Abstract
The purpose of this study is to reveal the impact of the plant hormone salicylic acid (SA) and methyl jasmonate (MeJA) on the growth, effective components accumulation, and related gene expression of the hairy root of Salvia przewalskii Maxim. Various concentrations of SA (0, 25, 50, 100, 200 μM) or MeJA (0, 50, 100, 200, 400, 600 μM) were added to the culture medium of Salvia przewalskii Maxim. Low concentrations of SA promoted the growth of hairy root, while a high concentration inhibited it. 0 to 400 μM MeJA promoted the growth of hairy root, but 600 μM MeJA starts to inhibit its growth. 50 μM SA and 400 μM MeJA significantly enhanced the production of caffeic acid, rosmarinic acid, salvianolic acid B, cryptotanshinone, and tanshinone IIA. In general, 50 μM SA can be used to accumulate of tanshinone in hairy roots of S. przewalskii with 6 days. 400 μM MeJA can be used to accumulate of phenolic acids in hairy roots of S. przewalskii with 3 days. The selected genes in the tanshinone and phenolic acid biosynthetic pathway were upregulated with elicitation. To obtain a higher yield and content of secondary metabolites, it is advisable to use 50 μM SA or 400 μM MeJA as the optimal doses to cultivate the hairy root of S. przewalskii. This study provides, for the first time, an efficient tanshinone and phenolic acid production method for S. przewalskii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
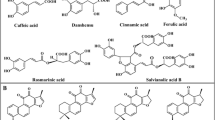
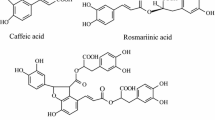
Salvia przewalskii Maxim. (family Lamiacease), is a herbaceous perennial plant commonly known as Hong Qin Jiao or Gansu Danshen [1, 2], and is endemic to the southwestern and northwestern regions of China [3, 4]. S. przewalskii and S. miltiorrhiza Bunge belong to the Salvia genus, and have a similar chemical composition and pharmacological effects. S. przewalskii is used as a substitute for S. miltiorrhiza [5,6,7]. Based on the chemical structures and pharmacological activities, the major constituents in S. przewalskii can be divided into two categories; phenolic acid components and tanshinone compounds. The phenolic acid components include rosmarinic acid, caffeic acid, fumaric acid, salvianolic acid B, and danshensu [8, 9], which have a variety of pharmacological properties, such as strong antioxidant activities [10,11,12]. The tanshinone compounds are abietane-type diterpene pigments-tanshinone, such as tanshinone I, tanshinone IIA, Tanshinone IIB, cryptotanshinone, and dihydrotanshinone [13,14,15], which have demonstrated antidermatophytic, anti-inflammatory, antioxidant, antimutagenic, and antiplatelet aggregation activities. Furthermore, tanshinones also exhibit cardiovascular effects and are used for the treatment of some coronary heart diseases, and have been shown to exhibit antiproliferative activity against various human tumor cells [16,17,18,19]. Among them, tanshinone I and cryptotanshinone prevent the complications of myocardial ischemia [20], tanshinone IIB and cryptotanshinone have bacteriostatic activity against Staphylococcus aureus [21], and tanshinone IIA and cryptotanshinone have an inhibitive effect against H37RV [22].
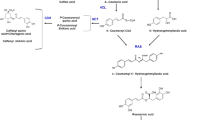
The biosynthesis of phenolic acids can be classified into two pathways, the phenylpropanoid pathway and the tyrosine-derived pathway [23, 24]. The phenylalanine ammonia lyase gene family (PAL) plays a key role in initiating the phenylpropanoid pathway, and tyrosine aminotransferase (encoded by the gene TAT) is the first enzyme in the tyrosine-derived pathway (Fig. 1). The biosynthesis of tanshinone can be divided into two pathways, the mevalonate (MVA) pathway and the 1-deoxy-d-xylulose 5-phosphate (DXP) pathway, the former occurring in the cytoplasm and the latter in the plastids of the cell [16, 25]. HMG-CoA reductase (encoded by the gene HMGR) is an initial and rate-limiting enzyme in the MVP pathway, 1-deoxy-d-xylulose 5-phosphate synthase (DXS) and 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXR) are key enzymes in the DXP pathway. Isopentenyl-diphosphate delta-isomerase (IPPI) and geranylgeranyl diphosphate synthase (GGPPS) also play important roles in the tanshinone biosynthetic pathway (Fig. 2) [26,27,28].
In recent years, numerous elicitors have been shown to improve the accumulation of various secondary metabolites in many plant species. For example, methyl jasmonate (MeJA) treatment increases tanshinone production in S. miltiorrhiza hairy roots [29] and phenolic acid content in S. miltiorrhiza [30], salicylic acid (SA) and MeJA were reported to stimulate the tropane alkaloid content in the transgenic Atropa baetica [31], and to significantly improve phenolic acid content in S. miltiorrhiza cell cultures and Lithospermum erythrorhizon suspension cells [32, 33]. Although elicitors have been used in the growth and the production of secondary metabolites of various plants, the influence of elicitors on S. przewalskii hairy roots has rarely been investigated. Thus, the purpose of this study was to examine the effects of SA and MeJA on the growth, the accumulation of phenolic acid and tanshinone in S. przewalskii hairy roots. In addition, we assessed the expression levels of thirteen genes, including PAL, TAT, HPPR, 4CL1, RAS, CYP98A14, HMGR, DXR, IPPI, GGPPS, CPS, KSL, and CYP76AH1, which are involved in phenolic acids and tanshinone biosynthetic pathway with diverse elicitation.
Materials and methods
Plant material
The hairy root of S. przewalskii Maxim. was induced by Agrobacteriom rhizogenes ATCC 15834 with a modified method from previous work [34]. Hairy root was cultivated in a 100 mL flask containing 50 mL of 6,7-V medium (with 30 g L−1 sucrose), and was placed on an gyratory shaker at 25 °C and 120 rpm in the dark [35, 36]. For elicitation experiments, 0.2 g of fresh hairy roots was prepared. Eighteen day cultured hairy roots were applied for MeJA and SA elicitation.
Confirmation of hairy root induction
Total DNA of the normal root of plant ( control) and the putative S. przewalskii hairy root lines were extracted by the CTAB method with some modification [37]. Primers of rolB and rolC gene fragments for PCR amplification were designed based on aprevious work [38]. It’s listed as follows: rolB, forward, 5′-GCT CTT GCA GTG CTA GAT TT-3′, and reverse, 5′-GAA GGT GCA AGC TAC CTC TC-3′; rolC, forward, 5′-CTC CTG ACA TCA AAC TCG TC-3′, and reverse, 5′-TGC TTC GAG TTA TGG GTA CA-3′. One microliter of DNA template, 1 μL each of the forward and reverse primers (concentration: 10 μM μL−1), 25 μL of 2X PCR Premix (Tiangen, Beijing), and 22 μL dH2O were added to the 50 μL amplification system. The conditions of PCR were 5 min for predenaturation at 94 °C, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 50 s, and extension at 72 °C for 1 min, after 35 cycles, at 72 °C for 6 min. The amplified PCR products were subjected to 1.5% (w/v) agarose gel electrophoresis and checked on the Gel Imaging System (Bio-Rad, USA) after stained by ethidium bromide. Predicted products of rolB and rolC were obtained apart from the control (noninduced root).
Preparation and application of MeJA and SA
MeJA and SA were applied to the hairy roots elicitation. MeJA (Sigma, USA) and SA ( Sigma, USA) were dissolved in ethanol and filtered through sterile filter, respectively. After 18 days-old hairy roots cultured, MeJA and SA were added to 6,7-V medium to give the final concentrations of 50, 100, 200, 400, 600 μM for MeJA; and 25, 50, 100, 200 μM for SA, hairy root of S. przewalskii without elicitation as control, then hairy roots were acquired after adding elicitors for 6 days. For hairy root culture time, the hairy roots were acquired from 0 h, 12 h, 24 h, 3 day, 6 day after adding the optimum concentrations of the elicitors, respectively.
Total RNA isolation and qRT-PCR
The hairy roots of S. przewalskii stored in a − 80 °C refrigerator were used for total RNA isolation. Total RNA of hairy roots was isolated using the Biospin Plant Total RNA Extraction Kit (BioFlux, China). The quality and concentration of RNA were determined by agarose gel electrophoresis and NanoDrop spectrophotometry (Thermo Fisher Scientific Inc, USA). cDNA was synthesized by reverse transcription using the PrimeScrip™ RT reagent Kit (Takara, Japan), initially incubated at 37 °C for reverse transcription (15 min), and then carried out at 85 °C for 5 s to inactivate the reverse transcriptase. The primers of relevant genes used for real-time quantitative PCR (qRT-PCR) are listed in Table 1. β-Actin was taken as the reference gene. qRT-PCR assay was performed using SYBR Premix Ex Taq™ II (Tli RNaseH Plus) kit (Takara, Japan) on the qTOWER 2.2 Real-time PCR Detection System (Analytik Jena, Germany). The conditions of qRT-PCR were 2 min for predenaturation and 5 s for denaturation at 95 °C, and then 30 s for annealing at 58 °C, 40 cycles in total. Quantification of gene expressions was obtained by a comparative CT method, relative transcripts of related genes in the hairy roots of S. przewalskii acquired at 0 day were as 1.
HPLC determination of phenolic acid and tanshinone
0.1 g of dried hairy roots was weighed accurately and soak for 12 h with 5 mL 70% methanol, then the samples were treated by ultrasound for 45 min and centrifuged at 13,000×g for 10 min, the supernatant was filtered through a 0.22 μm millipore filter and analyzed by HPLC system equipped with e2695 separations module and 2998 PDA detector (Waters, USA). HPLC conditions were as follows, column, Symmetry C18 column (250 × 4.6 mm, 5 μm); solvent system, HPLC grade acetonitrile (A)-ultrapure dH2O with 0.02% phosphoric acid (B) gradient elution: 0–18 min, 10–30% A (v/v); 18–30 min, 30–40% A (v/v); 30–40 min, 40–68% A (v/v); 40–50 min, 68–75%A (v/v); 50–60 min, 75–60%A (v/v); flow rate, 1.0 mL/min; column temperature, 30 °C; detection, 270 nm; Injection volume, 10 μL; HPLC grade acetonitrile (Fisher, Beijing, China) and phosphoric acid (Kermel, Tianjin, China) were used in the this study. Each compounds were confirmed by comparing the retention time with the standard substances, which were purchased from the National Institutes for Food and Drug Control (Beijing, China) under the identical HPLC condition. The standard curve of each component is listed in Table 2.
Data analysis
Graphics were produced by the OriginPro software version 9.3. Significance analysis were determined by analysis of variance (ANOVA) using the “Statistical Package” for Social Sciences program (SPSS 16.0, SPSS Inc. USA). All the data were expressed as mean ± standard deviation (SD) of three replicates.
Results
Induction and confirmation of hairy root cultures
As shown in Fig. 3, the leaf explants of S. przewalskii Maxim. responded to induction of Agrobacteriom rhizogenes ATCC 15,834 and formed hairy roots finally. About 400 bp band for rolB and 600 bp band for rolC were obtained from the putative hairy root lines, while no band was detected in the root sample of nontransformed contral plant (Fig. 4). Thus, rolB and rolC genes in the plasmid (pRi) of the bacteria were integrated into the explants successfully and induced hairy roots of S. przewalskii completely.
rolB and rolC gene fragments of putative hairy root lines amplified by PCR. Lane M: D2000 DNA marker (Tiangen, Beijing), lane 1, 5: Hairy root sample 1 of S. preziwalskii, lane 2, 6: Hairy root sample 2 of S. przewalskii, lane 3, 7: root sample 1 of nontransformed control plant, lane 4, 8: root sample 2 of nontransformed control plant
Effect of elicitor concentration on hairy root cultures
The effect of different concentrations of SA and MeJA on S. przewalskii hairy root is shown in Figs. 5 and 6. SA and MeJA affect the growth and the production of phenolic acid and tanshinone in S. przewalskii hairy root. As shown in Table 3, fresh weight and dry weight increased initially and then decreased with increasing concentrations of SA. SA (50 μM) markedly promoted the fresh weight and dry weight of hairy root with 61.69% and 7.17% increment, respectively. As shown in Fig. 7a, the addition of 50 μM SA promoted the content of caffeic acid, rosmarinic acid, salvianolic acid B, cryptotanshinone, and tanshinone IIA by 1.82-, 1.41-, 2.67-, 1.38-, and 2.61-fold, respectively. Thus, 50 μM SA was appropriate for S. przewalskii hairy root growth and the accumulation of chemical components.
As shown in Table 3, treatment with MeJA promoted the fresh weight and dry weight of hairy root culture with increasing concentrations, but it decreased when then cincentration was 600 MeJA. Treatment with 400 μM enhanced the fresh weight and dry weight of hairy root with 64.92% and 105.68% increment, respectively. As shown in Fig. 7b, addition of 400 μM MeJA increased caffeic acid, rosmarinic acid, salvianolic acid B, cryptotanshinone and tanshinone IIA contents significantly by 1.78-, 1.27-, 8.14-, 3.00- and 11.04-fold, respectively. Thus, 400 μM MeJA was the optimal concentration for S. przewalskii hairy root growth and effective components accumulation.
Effect of induction time on hairy root cultures
In Table 4, the fresh weight and dry weight of hairy root at different culture time were listed. Fresh weight showed a gradual increase in response to treatment with SA (50 μM) and MeJA (400 μM) within 6 days. SA inhibited caffeic acid content within 12 h, and then showed a significant enhancement at 24 h. Finally, a 0.07-fold (0.2506 ± 0.03 mg·g−1 DW) increase of caffeic acid was acquired at 6 days compared with the control after SA treatment (Fig. 8a). Caffeic acid accumulation showed a gradually increase and reached its maximum (0.3071 ± 0.01 mg·g−1 DW) at 6 days after the addition of 400 μM MeJA (Fig. 8a).
The amount of rosmarinic acid increased after treatment with SA and MeJA for 12 h (Fig. 8b). The highest concentration of rosmarinic acid was observed at three days after SA (44.0306 ± 0.08 mg·g−1 DW) and MeJA (67.1273 ± 0.41 mg g−1 DW) treatment; however, it decreased significantly on the sixth day. Therefore, SA and MeJA consistently enhanced rosmarinic acid accumulation in the hairy root cultures of S. przewalskii during the first three days.
The contents of salvianolic acid B showed a significant increase during the first three days after SA treatment and reached its maximum (2.5138 ± 0.07 mg g−1 DW) at three days, yet a sharp decrease was observed at six days. After the addition of 400 μM MeJA, the concentration of salvianolic acid B increased at first then decreased, and reached its maximum (21.4448 ± 0.34 mg g−1 DW) at three days after treatment (Fig. 8c).
50 μM SA treatment resulted in an inhibition of cryptotanshione and tanshinone IIA concentrations during the first 3 days, while a sharp increment was observed at 6 day. The treatment with 400 μM MeJA led to a short inhibition of cryptotanshione and tanshinone IIA concentrations at first, while a sharp increase appeared, the concentration of cryptotanshione reached its maximum (0.0674 ± 0.00 mg g−1 DW) at 6 day after treatment (Fig. 8d), while the concentration of tanshinone IIA reached its maximum (0.3791 ± 0.00 mg g−1 DW) at 3 day after treatment and slightly decreased after six days (Fig. 8e).
Effect of elicitors on expression of genes involved in the phenolic acid biosynthetic pathway
PAL expression levels showed a significant increase and reached its maximum on the sixth day after treatment with 50 μM SA, which was a 7.3-fold increase as compared with the control (Fig. 9a). 4CL1 expression levels showed a slight inhibition at 12 h after treatment with 50 μM SA, and then gradually increased and reached its maximum at 6 days after treatment, and the maximum increment of 4.2-fold was acquired after three days as compared with the control (Fig. 9b). SA promoted the expression levels of TAT and HPPR and reached the maximum expression at 3 day after the addition of the elicitor, which resulted in a 4.1- and 4.9-fold increase compared with control, respectively (Fig. 9c, d). SA had a significant inhibitory effect on the expression levels of RAS during the first three days, while a promotional effect was shown on the sixth day, which was 1.4-fold higher than that of the control (Fig. 9e). CYP98A14 expression levels increased during the first three days and reached its maximum of 2.8-fold at 3 day after treatment with SA, while it showed a relative decrease at 6 day after the treatment (Fig. 9f).
Relative expression levels of phenolic acid biosynthetic related genes in the hairy root culture of S. przewalskii by SA and MeJA treatment. SA concentration, 50 μM; MeJA concentration 400 μM; CK, the hairy root without elicitation. Gene expressions of the control at the initial treatment (0 day) were as relative 1. *P < 0.05, **P < 0.01
Elicitation with 400 μM MeJA led to significant inhibition of PAL expression levels at 12 h, then it showed a moderate promotion within 3 days and reached its maximum of a 5.4-fold increase compared with the control, yet it showed a slight inhibition after six days (Fig. 9a). 4CL1, TAT, and RAS responded to MeJA elicitation sensitively at 12 h, about 29.8-, 4.2-, and 3.3-fold higher expression levels than that of the control were observed, respectively, and then showed a gradual decrease (Fig. 9b, c, e). HPPR expression levels were promoted after the addition of MeJA, which showed a relatively higher level of 7.5-fold after three days compared with the control (Fig. 9d). CYP98A14 expression levels increased during the first three days and reached its maximum of 1.7-fold at 3 day after treatment with MeJA, and then it showed a slight inhibition compared with the control at 6 day (Fig. 9f).
Effect of elicitors on genes expression of tanshinone biosynthetic pathway
SA (50 μM) caused enhancement of HMGR expression levels and reached its maximum of 17.3-fold higher level at three days (Fig. 10a). DXR, IPPI, GGPPS, KSL, and CYP76AH1 expression levels showed a gradual increase and reached the maximums at 6 day. DXR and KSL indicated a 5.3- and 12.1-fold increase on 6 day, respectively (Fig. 10b, f). The expression levels of IPPI and CYP76AH1 showed 10.1- and 3.9-fold increments compared with the control at 3 day, respectively (Fig. 10c, g). GGPPS indicated an 11.7-fold increase compared with the control after 12 h (Fig. 10d). CPS expression levels were significantly inhibited within first three days and then showed a sharp enhancement on 6 day, which was 9.3-fold higher than that of the control (Fig. 10e).
Relative expression levels of tanshinone biosynthetic related genes in the hairy root culture of S. przewalskii by SA and MeJA treatment. SA concentration, 50 μM; MeJA concentration 400 μM; CK, the hairy root without elicitation. Gene expressions of the control at the initial treatment (0 day) were as relative 1. *P < 0.05, **P < 0.01
After the addition of 400 μM MeJA, HMGR expression levels were slightly inhibited (Fig. 10a). DXR expression levels were slightly inhibited at 12 h, then showed a slight promotion within 3 days. After 6 days of treatment, DXR expression levels were slightly inhibited again (Fig. 10b). IPPI expression levels gradually increased during the first three days and then slightly decreased on the sixth day, with a relatively higher level of 17.2-fold compared with the control on 3 days (Fig. 10c). GGPPS expression levels were slightly inhibited at 12 h, and then showed a gradual increase and reached its maximum at 6 day, which was 1.2-fold higher than that of the control (Fig. 10d). CPS expression levels showed significant inhibition at 24 h, while increased and reached 5.9-fold higher levels than controls at 6 day (Fig. 10e). 400 μM MeJA caused the promotion of KSL expression levels and reached its maximum at 3 day, which was 13.6-fold higher than that of the control (Fig. 10f). CYP76A expression levels showed a gradual increase and reached the maximum at 6 day, which was 1.5-fold higher than that of the control (Fig. 10g).
Discussion
Generally, hairy root is obtained by infection of plants with Agrobacteriom rhizogenes and it is characterized by a high growth rate and genetic stability. Compared with the original plants, the hairy root can produce higher levels and more valuable secondary metabolites, which could be used as the continuous resource for the practical production [39, 40]. In the present study, the application of hairy root cultures to produce the active substances required in cosmetics or pharmaceuticals is reported, such as peanut [41], Catharanthus roseus [42], Valeriana wallichii DC [43], Atropa belladonna [44] and S. miltiorrhiza [45].
SA and MeJA, the effective elicitors, have been widely used to regulate growth and increase the accumulation of secondary metabolites in the medicinal plants. For instance, SA and MeJA have been confirmed to improve the content of total phenols in Bletilla striata seedings [46]. MeJA also promotes total flavonoid and phenolic contents of the callus of Phyllanthus pulcher [47]. SA increases the accumulation of phenolic acids in S. miltiorrhiza cell culture [32]. In this study, SA and MeJA were used to induce the hairy root growth and secondary metabolites of S. przewalskii.
Elicitation with SA is known to inhibit the growth of the hairy root cultures of Silybum marianum [48]. In this study, however, low concentrations of SA promoted the growth of hairy roots of S. przewalskii. a relative high concentration of SA (200 μM) had an inhibitory effect on the growth of the hairy root. This may be due to the variable tolerance of the plant species. In addition, SA is known to enhance the production of withanolide A, withanone, and withaferin A in the hairy root cultures of Withania somnifera (L.) Dunal [49]. In this study, the remarkable accumulation of caffeic acid, rosmarinic acid, salvianolic acid B, and tanshinone IIA were observed by using 50 μM SA treatment.
According to a previous report, MeJA was found to be the most effective elicitor for astragaloside biosynthesis of Astragalus membranceus hairy root [50], and 100 μM MeJA could enhance the massive accumulation of anthraquinones in the hairy root culture of Rubia tinctorum [51]. Additionally, MeJA was found to improve the fresh weight and tanshinone IIA contents of hairy root cultures of S. castanea Diels f. tomentosa Stib [34]. In our study, we achieved a similar result with variable concentrations of MeJA treatment, which promoted the growth of hairy roots, and the promotion effect was stronger in pace with increasing elicitor concentrations. When the concentration of MeJA was 400 μM, the growth rate reached its maximum. Furthermore, the content of caffeic acid, salvianolic acid B, cryptotanshinone, and tanshinone IIA reached the highest value under the same conditions.
After addition 50 μM SA, the content of caffeic acid, cryptotanshione and tanshinone IIA reached there maximum at 6 days, rosmarinic acid and salvianolic acid B reached the highest values at 3 days. After addition 400 μM MeJA, the content of caffeic acid and cryptotanshione reached there maximum at 6 days, tanshinone IIA, rosmarinic acid and salvianolic acid B reached the highest values at 3 days. The experimental results show that in the treatment group, the chemical components accumulated under the optimal time node are higher than those in the control group. In general, 400 μM MeJA has a greater effect on the accumulation of phenolic acids than 50 μM SA, but 50 μM SA has a greater effect on the accumulation of tanshinones than 400 μM MeJA. Therefore, 50 μM SA can be used to accumulate of tanshinone in hairy roots of S. przewalskii with 6 days. 400 μM MeJA can be used to accumulate of phenolic acids in hairy roots of S. przewalskii with 3 days.
PAL and TAT are the key genes at the early stages of two parallel phenolic acids biosynthetic pathways, and the expression of PAL is related to the biosynthesis of caffeic acid, lignin, anthocyanidin, and other phenolic compounds [52]. 4CL1 and HPPR play key roles in the middle stages of the phenolic acid biosynthetic pathway. RAS and CYP98A14 are the rate-limiting enzymes in the final stages of the rosmarinic acid biosynthetic pathway [23, 24]. In this study, the expression levels of rate-limiting enzyme CYP98A14 were increased on 3 day with 50 μM SA and 400 μM MeJA treatments respectively, meanwhile, the content of rosmarinic acid was also observed to reach the maximum.
In the two biosynthetic pathways of tanshinone, HMGR is a crucial enzyme in the early MVA pathway, and DXR is a key enzyme in the early DXP pathway. IPPI and GGPPS are also critical genes in the middle stages of the tanshinone biosynthetic pathway. CPS, KSL, and CYP76AH1 are the rate-limiting enzymes in the downstream of tanshinone biosynthetic pathway [26,27,28]. In our study, with 50 μM SA treatment, HMGR, DXR, IPPI, GGPPS, CPS, KSL, and CYP76AH1 gene expression levels were higher at the end of the treatment period, and the most striking increments of cryptotanshinone and tanshinone IIA were also observed. These genes are involved in tanshinone biosynthetic pathway of S. przewalskii hairy root cultures. With the application of 400 μM MeJA in the hairy root culture, the expression levels of CPS and CYP76AH1 were enhanced at the end of the treatment stage, while KSL expression level was enhanced at the mid-term of the hairy culture. Additionally, cryptotanshinone content achieved the maximum at the end of the treatment stage, while the accumulation of tanshinone IIA was found markedly at the mid-term period.
As an effective tool for regulating plant secondary metabolites, elicitors have been widely used in the regulation of secondary metabolites in S. miltiorrhiza. However, the effects of elicitors on the synthesis of secondary metabolites in the hairy roots of S. przewalskii are rarely reported. In this work, the growth, phenolic acids and tanshinones accumulation of hairy root treated by different concentration of SA and MeJA were studied. It is conclude that low concentration of SA promoted the growth of hairy root while high concentration inhibited. MeJA promoted the growth of hairy root. 50 μM SA and 400 μM MeJA significantly enhanced the production of caffeic acid, rosmarinic acid, salvianolic acid B, cryptotanshinone and tanshinone IIA. The selected genes in the tanshinone and phenolic acids biosynthetic pathways were remarkably upregulated with the elicitation. This study provide a reference for the selection of elicitors to improve the industrial production of phenolic acids and tanshinone in the hairy root culture of S. przewalskii.
Abbreviations
- SA:
-
Salicylic acid
- MeJA:
-
Methyl jasmonate
- FW:
-
Fresh weight
- DW:
-
Dry weight
- PAL :
-
Phenylalanine ammonia-lyase
- TAT :
-
Tyrosine aminotransferase
- HPPR :
-
4-Hydroxyphenylpyruvate reductase
- 4CL1 :
-
4-Coumaric acid CoA-ligase 1
- RAS :
-
Rosmarinic acid synthase
- CYP98A14 :
-
Acytochrome P450-dependent monooxygenase
- HMGR :
-
3-Hydroxy-3-methylglutaryl CoA reductase
- DXR :
-
1-Deoxy-d-xylulose 5-phosphate reductoisomerase
- DXS :
-
1-Deoxy-d-xylulose 5-phosphate synthase
- IPPI :
-
Isopentenyl-diphosphate delta-isomerase
- GGPPS :
-
Geranylgeranyl diphosphate synthase
- CPS :
-
Copalyl diphosphate synthase
- KSL :
-
Entkaurene synthase like
- qRT-PCR:
-
Real-time quantitative PCR
References
Ewa S, Halina W (2005) Tanshinone production in roots of micropropagated Salvia przewalskiiMaxim. Z Naturfr C 60:7–8
Li X, Luo Y, Wang L et al (2010) Acute and subacute toxicity of ethanol extracts from Salvia przewalskii Maxim in rodents. J Ethnopharmacol 131(1):110–115
Liu J, Nan P, Tsering Q et al (2010) Volatile constituents of the leaves and flowers of Salvia przewalskii Maxim. from Tibet. Flavour Fragr J 21(3):435–438
Wang L, Jiang YY, Zhang L et al (2017) High-performance liquid chromatography fingerprints and simultaneous quantification of bioactive compounds in Salvia przewalskii maxim. Acta Chromatogr 29(3):1–18
Matkowski A, Zielińska S, Oszmiański J et al (2008) Antioxidant activity of extracts from leaves and roots of Salvia miltiorrhiza Bunge, S. przewalskii Maxim. and S. verticillata L. Bioresour Technol 99(16):7892–7896
Jassbi AR, Zare S, Firuzi O et al (2016) Bioactive phytochemicals from shoots and roots of Salvia species. Phytochem Rev 15(5):829–867
Gryszczynska A, Opala B, Lowicki Z et al (2015) Bioactive compounds determination in the callus and hydroalcoholic extracts from Salvia miltiorrhiza and Salvia przewalskii—preliminary study on their anti-alcoholic activity effects. Phytochem Lett 11:399–403
Yang D, Huang Z, Xing B et al (2016) Regulation of folic acid on phenolic acids production in Salvia miltiorrhizahairy roots. Plant Cell Tissue Organ Cult 127(1):175–185
Wang M, Dai H, Li X et al (2010) Structural elucidation of metabolites of tanshinone I and its analogue dihydrotanshinone I in rats by HPLC-ESI-MSn. J Chromatogr B 878(13–14):915–924
Petersen M, Abdullah Y, Benner J et al (2009) Evolution of rosmarinic acid biosynthesis. Phytochemistry 70(15–16):1663–1679
Wu J, Shi M (2008) Ultrahigh diterpenoid tanshinone production through repeated osmotic stress and elicitor stimulation in fed-batch culture of Salvia miltiorrhiza hairy roots. Appl Microbiol Biotechnol 78(3):441–448
Gülçin I (2006) Antioxidant activity of caffeic acid (3, 4-dihydroxycinnamic acid). Toxicology 217(2):213–220
Yang L, Li X, Liu C et al (2011) Chemical constituents from Salvia przewalskii Maxim. Acta Pharm Sin 46(7):818–821
Xue M, Shi Y, Cui Y et al (2000) Study on the chemical constituents from Salvia przewalskii Maxim. Tianran Chanwu Yanjiu Yu Kaifa 12:27–32
Wan C, Ming JX, Dong ZW et al (2003) Chemical constituents in the roots of Salvia przewalskii Maxim. Acta Pharm Sin 38(5):354
Liao P, Zhou W, Zhang L et al (2009) Molecular cloning, characterization and expression analysis of a new gene encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase from Salvia miltiorrhiza. Acta Physiol Plant 31(3):565–572
Park EJ, Zhao YZ, Kim YC et al (2009) Preventive effects of a purified extract isolated from Salvia miltiorrhiza enriched with tanshinone I, tanshinone IIA and cryptotanshinone on hepatocyte injury in vitro and in vivo. Food Chem Toxicol 47:2742–2748
Ling S, Dai A, Guo Z et al (2010) Effects of a chinese herbal preparation on vascular cells in culture: mechanisms of cardiovascular protection. Clin Exp Pharmacol Physiol 32(7):571–578
SkaŁA E, Mielicki W, Halina W (2014) Tanshinones in culture of salvia przewalskii maxim in vitro. Acta Biol Crac Ser Bot 56(1):104–110
Chen H, Yuan JP, Chen F et al (1997) Tanshinone production in Ti-transformed Salvia miltiorrhiza cell suspension cultures. J Biotechnol 58(3):147–156
Dweck AC (2000) The folklore and cosmetic use of various Salvia species. Harwood Academic Publishers, Reading, pp 1–25
Hu ZD, Jia L, Zhang ZP et al (1997) Studies of Crude ethanol extract of roots of Salvia przewalskii maxim by micellar electrokinetic capillary chromatography. J Liq Chromatogr Relat Technol 20(8):1211–1220
Wang Z, Cui L, Chen C et al (2012) Down regulation of cinnamoyl CoA teductase affects lignin and phenolic acids biosynthesis in Salvia miltiorrhiza Bunge. Plant Mol Biol Rep 30(5):1229–1236
Zhang S, Yan Y, Wang B et al (2014) Selective responses of enzymes in the two parallel pathways of rosmarinic acid biosynthetic pathway to elicitors in Salvia miltiorrhiza hairy root cultures. J Biosci Bioeng 117(5):645–651
Laule O, Fürholz A, Chang HS et al (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci 100(11):6866–6871
Yan X, Zhang L, Wang J et al (2019) Molecular characterization and expression of 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXR) gene from Salvia miltiorrhiza. Acta Physiol Plant 31:1015–1022
Kai G, Liao P, Zhang T et al (2010) Characterization, expression profiling, and functional identification of a gene encoding geranylgeranyl diphosphate synthase from Salvia miltiorrhiza. Biotechnol Bioprocess Eng 15(2):236–245
Ma P, Liu J, Osbourn A et al (2015) Regulation and metabolic engineering of tanshinone biosynthesis. RSC Adv 5(23):18137–18144
Zhao J, Zhou L, Wu J (2010) Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl Microbiol Biotechnol 87(1):137–144
Xiao Y, Gao S, Di P et al (2009) Methyl jasmonate dramatically enhances the accumulation of phenolic acids in Salvia miltiorrhiza hairy root cultures. Physiol Plant 137(1):1–9
El JN, Barres ML, Ravelo ÁG et al (2008) Effects of elicitors on tropane alkaloids and gene expression in Atropa baetica transgenic hairy roots. J Nat Prod 71(12):2026–2031
Dong J, Wan G, Liang Z (2010) Accumulation of salicylic acidinduced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol 148(2–3):99–104
Mizukami H, Tabira Y, Ellis BE (1993) Methyl jasmonate-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Plant Cell Rep 12(12):706–709
Li B, Wang B, Li H et al (2016) Establishment of Salvia castanea Diels f. tomentosa Stib. hairy root cultures and the promotion of tanshinone accumulation and gene expression with Ag(+), methyl jasmonate, and yeast extract elicitation. Protoplasma 253(1):87–100
Ru M, An Y, Wang K et al (2016) Prunella vulgaris L. hairy roots: culture, growth, and elicitation by ethephon and salicylic acid. Eng Life Sci 6:494–502
Xing B, Yang D, Guo W et al (2014) Ag+ as a more effective elicitor for production of tanshinones than phenolic acids in Salvia miltiorrhiza hairy roots. Molecules 20(1):309–324
Porebski S, Bailey LG, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15:8–15
Królicka A, Staniszewska I, Bielawski K et al (2001) Establishment of hairy root cultures of Ammi majus. Plant Sci 160:259–264
Giri A, Narasu ML (2000) Transgenic hairy roots. Recent trends and applications. Biotechnol Adv 18(1):1–22
Liu J, Li Y, Chen W et al (2010) Transgenic plant hairy root: future and application. Acad J Second Mil Med Univ 30(4):433–437
Condori J, Sivakumar G, Hubstenberger J et al (2010) Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin-1 and arachidin-3 in hairy root cultures of peanut: Effects of culture medium and growth stage. Plant Physiol Biochem 48(5):310–318
Rodriguez S, Compagnon V, Crouch NP et al (2003) Jasmonate-induced epoxidation of tabersonine by a cytochrome P-450 in hairy root cultures of Catharanthus roseus. Phytochemistry 64(2):401–409
Banerjee S, Rahman L, Uniyal GC et al (1998) Enhanced production of valepotriates by Agrobacterium rhizogenes induced hairy root cultures of Valeriana wallichii DC. Plant Sci 131(2):203–208
Fukami H, Asakura T, Hirano H et al (2002) Salicylic acid carboxyl methyltransferase induced in hairy root cultures of atropa belladonna after treatment with exogeneously added salicylic acid. Plant Cell Physiol 43(9):1054
Kai G, Liao P, Xu H et al (2012) Molecular mechanism of elicitor-induced tanshinone accumulation in Salvia miltiorrhizahairy root cultures. Acta Physiol Plant 34(4):1421–1433
Yang J, Wang K, Liang J et al (2016) Effects of exogenous MeJA, SA and two kinds of endophytic fungi on physiology and total phenols content of seedlings of Bletilla striata. China J Chin Materia Med 41(15):2794
Danaee M, Farzinebrahimi R, Kadir AF et al (2015) Effects of MeJA and SA elicitation on secondary metabolic activity, antioxidant content and callogenesis in Phyllanthus pulcher. Braz J Bot 38(2):265–272
Khalili M, Hasanloo T, Kazemi Tabar SK et al (2009) Influence of exogenous salicylic acid on flavonolignans and lipoxygenase activity in the hairy root cultures of Silybum marianum. Cell Biol Int 33(9):988–994
Sivanandhan G, Dev GK, Jeyaraj M et al (2013) Increased production of withanolide A, withanone, and withaferin A in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Cult 114(1):121–129
Jiao J, Gai Q, Wang W et al (2016) Enhanced astragaloside production and transcriptional responses of biosynthetic genes in Astragalus membranaceus hairy root cultures by elicitation with methyl jasmonate. Biochem Eng J 105:339–346
Perassolo M, Cardillo AB, Mugas ML et al (2017) Enhancement of anthraquinone production and release by combination of culture medium selection and methyl jasmonate elicitation in hairy root cultures of Rubia tinctorum. Ind Crops Prod 105:124–132
Yu H, Liu X, Gao S et al (2014) Molecular cloning and functional characterization of a phenylalanine ammonia-lyase from liverwort Plagiochasma appendiculatum. Plant Cell Tissue Organ Cult 117(2):265–277
Acknowledgements
This work was supported by: (1) Subject Innovation Team of Quality Control and Resources Development of “Qin drug” of Shaanxi University of Chinese Medicine (2019-QN01). (2) Subject of Standardization construction of the Tussilago farfara L.(202410001). (3) Subject of Study on Breeding of Excellent Varieties and Quality Control Techniques of Polygala tenuifolia Willd (201330001). (4) Special Project for Construction of Modern Agricultural Industrial Technology System (CARS-21). (5) Scientific Research Project of Education Department of Shaanxi Provincial Government (18JK0214).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Li, B., Luo, L. et al. Increased phenolic acid and tanshinone production and transcriptional responses of biosynthetic genes in hairy root cultures of Salvia przewalskii Maxim. treated with methyl jasmonate and salicylic acid. Mol Biol Rep 47, 8565–8578 (2020). https://doi.org/10.1007/s11033-020-05899-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05899-1