 6890N/5975B gas-mass analyzer (Agilent, US).
Vector Construction
The chicken ACSBG2 coding region was amplified from chicken abdominal adipose tissue cDNA by PCR, isolated with NheI and EcoRI, and then cloned into the pcDNA3.1 vector to obtain pcDNA3.1-ACSBG2. The 3′ untranslated region (3′ UTR) of chicken ACSBG2 containing the predicted binding site, including wild type and mutant type (referred to as ACSBG2-3′ UTR WT and ACSBG2-3′ UTR MUT, respectively), was amplified from abdominal adipose tissue by PCR and then subcloned into the XhoI-NotI site of the psiCHECK-2 vector (Promega, Madison, WI, USA). The primers used for vector construction are listed in Table S2.
Luciferase Reporter Assay
Chicken fibroblasts (DF1) were used in luciferase reporter experiments. Cells were inoculated into a 24-well plate at a density of 5 × 105 cells/well and cultured with 10% FBS (Gibco, USA) under normal conditions. When cell fusion reached 70%, ACSBG2-3'UTR-WT or ACSBG2-3'UTR-MUT were cotransfected with the miR-125b-5p mimic and mNC, respectively. The luciferase activities were determined by a dual-luciferase reporter gene detection system (Promega) using a Fluoroskan Ascent FL instrument (Thermo Fisher Science, US) at 48 h posttransfection. Each group was transfected three times in total, and three biological replications were performed for each transfection experiment.
Data Analysis
All data are presented as the mean ± SEM based on at least three independent replicates for each treatment. Graphics were drawn using GraphPad Prism 7 software (San Diego, CA, USA). Differences among groups were analyzed using Student’s test by SPSS 19.0 (IBM, Chicago, IL, USA). The t-test was used to evaluate whether the values of two groups had statistically significant differences (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
Temporal Expression of miR-125b-5p and ACSBG2 Gene During Adipogenesis
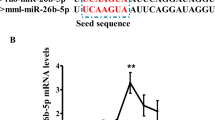
The expression level of miR-125b-5p in abdominal adipose tissue at different developmental stages of chicken was detected by qRT-PCR. The results show that the expression level of miR-125b-5p was upregulated dramatically with the different developmental stages of chicken in abdominal adipose tissue (Fig. S1). This suggests that miR-125b-5p may be involved in the regulation of abdominal fat deposition in chickens. Meanwhile, the expression of miR-125b-5p during primary preadipocyte differentiation was also analyzed by qRT-PCR. For the purpose, a preadipocyte differentiation model was constructed from Gushi chicken abdominal adipose tissue. As shown in Fig. S1, the expression level of miR-125b-5p gradually increased with the differentiation of chicken preadipocytes and then reached a maximum at day 10. This suggests that miR-125b-5p may be closely correlated with adipogenesis in chicken abdominal adipose tissue.
In addition, the expression of ACSBG2 was detected during preadipocyte differentiation by qRT-PCR. The results showed that ACSBG2 expression decreased gradually in the early stage of preadipocyte differentiation (0–4 days), which was contrary to miR-125b-5p expression (Fig. S1). Interestingly, miR-125b-5p expression continued to increase in the late stage of preadipocyte differentiation (6-10 days), but ACSBG2 expression remained stable. This suggests that there may be potential interactions between ACSBG2 and miR-125b-5p in the early stage of chicken preadipocyte differentiation.
miR-125b-5p Inhibits the Proliferation of Chicken Preadipocytes
To determine the effect of miR-125b-5p on chicken preadipocyte proliferation, a synthetic miR-125b-5p mimic/inhibitor and mNC/iNC was transfected into preadipocytes isolated from the abdominal adipose tissues of Gushi chickens, respectively. After 48 h of transfection, the expression level of miR-125b-5p was remarkably increased by transfection with the miR-125b-5p mimic, while the expression of miR-125b-5p in miR-125b-5p inhibitor-transfected preadipocytes was significantly inhibited (Fig. S2). Moreover, growth status of cells transfected with miR-125b-5p mimics or inhibitors were also significantly different. The density of preadipocyte transfected with miR-125b-5p mimics was lower than that of the mNC. In contrast, the cell density of the miR-125b-5p inhibitor group was significantly higher than that of the iNC group (Fig. S2). Additionally, the effect of miR-125b-5p on the proliferation of preadipocytes was monitored by using EdU and CCK-8 assays at different time points. The CCK-8 detection suggested that the proliferation rate of preadipocytes in the miR-125b-5p mimic-treated group was reduced compared with that of mNC-treated group (Fig. 1a), while the proliferation rate of preadipocytes transfected with the inhibitor significantly increased compared with those transfected with the iNC (Fig. 1b). Likewise, EdU analysis also showed that the average percentage of proliferating preadipocytes transfected with miR-125b-5p mimic was significantly reduced and was 26% lower than that of the mNC group (Fig. 1c, d). In contrast, the average percentage of proliferating preadipocytes transfected with miR-125b-5p inhibitor increased by 13% compared with that of the iNC group (Fig. 1e, f).
Furthermore, flow cytometry analysis was performed to assess the effect of miR-125b-5p on the cell cycle distribution of preadipocytes. miR-125b-5p overexpression significantly reduced the number of cells in S phase and G2/M phase and increased the number of preadipocytes in G0/G1 phase (Fig. 2a, c). Conversely, transfection with miR-125b-5p inhibitors remarkably increased the number of preadipocytes in S phase and G2/M phase and decreased the number of cells in G0/G1 phase (Fig. 2b, d). This suggests that miR-125b-5p induced cell cycle arrest in chicken preadipocytes. Proliferating cells can enter S phase from G1 phase in time, and maintain their vigorous division ability. Therefore, the low proportion of cells in S phase and M phase (mitotic period) can indicate the weak proliferation activity of cells. Taken together, these results indicated that miR-125b-5p might inhibit the proliferation of preadipocytes in chicken abdominal adipose tissue.
miR-125b-5p induced cell-cycle arrest of preadipocytes. (a, b) Flow cytometry after 48 h of transfection with miR-125b-5p mimic/inhibitor and mNC/iNC, respectively. (c) Percentage of G1, S and G2 cells transfection with miR-125b-5p mimic. (d) Percentage of G1, S and G2 cells transfection with miR-125b-5p inhibitors. Data are expressed as mean ± SEM (n = 6), *p < 0.05, **p < 0.01
miR-125b-5p Promotes the Differentiation of Chicken Preadipocytes
To verify the potential effect of miR-125b-5p on chicken preadipocyte differentiation, preadipocytes isolated from abdominal adipose tissue were transfected with the miR-125b-5p mimic/inhibitor and mNC/iNC, and then differentiation was induced by adding differentiation induction medium. The miR-125b-5p mimic transfection significantly increased the expression level of miR-125b-5p (Fig. 3a) as well as the mRNA (Fig. 3b) and protein (Fig. 3c) levels of the marker genes C/EBP α and PPAR γ in adipocytes. The TG content (Fig. 3d) and number of lipid droplets (Fig. 3e, f) in the miR-125b-5p mimic group were significantly higher than that of the mNC group. These suggest that miR-125-5p overexpression promoted preadipocyte differentiation. In contrast, miR-125b-5p inhibitor transfection significantly decreased the expression level of miR-125b-5p (Fig. 3g), and the miR-125b inhibitor group showed a significant decrease in the TG concentration (Fig. 3h) and lipid droplets (Fig. 3i, j) in adipocytes.
miR-125b-5p promotes the differentiation of chicken preadipocyte (a, g). The relative expression level of miR-125b-5p in adipocytes transfected with miR-125b-5p mimic and inhibitor, respectively. (b, c) The mRNA and protein level of adipogenic marker genes PPAR γ and C/EBP α in adipocytes transfected with miR-125b-5p mimic, respectively. (d, h) Triglycerides concentration of adipocytes after 8 days of transfection with miR-125b-5p mimic and inhibitor, respectively (e, f, i, j). Number of lipid droplets in adipocytes stained with Oil Red O after 2 days of transfection with miR-125b-5p mimic and inhibitor, respectively. All data are expressed as mean ± SEM (n = 3), * p < 0.05, **p < 0.01
miR-125b-5p Directly Targets the ACSBG2 Gene
The above results showed that the expression level of miR-125b-5p and ACSBG2 was negatively correlated in the early stage of preadipocyte differentiation (Fig. S1). When miR-125b-5p was overexpressed in adipocytes, ACSBG2 expression was significantly reduced at the mRNA and protein level (Fig. 4a, b). In addition, target gene prediction revealed the presence of miR-125b-5p binding sites on ACSBG2 gene. These findings suggest that ACSBG2 may be a direct target of miR-125b-5p during the early differentiation of chicken preadipocytes. Based on this, dual-luciferase reporter vectors of wild-type ACSBG2 3′UTR and mutant ACSBG2 3′UTR were constructed (Fig. 4c), and a luciferase reporter assay was performed in DF-1 cells. The results showed that miR-125b-5p overexpression observably decreased the luciferase activity of the wild-type ACSBG2 3′UTR reporter compared with that of the mutant reporter (Fig. 4d). Thus, ACSBG2 was confirmed as the direct target of miR-125b-5p.
miR-125b-5p directly targets ACSBG2 during chicken adipogenesis. (a, b) The mRNA and protein levels of ACSBG2 gene after the miR-125b-5p overexpression on the second day of preadipocyte differentiation. (c) Diagram of the dual luciferase reporter psi-CHECK2.0-ACSBG2 3′UTR. (d) Relative luciferase activity after co-transfection of miR-125b-5p mimic with ACSBG2-3′UTR wild-type vectors or mutant vectors in DF1 cells. Data are expressed as mean ± SEM (n = 6), *p < 0.05, **p < 0.01
ACSBG2 and miR-125b-5p Affect Lipid Accumulation and FA Content During the Early Differentiation of Preadipocytes
To reveal the effect of ACSBG2 itself on the differentiation of chicken preadipocytes, we completed transient overexpression of ACSBG2 in the early stage of adipocyte differentiation. After 48 h of overexpression, the expression level of ACSBG2 was significantly upregulated (Fig. 5a), and the accumulation of TGs (Fig. 5b) and lipid droplets (Fig. 5c, d) was markedly reduced in adipocytes. However, ACSBG2 overexpression did not significantly change the expression levels of the adipogenic marker genes PPAR γ and C/EBP α. This suggests that the ACSBG2 gene itself does not affect preadipocyte differentiation, but it can reduce lipid accumulation in adipocytes.
miR-125b-5p and ACSBG2 affect lipid accumulation in chicken adipocyte. (a) The mRNA expressions levels of ACSBG2 in the early stage of preadipocyte differentiation after 48 h overexpression. (b) The triglyceride accumulation was quantified at 48 h of overexpression. (c, d) The cells differentiated 2 days after overexpression were stained with Oil Red O and determined by spectrophotometry. These data are expressed as mean ± SEM (n = 3), *p < 0.05, **p < 0.01
The above results showed that miR-125-5p overexpression significantly increased lipid accumulation (Fig. 3d–f). Therefore, the fatty acid (FA) composition in adipocytes transfected with the miR-125b-5p mimic was further determined by the Agilent 6890N/5975B gas-mass analyzer (Agilent, US). A total of 13 saturated fatty acids (SFA), 9 monounsaturated fatty acids and 9 polyunsaturated fatty acids were detected in differentiated adipocytes. Besides the content of C6:0 and C20:2, the contents of other fatty acids were significantly increased after overexpression of miR-125b-5p (p < 0.05) (Fig. S3). The results suggested that miR-125b-5p may promote the synthesis of fatty acids by decreasing the activity of ACSBG2 gene.
Discussion
Increasing evidence has shown that miRNAs have a significant effect on adipogenesis. Understanding the role of miRNA in adipogenesis regulation is conducive to exploring the mechanisms of lipid deposition and adipose tissue development. Thus, the aim of this study was to explore the regulatory effect of miR-125b-5p on adipogenesis in abdominal adipose tissue. Our results showed that the expression level of miR-125b-5p was gradually increased during the development of abdominal adipose tissue and during the differentiation of preadipocytes in chickens (Fig. S1). This upregulated expression trend in chicken adipogenesis is consistent with the results of other studies, which showed that miR-125b was upregulated in adipogenesis in 3T3-L1 preadipocytes [21] and human preadipocytes [19] and in subcutaneous adipose tissue [27]. In fact, previous studies have confirmed that miR-125b can significantly affect adipogenesis in porcine [18] and human [19] adipocytes as well as in 3T3-L1 preadipocytes [21]. Therefore, these studies indicate that miR-125b-5p may have an indispensable role during adipogenesis in chicken abdominal adipose tissue.
Adipogenesis mainly includes preadipocyte proliferation and adipocyte differentiation, which are strictly regulated by differentiating factor interactions and cell cycle regulators [28, 29]. Previous studies have shown that miR-125b plays a regulatory role in the proliferation and differentiation of a variety of cells [30,31,32,33,34]. For example, miR-125b can suppress cell proliferation in infantile hemangioma cells [30], osteosarcoma cells [31] and primary human keratinocytes [32], while it can promote muscle differentiation in bovine skeletal muscle [33]. Of note, the transfection of exogenous miR-125b inhibited proliferation and promoted differentiation in neural stem/progenitor cells [34]. In the present study, the results showed that miR-125b-5p can typically inhibit the proliferation (Figs. S2, 1, and 2) and promote the adipogenic differentiation (Fig. 3) of chicken preadipocytes. This result is consistent with those of previous studies that demonstrated that miR-125b-5p inhibits the proliferation and promotes the differentiation of 3T3-L1 preadipocytes [21]. These data indicated that miR-125b-5p significantly affects adipogenesis in chicken abdominal adipose tissue and is an important positive regulator of the adipogenic differentiation of preadipocytes. However, the results of some previous studies demonstrated that miR-125b could negatively regulate adipogenesis in porcine adipocytes [18] and human preadipocytes [19]; thus, the role of miR-125b-5p in chicken adipogenesis is inconsistent with the results of these previous studies. The reason may be that different members of the miRNA family have different regulatory effects on different cell types.
In organisms, miRNAs regulate the expression of genes at the transcriptional level by interacting with their target genes [35]. Many studies have shown that miRNA expression is negatively correlated with target gene expression in animals [36, 37]. Our study showed that miR-125b-5p was negatively correlated with ACSBG2 in the early stage (0–4 days) of adipocyte differentiation (Fig. S1). This indicated the potential target relationship between miR-125b-5p and ACSBG2 in chicken adipocytes. Furthermore, overexpression of miR-125b-5p in chicken adipocytes significantly downregulated the ACSBG2 mRNA (Fig. 4a) and protein (Fig. 4b) expression levels, which suggests that miR-125b-5p may directly regulate the expression of ACSBG2. MiRNAs act by completely or incompletely pairing with the 3′-UTRs of their target genes [38, 39]. Further, our luciferase assay results confirmed that miR-125b-5p could target the seed sequence in the 3′-UTR of ACSBG2 (Fig. 4d). Overall, these data indicated that miR-125b-5p affects adipogenesis by suppressing ACSBG2 expression in adipocytes of chicken abdominal adipose tissues, at least in the early stages of differentiated adipocytes.
Fatty acid (FA) activation is a critical rate-limiting step of FA metabolism [40]. The ACSBG gene family includes 2 members of ACSBG1 and ACSBG2 [41, 42], which activate C16 to C24 FAs [25, 26]. In particular, previous studies have found that ACSBG2 plays a role in adipogenesis in chickens. For example, Guo et al. (2018) demonstrated that the expression of ACSBG2 was upregulated by coculture of skeletal muscle satellite cells and intramuscular preadipocytes and was closely related to lipid deposition in chickens [43]. D’Andre et al. (2013) reported that the ACSBG2 gene was significantly associated with abdominal fat weight in chickens [44]. Based on the role of ACSBG2 in adipogenesis, we were interested in whether ACSBG2 itself has an effect on the differentiation of chicken preadipocytes. For this purpose, we overexpressed ACSBG2 on day 2 in preadipocytes induced to differentiate. The results showed that ACSBG2 itself may not affect the differentiation of adipocytes but only affects the lipid content of adipocytes in the early stage of preadipocyte differentiation (Fig. 5b–d). The ACSBG2 gene is mainly involved in the following pathways: FA biosynthesis, FA degradation and adipocytokine signaling. Combined with the ACSBG2 overexpression results and its temporal expression during adipocyte differentiation, we speculated that ACSBG2 might mainly participate in pathways including the adipocytokine signaling pathway and FA degradation at early stages of chicken adipocyte differentiation, thereby leading to FA degradation and β-oxidation. In contrast to the early stage, ACSBG2 might be mainly involved in FA biosynthesis in the late stages of adipogenesis.
In addition, the ACSBG2 gene is also involved in the PPAR signaling pathway. In this pathway, saturated or unsaturated fatty acids, as ligands, can activate the expression of PPARs, while downstream genes such as ACSBG1, ACSBG2, LPL and FABPs can promote fatty acid transport through PPAR activation. At the same time, the PPAR activated downstream, such as aP2, CAP and PGAR, can also promote the differentiation of adipocytes. Interestingly, we note that the ACSBG2 gene targeted by miR-125b-5p in this study is a downstream target gene of PPARs and is closely associated with FA transport. Combined with the above discussion, we hypothesize that miR-125b-5p can regulate the function of the ACSBG2-mediated pathway axis (including FA degradation, the adipocytokine signaling pathway and the PPAR signaling pathway) in the early stage of chicken preadipocyte differentiation. miR-125b-5p can inhibit the expression of the ACSBG2 gene to reduce the role of FA oxidation, degradation and transport in the above three pathways, thus increasing the FA content in adipocytes (Fig. S3). These accumulated FAs can further promote the expression of PPARs and their downstream genes, leading to chicken adipocyte differentiation (Fig. S4). ACSBG2 itself does not affect the differentiation of chicken adipocytes. Thus, miR-125b-5p promotes adipocyte differentiation by targeting ACSBG2 and increasing the accumulation of FAs, which is an indirect, positive feedback effect. However, this hypothesis still needs to be further tested.
Many target genes of miR-125b-5p have been identified in different biological contexts, such as SCD1 in porcine adipocytes [18], MMP11 during human adipocyte differentiation [19], Smad4 in 3T3-L1 preadipocytes [21], p53 in lens epithelial cells [45], and E2F2 in glioblastoma stem cells [46]. Unfortunately, there is no binding site for miR-125b-5p in these genes in chickens. In this study, the miR-125b-5p/ACSBG2 interaction was demonstrated in only the early stage of preadipocyte differentiation, which does not represent a key regulatory stage in adipogenesis. Therefore, at this point, we cannot conclusively and fully clarify the mechanism by which miR-125b-5p affects adipogenesis in chicken abdominal adipose tissues by inhibiting preadipocyte proliferation and by promoting preadipocyte differentiation. In fact, one miRNA can interact with hundreds of targets in organisms. The miRNA-mRNA targeting and interaction vary in different tissues, cell types and physiological conditions [47]. Thus, we speculated that miR-125b-5p might have unknown target genes in chicken abdominal adipose tissue and adipocytes. Further research will focus on the determination of these miR-125b-5p targets and their regulatory mechanism in the process of adipogenesis in chickens.
In conclusion, our observations indicate that miR-125b-5p is a regulator of adipogenesis in chicken abdominal adipose tissue and plays role at least a partly by downregulating ACSBG2. This evidence will provide new clues for understanding the molecular mechanism of abdominal fat deposition in chickens.
Data Availability
The data and material used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bai S, Wang G, Zhang W, Zhang S, Rice BB, Cline MA, Gilbert ER (2015) Broiler chicken adipose tissue dynamics during the first two weeks post-hatch. Comp Biochem Physiol A Mol Integr Physiol 189:115–123
Abdalla BA, Chen J, Nie Q, Zhang X (2018) Genomic insights into the multiple factors controlling abdominal fat deposition in a chicken model. Front Genet 9:262
Ahn J, Lee H, Jung CH, Jeon TI, Ha TY (2013) MicroRNA-146b promotes adipogenesis by suppressing the SIRT1-FOXO1 cascade. EMBO Mol Med 5:1602–1612
Chen L, Hou J, Ye L, Chen Y, Cui J, Tian W, Li C, Liu L (2014) MicroRNA-143 regulates Adipogenesis by modulating the MAP2K5–ERK5 signaling. Sci Rep 4:3819
Ling HY, Wen GB, Feng SD, Tuo QH, Ou HS, Yao CH, Zhu BY, Gao ZP, Zhang L, Liao DF (2011) MicroRNA-375 promotes 3T3-L1 adipocyte differentiation through modulation of extracellular signal-regulated kinase signalling. Clin Exp Pharmacol Physiol 38:239–246
Jeong BC, Kang IH, Koh JT (2014) MicroRNA-302a inhibits adipogenesis by suppressing peroxisome proliferator-activated receptor γ expression. FEBS Lett 588:3427–3434
Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, Dani C, Amri EZ, Scheideler M (2009) microRNA miR-27b impairs human adipocyte differentiation and targets PPARγ. Biochem Biophys Res Commun 390:247–251
Peng Y, Li H, Li X, Yu S, Xiang H, Peng J, Jiang S (2016) MicroRNA-215 impairs adipocyte differentiation and co-represses FNDC3B and CTNNBIP1. Int J Biochem Cell Biol 79:104–112
Cortinas L, Barroeta A, Villaverde C, Galobart J, Guardiola F, Baucells M (2005) Influence of the dietary polyunsaturation level on chicken meat quality: lipid oxidation. Poult Sci 84:48–55
Wang L, Cheng B, Li H, Wang Y (2019) Proteomics analysis of preadipocytes between fat and lean broilers. Br Poult Sci 60:522–529
Huang H, Liu R, Zhao G, Li Q, Zheng M, Zhang J, Li S, Liang Z, Wen J (2015) Integrated analysis of microRNA and mRNA expression profiles in abdominal adipose tissues in chickens. Sci Rep 5:16132
Yao J, Wang Y, Wang W, Wang N, Li H (2011) Solexa sequencing analysis of chicken pre-adipocyte microRNAs. Biosci Biotechnol Biochem 75:54–61
Wang W, Du ZQ, Cheng B, Wang Y, Yao J, Li Y, Cao Z, Luan P, Wang N, Li H (2015) Expression profiling of preadipocyte microRNAs by deep sequencing on chicken lines divergently selected for abdominal fatness. PLoS One 10:e0117843
Ma X, Sun J, Zhu S, Zhenwei D, Li D, Li W, Li Z, Tian Y, Kang X, Sun G (2020) MiRNAs and mRNAs analysis during abdominal preadipocyte differentiation in chickens. Animals 10:468
Sun YM, Lin KY, Chen YQ (2013) Diverse functions of miR-125 family in different cell contexts. J Hematol Oncol 6:6
Guan Y, Yao H, Zheng Z, Qiu G, Sun K (2011) MiR-125b targets BCL3 and suppresses ovarian cancer proliferation. Int J Cancer 128:2274–2283
Wang YD, Cai N, Wu X, Cao H, Xie L, Zheng P (2013) OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis 4:e760
Cheng X, Xi QY, Wei S, Wu D, Ye RS, Chen T, Qi QE, Jiang QY, Wang SB, Wang LN (2016) Critical role of miR-125b in lipogenesis by targeting stearoyl-CoA desaturase-1 (SCD-1). J Anim Sci 94:65–76
Rockstroh D, Löffler D, Kiess W, Landgraf K, Körner A (2016) Regulation of human adipogenesis by miR125b-5p. Adipocyte 5:283–297
Giroud M, Pisani DF, Karbiener M, Barquissau V, Ghandour RA, Tews D, Fischer-Posovszky P, Chambard JC, Knippschild U, Niemi T (2016) miR-125b affects mitochondrial biogenesis and impairs brite adipocyte formation and function. Mol Metab 5:615–625
Ouyang D, Ye Y, Guo D, Yu X, Chen J, Qi J, Tan X, Zhang Y, Ma Y, Li Y (2015) MicroRNA-125b-5p inhibits proliferation and promotes adipogenic differentiation in 3T3-L1 preadipocytes. Acta Biochim Biophys Sin 47:355–361
He J, Xu Q, Jing Y, Agani F, Qian X, Carpenter R, Li Q, Wang XR, Peiper SS, Lu Z (2012) Reactive oxygen species regulate ERBB2 and ERBB3 expression via miR-199a/125b and DNA methylation. EMBO Rep 13:1116–1122
Ren C, Xie R, Yao Y, Yu M, Chang F, Xing L, Zhang Y, Liu Y, Wang S, Farooque M (2019) MiR-125b suppression inhibits apoptosis and negatively regulates sema4d in avian leukosis virus-transformed cells. Viruses 11:728
Chen Y, Zhao Y, Jin W, Li Y, Zhang Y, Ma X, Sun G, Han R, Tian Y, Li H (2019) MicroRNAs and their regulatory networks in Chinese Gushi chicken abdominal adipose tissue during postnatal late development. BMC Genomics 20:778
Moriya-Sato A, Hida A, Inagawa-Ogashiwa M, Wada MR, Sugiyama K, Shimizu J, Yabuki T, Seyama Y, Hashimoto N (2000) Novel acyl-CoA synthetase in adrenoleukodystrophy target tissues. Biochem Biophys Res Commun 279:62–68
Steinberg SJ, Morgenthaler J, Heinzer AK, Smith KD, Watkins PA (2000) Very long-chain acyl-CoA synthetases. human “bubblegum” represents a new family of proteins capable of activating very long-chain fatty acids. J Biol Chem 275:35162–35169
Chen K, He H, Xie Y, Zhao L, Zhao S, Wan X, Yang W, Mo Z (2015) miR-125a-3p and miR-483-5p promote adipogenesis via suppressing the RhoA/ROCK1/ERK1/2 pathway in multiple symmetric lipomatosis. Sci Rep 5:11909
Fajas L (2003) Adipogenesis: a cross-talk between cell proliferation and cell differentiation. Ann Med 35:79–85
Lefterova MI, Lazar MA (2009) New developments in adipogenesis. Trends Endocrinol Metab 20:107–114
Huang J, Jiang D, Zhao S, Wang A (2019) Propranolol suppresses infantile hemangioma cell proliferation and promotes apoptosis by upregulating miR-125b expression. Anti-Cancer Drugs 30:501–507
Liu LH, Li H, Li JP, Zhong H, Zhang HC, Chen J, Xiao T (2011) miR-125b suppresses the proliferation and migration of osteosarcoma cells through down-regulation of STAT3. Biochem Biophys Res Commun 416:31–38
Xu N, Brodin P, Wei T, Meisgen F, Eidsmo L, Nagy N, Kemeny L, Ståhle M, Sonkoly E, Pivarcsi A (2011) MiR-125b, a microRNA downregulated in psoriasis, modulates keratinocyte proliferation by targeting FGFR2. J Invest Dermatol 131:1521–1529
Sun X, Li M, Sun Y, Cai H, Lan X, Huang Y, Bai Y, Qi X, Chen H (2016) The developmental transcriptome sequencing of bovine skeletal muscle reveals a long noncoding RNA, lncMD, promotes muscle differentiation by sponging miR-125b. Biochim Biophys Acta 1863:2835–2845
Cui Y, Xiao Z, Han J, Sun J, Ding W, Zhao Y, Chen B, Li X, Dai J (2012) MiR-125b orchestrates cell proliferation, differentiation and migration in neural stem/progenitor cells by targeting Nestin. BMC Neurosci 13:116
Wahid F, Shehzad A, Khan T, Kim YY (2010) MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta 1803:1231–1243
Ren J, Jin P, Wang E, Marincola FM, Stroncek DF (2009) MicroRNA and gene expression patterns in the differentiation of human embryonic stem cells. J Transl Med 7:20
Xin F, Li M, Balch C, Thomson M, Fan M, Liu Y, Hammond SM, Kim S, Nephew KP (2008) Computational analysis of microRNA profiles and their target genes suggests significant involvement in breast cancer antiestrogen resistance. Bioinformatics 25:430–434
Lal A, Navarro F, Maher CA, Maliszewski LE, Yan N, O'Day E, Chowdhury D, Dykxhoorn DM, Tsai P, Hofmann O (2009) miR-24 inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3′UTR microRNA recognition elements. Mol Cell 35:610–625
Kim YJ, Hwang SH, Cho HH, Shin KK, Bae YC, Jung JS (2012) MicroRNA 21 regulates the proliferation of human adipose tissue-derived mesenchymal stem cells and high-fat diet-induced obesity alters microRNA 21 expression in white adipose tissues. J Cell Physiol 227:183–193
Lopes-Marques M, Machado AM, Ruivo R, Fonseca E, Carvalho E, Castro LFC (2018) Expansion, retention and loss in the Acyl-CoA synthetase “Bubblegum”(Acsbg) gene family in vertebrate history. Gene 664:111–118
Watkins PA, Maiguel D, Jia Z, Pevsner J (2007) Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J Lipid Res 48:2736–2750
Pei Z, Oey NA, Zuidervaart MM, Jia Z, Li Y, Steinberg SJ, Smith KD, Watkins PA (2003) The acyl-CoA synthetase “bubblegum” (lipidosin): further characterization and role in neuronal fatty acid beta-oxidation. J Biol Chem 278:47070–47078
Guo L, Cui H, Zhao G, Liu R, Li Q, Zheng M, Guo Y, Wen J (2018) Intramuscular preadipocytes impede differentiation and promote lipid deposition of muscle satellite cells in chickens. BMC Genomics 19:838
D’Andre HC, Paul W, Shen X, Jia X, Zhang R, Sun L, Zhang X (2013) Identification and characterization of genes that control fat deposition in chickens. J Anim Sci Biotechnol 4:43
Qin Y, Zhao J, Min X, Wang M, Luo W, Wu D, Yan Q, Li J, Wu X, Zhang J (2014) MicroRNA-125b inhibits lens epithelial cell apoptosis by targeting p53 in age-related cataract. Biochim Biophys Acta 1842:2439–2447
Wu N, Xiao L, Zhao X, Zhao J, Wang J, Wang F, Cao S, Lin X (2012) miR-125b regulates the proliferation of glioblastoma stem cells by targeting E2F2. FEBS Lett 586:3831–3839
Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, Da Piedade I, Gunsalus KC, Stoffel M (2005) Combinatorial microRNA target predictions. Nat Genet 37:495
Funding
This study was funded by a grant from the National Natural Science Foundation of China (Nos. 32072692 and 31572356), the Program for Innovation Research Team of Ministry of Education (IRT16R23) and the Scientific Studio of Zhongyuan Scholars (No. 30601985).
Author information
Authors and Affiliations
Contributions
Conceptualization, G.L.; data curation, Y.L. and G.S.; formal analysis, W.J.; funding acquisition, G.L., Y.T., and X.K.; investigation, G.L., Y.C., W.J., and B.Z.; resources, H.L. and X.K.; software, Z.L. and W.L.; validation, Y.Z. and B.Z.; visualization, Y.C.; writing—original draft, S.F.; writing—review and editing, G.L. and Y.C.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
We confirm that this manuscript has not been published elsewhere and all authors agree to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, G., Chen, Y., Jin, W. et al. Effects of miR-125b-5p on Preadipocyte Proliferation and Differentiation in Chicken. Mol Biol Rep 48, 491–502 (2021). https://doi.org/10.1007/s11033-020-06080-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-06080-4