Abstract
Adaptogens are substances that act nonspecifically to combat stress by regulating the key elements involved in stress-induced pathologies. d-Ribose–l-cysteine (DRLC), a potent glutathione (GSH) booster, has been recommended for relief of stress. Hence, we investigated its adaptogenic-like effect in mice subjugated to unpredictable chronic mild stress (UCMS). Thirty six male Swiss mice were assigned to 6 groups (n = 6): group 1 received saline (p.o, non-stress control), group 2 (stress-control) also had saline, groups 3 to 5 received DRLC (25, 50 and 100 mg/kg, p.o) whereas group 6 had ginseng (50 mg/kg, p.o). The animals in groups 2–6 were subjugated to UCMS 30 min later, daily for 21 days and afterwards, tested for memory and anxiety. Blood glucose, serum corticosterone concentrations and adrenal weight were determined. The brain tissues were processed for estimation of malondialdehyde (MDA), GSH, superoxide-dismutase (SOD), catalase, tumor necrosis factor-alpha (TNF-α), interleukin-6, acetyl-cholinesterase, and caspase-3 activities. The histomorphologic features and neuronal viability of the hippocampus, amygdala and prefrontal cortex were also determined. DRLC (25–100 mg/kg) reduces anxiety, memory deficit, adrenal gland enlargement, glucose, and corticosterone concentrations in UCMS-mice. The increased brain contents of MDA, TNF-α, interleukin-6, acetyl-cholinesterase and decreased antioxidant (GSH, SOD and catalase) status induced by UCMS were attenuated by DRLC. The DRLC increased caspase-3 activity and reduces histomorphological distortions of neuronal cells of the hippocampus, amygdala and prefrontal cortex of stressed-mice. These findings suggest that DRLC has adaptogenic-like effect which might be related to modulation of corticosterone-mediated oxido-inflammatory processes and altered caspase-3 activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adaptogens are substances with multiple health benefits and with multipronged mechanisms of action that act nonspecifically to combat deleterious effects of chronic stress by regulating the key elements involved in stress-induced pathological derangements [1, 2]. Reports have shown that protracted stress contributes to increased vulnerability risk factor for the development of neuropathological conditions including cognitive impairment, anxiety, depression and addictive disorders [2, 4]. It has also been implicated in cardiovascular disorders, loss of sexual motivation and accelerated ageing in susceptible individuals [2, 3]. Nevertheless, the health promoting effects of adaptogens have been ascribed to their ability to positively regulate metabolic activity and increased adaptation thereby providing resilience and protection against psychosocial and environmental stress [1, 4].The uniqueness of adaptogens is premised on their ability to act nonspecifically to normalize the homeostatic mechanisms of the organism and strengthened the cellular defense machinery compromised by chronic stress [1, 2].
The defensive effects of adaptogens against stress-induced pathologies have been connected to the restoration of deregulated homeostasis through multiple actions tied around decreased hypothalamic–pituitary–adrenal (HPA) axis-mediated release of cortisol with consequential inhibition of oxidant molecules, pro-inflammatory cytokines, and up-regulation of stress resilient brain areas [1, 2, 5]. Adaptogens have been reported to normalize increased cortisol concentrations in humans and animals, and also modulated apoptotic proteins, neuroprotective mechanisms, and antioxidant enzymes in stressed states [1, 2, 4]. Studies have established the adaptogenic property of a variety of herbs including Panax ginseng, with some of them already available for relief of stress [4, 6]. Precisely, ginseng traditionally obtained from Panax ginseng has been confirmed as an anti-stress compound for stress-related neurological impairments [1, 6].
The UCMS is a well-known model used to mimic long-term stress in humans and for elucidation of the interplays between psychopathological derangements and the deregulated biochemical signaling pathways associated with stress [7, 8]. The UCMS model is known to cause more devastating effects on human than the predictable stress paradigm [8,9,10]. Moreover, exposures to UCMS have also been shown to induce depression, anxiety, memory loss and loss of pleasure in rodents [8, 10].
d-Ribose–l-cysteine (DRLC), a potent antioxidant molecule, is an analogue of cysteine developed as a pro-drug to boost glutathione biosynthesis [11, 12]. GSH, a major endogenous antioxidant, mediates its cytoprotective effect by scavenging free radicals and detoxifying injurious cellular oxidant molecules [13]. Studies have shown that DRLC exhibited cardioprotective [11], chemoprotective [12], activities, and ameliorated reproductive organ damage [14]. d-Ribose, a major component of DRLC, is an important part of the DNA, RNA, ATP and NADH needed by the mitochondria to maintain cellular functions [15]. d-Ribose has been used in patients for chronic fatigue syndrome and as an energy source to improve athletic performance [16], suggesting enhanced cellular capacity of cysteine to mitigate stress, when combined together as DRLC. Impaired cysteine transporter that resulted in neuronal GSH deficiency has been identified as a factor for age-dependent neurodegeneration in mice [13], which further suggest the benefit of DRLC in memory-related disorders. Recently, we have also reported that DRLC attenuated memory deficit induced by lipopolysaccharide (LPS) via inhibition of pro-inflammatory cytokines, oxidative stress and neurodegeneration in mice [17]. The potent antioxidant, anti-inflammatory and neuroprotective effects of DRLC suggest that it might exhibit polyvalent protective effects against chronic stress. Thus, this current study described the adaptogenic-like activity of DRLC and the putative mechanisms involved in its action in mice exposed to UCMS.
Materials and methods
Experimental animals
Thirty six male Swiss mice weighing between 20–24 g (6–8 weeks) procured from the Central Animal Housing facility of University of Ibadan and housed in a standard animal cage at standard conditions were used for the experiment. Mice were adapted for a minimum of 14 days with free feeding and drinking water before the commencement of the study. The experimental procedures were in line with the NIH protocols for the use and care of animals and the institutional ethical committee of the University of Ibadan (UI-ACURE/18/0086).
Drugs and chemicals
d-Ribose-l-cysteine was obtained from Sigma-Aldrich, St. Louis, USA. ELISA corticosterone kit (Oxford Biomedical Research, USA), TNF-α, IL-6 (BioLegend, USA), caspase 3 (R&D Systems, USA) ELISA Kits were purchased from BioLegend (USA), and ginseng (Mega life Sciences, Australia) were used in the study. DRLC and ginseng were freshly dissolved in saline before use. The doses of DRLC (25, 50 and 100 mg/kg) were chosen based on earlier findings [17].
UCMS procedure and Experimental design
The UCMS was induced as earlier described [10], which entails unpredictable and random exposure of rodents to different stressors. Thirty six male Swiss mice were assigned into 6 groups (n = 6): group 1, which served as non-stress control received saline (10 mL/kg, p.o), group 2, which functioned as stress-control also had saline (10 mL/kg, p.o), groups 3, 4 and 5 received DRLC (25, 50 and 100 mg/kg, p.o) whereas group 6 had ginseng (50 mg/kg, p.o). After 30 min, the mice in groups 2 to 6 were subjected to various stressors daily for 21 days (Table 1) before the behavioral and biochemical assays were performed (Scheme 1). The animals in group 1 also had the same treatment procedure but were not subjected to the UCMS and vehicular treatment was done in a stress free manner. Behavioral testing for memory and anxiety were performed in all the groups 24 h after the last treatments and stress session.
Behavioral assays
Test for spatial and non-spatial memories
Alternation behavior, a spatial cognitive function, was performed in both stressed and non-stress mice using the Y-maze apparatus as earlier described [10]. Each mouse was placed at arm A of the Y-maze and allowed to explore the three arms (A, B, C) for 5 min and the percentage alternation, a measure of memory, was calculated as described [10]. Non-spatial memory was assessed using the trial and test phases of the novel object recognition test (NORT) [10]. The trial phase entails training of mice with two identical objects (A and B) in an open arena (60 cm × 50 cm × 40 cm) for 5 min, while the test phase consists of replacement of object B with a novel object, C for another 5 min testing period after 4 h. Time spent (s) in inspecting the objects were noted. The non-spatial (discrimination index) memory were calculated using the differential time of inspection between the familiar and novel objects [17].
Test for anxiety-like behavior
Light/dark box test for anxiety was performed by placing each mouse at the center of the bright compartment of the box and then observed for 5 min. Investigational time (s) in each of the compartments of the box was recorded and evaluated [10]. The elevated plus-maze (EPM) test was also used to examine the effect of DRLC on UCMS-induced anxiety-like behavior in mice based on the index of open arm avoidance (IOAA) as described [10].
Blood glucose and serum corticosterone levels
After the all behavioral tests, blood droplet from tail veins were used for the estimation of the glucose level using a commercial glucometer (Accu-Chek Actives, Roche Diagnostics India Pvt., Ltd). Serum corticosterone (ng/mL) assay was done according to manufacturer’s instructions of the ELISA kit (Oxford Biomedical Research, USA) with a Spectramax M-5 (Molecular Devices, Sunnyvale, CA) multifunctional plate reader.
Weight of adrenal gland
The body weight of each mouse was recorded before and after UCMS procedure. After euthanization of the mice with ether, the adrenal glands were excised and weighed. The relative organ weights were calculated as organ weight/100 g of body weight [10].
Preparation of brain tissues for biochemical and histological studies
The animals were euthanized under light ether anesthesia and the brains were rapidly removed and half of each brain was prepared for biochemical assays as described [10]. The other halves of the brains were preserved in 10% formaldehyde for the histological studies.
Estimation of brain oxidative and nitrergic activities
Reduced GSH level was assayed in the supernatant of the brain based on the presence of relatively yellow colour in the presence of 5,5-diothiobis-(2-nitrobenzoic acid (DTNB) to sulfhydryl agents [18] at 412 nm using ELISA plate reader and expressed as micromoles per milligram protein (μmol/mg protein). Brain marker of lipid peroxidation (MDA) was estimated based on previous protocol with thiobarbituric acid (TBA) and trichloroacetic acid (TCA) [19]. The absorbance was obtained with spectrophotometer at 535 nm using ELISA plate reader and expressed as TBARS (nmol/mg protein). Activity of brain SOD was evaluated as described by Misra and Fridovich [20], which was based on the blockade of auto-oxidative action of adrenaline by superoxide in spectrophotometer at 480 nm. Catalase (CAT) activity was assayed based on the depletion of hydrogen perioxide by an enzyme source (catalase) in the spectrophotometer (520 nm) as previously described [21]. The catalase enzyme activity unit was expressed as Unit/mg protein. Furthermore, nitrite level was also measured as an index of nitrergic stress using 100 µL Greiss reagent and 100 µL brain supernatant [22]. Brain protein was measured in brain supernatants according to the procedure of Gornall et al. [23] at 540 nm.
Acetyl-cholinesterase activity
Acetyl-cholinesterase (AChE) activity was measured as earlier described [24]. Aliquot of the brain homogenate (0.4 mL) was added to 2.6 mL of phosphate buffer (0.1 M, pH 7.4) and 0.1 mL of 5.5′-dithio-bis (2-nitrobenzoic acid) (DTNB followed by acetylthiocholine iodide (0.1 mL). The variation in absorbance per min of 412 nm was recorded and the degree of AChE activity was calculated and denoted as µmol/min/mg protein.
Estimation of pro-inflammatory cytokines (TNF-α and IL-6) contents and caspase-3 activity
ELISA kits were used for the determination of the brain concentrations of TNF-α and IL-6 (BioLegend, USA) and caspase-3 activity (R&D, USA) according to the producers’ guide. The concentrations (pg/mL) were determined from their standard curves respectively and expressed as pg/mg protein.
Histological studies of specific brain regions of mice exposed to UCMS
Brain tissue obtained from the animals allocated for histological studies in each group was fixed in 10% formaldehyde for 7 days. The transverse sections (5–6 m thick) of the hippocampus (cornu ammonis 3, CA3), prefrontal cortex and amygdala region were then obtained by microtomy and processed by the routine method for paraffin wax embedment sections [17] and a viable neuronal cells were estimated using binocular microscope (Olympus CH (Japan).
Statistical analysis
Data were analyzed using Graph pad prism 5 Biostatistics software (Graph pad Software, Inc., Lajolla, USA version 5.0). All data were presented as mean ± SEM. Further analysis was done by one or two-way analysis of variance (ANOVA) and post hoc test (Benferroni) for multiple comparisons were appropriate. The level of significance for all tests was set at p < 0.05.
Results
d-Ribose–l-cysteine improves memory performance and reduces anxiety-like behavior in stressed mice
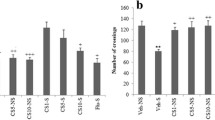
One-way ANOVA showed that there were significant effect between treatment group: spatial memory [F(5, 36) = 4.502, p = 0.0027] (Fig. 1a) and non-spatial memory [F(5, 36) = 7.152, p < 0.0001] (Fig. 1b). As shown in Fig. 1a-b, mice that were exposed to UCMS daily for 21 days had impaired spatial and non-spatial memories as indexed by reduced correct alternations (%) and DI when compared with controls. Oral treatment with DRLC (25, 50 and 100 mg/kg, p.o.) or ginseng markedly (p < 0.05) reversed the memory impairments in chronically stressed mice. As presented in Table 2, UCMS exposure for 21 days produced anxiety-like behavior due to increased duration of time spent in the closed arm and increased index of open arm avoidance (IOAA) in the EPM. Also, UCMS-mice exhibited anxiogenic behavior as shown by greater time spent in the dark side of the light/dark box (Fig. 1c). However, treatments with DRLC (25–100 mg/kg, p.o) in similar fashions to ginseng attenuated the anxiogenic behaviors due to UCMS exposure (Table 2 and Fig. 1c).
d-Ribose–l-cysteine improves spatial memory (a) and non-spatial memory (b) and reduced anxiety-like behavior (c) in mice exposed to unpredictable chronic mild stress. Bar signifies the mean ± SEM of 6 animals per group. *P < 0.05 relative to vehicle group and #p < 0.05 relative to UCMS group (one/two-way ANOVA ANOVA followed by Bonferroni post-hoc test). VEH Vehicle, UCMS unpredictable chronic mild stress, DRLC d-Ribose–l-cysteine
Effects of d-Ribose–l-cysteine on adrenal weight, glucose and corticosterone concentrations in chronically stressed mice
The effects of DRLC on UCMS-induced changes in adrenal weight, blood glucose and serum corticosterone concentrations in mice are presented in Fig. 2a–c. As shown in Fig. 2a, daily UCMS induction for 21 consecutive days resulted in a profound (p < 0.05) increase in adrenal weight. DRLC (25 and 50 mg/kg, p.o.) or ginseng mitigated the UCMS-induced increase in adrenal weight [F(5, 36) = 4.507, p = 0.0027] in mice (Fig. 2a). Mice exposed to UCMS showed marked elevation in blood glucose (Fig. 2b) and serum corticosterone (Fig. 2c) levels in comparison with non-stress controls. It is evident from Fig. 2b that the oral dose of 25 mg/kg of DRLC significantly (p < 0.05) [F(5,36) = 5.935, p = 0.0004] lowered UCMS-induced increased blood glucose levels caused by UCMS in mice. Meanwhile, at doses of 25 and 50 mg/kg, DRLC (p.o.) showed a significant [F(5, 36) = 5.388, p = 0.0011] decrease in the serum concentrations of corticosterone in chronically stressed mice (Fig. 2c).
Effect of d-ribose-l-cysteine on adrenal gland (a), blood glucose (b) and serum corticosterone (c) contents in mice subjected to unpredictable chronic mild stress. Bar represents the mean ± SEM of 6 animals per group. *P < 0.05 relative to vehicle group and #p < 0.05 relative to UCMS group (one-way ANOVA followed by Bonferroni post-hoc test). VEH Vehicle, UCMS unpredictable chronic mild stress, DRLC d-Ribose–l-cysteine
d-Ribose–l-cysteine boosts brain antioxidant activities and reduces brain concentration of malondialdehyde in mice exposed to UCMS
Figure 3a–d showed the effects of DRLC on UCMS-induced depletion of antioxidant ctivities (SOD and catalase, GSH) and increased MDA in mice. Animals exposed to UCMS had a profound decrease in brain levels of SOD [F(5, 36) = 7.504, p < 0.0001] (Fig. 3a), catalase [F(5, 36) = 5.918, p = 0.0004] (Fig. 3b) and GSH [F(5, 36) = 5.675, p = 0.0011] (Fig. 3c). Also, UCMS produced a significant rise in the level of MDA [F(5, 36) = 4.143, p < 0.0001] (Fig. 3c) relative to control. However, treatment with DRLC (25–100 mg/kg, p.o.) significantly (p < 0.05) boosts the brain SOD (Fig. 3a), catalase (Fig. 3b) and GSH (Fig. 3c) levels and reduced MDA concentrations (Fig. 3d) relative to stress controls.
d-Ribose–l-cysteine boosts brain activities of superoxide dismutase (a) catalase (b), and glutathione (c) and reduced brain concentration of malondialdehyde (d) in stressed mice. Bar represents the mean ± SEM of 6 animals/group. #P < 0.05 relative to vehicle group; *P < 0.05 relative to UCMS control (One-way ANOVA followed by Bonferroni post-hoc test). VEH Vehicle, UCMS unpredictable chronic mild stress, DRLC d-Ribose–l-cysteine
d-Ribose–l-cysteine reduces brain nitrite content and acetyl-cholinesterase activity in UCMS-mice
The effects of DRLC on brain nitrite content and AChE activity in UCMS-mice are shown in Fig. 4a, b. As presented in Fig. 4a, the increase in brain nitrite concentrations [F(5, 24) = 5.470, p = 0.0011] caused by UCMS were expressively (p < 0.05) reduced by DRLC (25, 50 and 100 mg/kg, p.o.) or ginseng. But, only 25 and 50 mg/kg of DRLC was able to reduce the elevated brain AChE activity [F(5, 24) = 6.347, p = 0.0004] in chronically stressed mice (Fig. 4b).
d-Ribose–l-cysteine reduces brain nitrite content (a) and acetyl-cholinesterase activity (b) in mice subjected to unpredictable chronic mild stress. Bar signifies the mean ± SEM of 6 animals/group. *P < 0.05 relative to vehicle group; #P < 0.05 relative to UCMS control (One-way ANOVA followed by Bonferroni post-hoc test). VEH Vehicle, UCMS Unpredictable chronic mild stress, DRLC d-Ribose–l-cysteine
d-Ribose–l-cysteine attenuates unpredictable chronic mild stress-induced changes in the brain concentrations of proinflammatory cytokines and apoptotic factor in mice
Mice exposed to UCMS for 21 days displayed increased brain concentrations of TNF-α [F(5, 24) = 5.470, p = 0.0011] (Fig. 5a) and IL-6 [F(5, 24) = 5.470, p = 0.0011] (Fig. 5b) relative to non-stress controls. However, DRLC (25, 50 and 100 mg/kg, p.o.) significantly (p < 0.05) decreased the brain concentrations of TNF-α and IL-6 in the stressed mice (Fig. 5a, b). As presented in Fig. 5c, UCMS exposure for 21 days caused a significant [F(5, 36) = 4.143, p < 0.0001] diminution in brain caspase-3 activity. Administration of DRLC (25 and 50 mg/kg, p.o.) or ginseng significantly (p < 0.05) reversed the UCMS-induced elevation of the brain caspase-3 activity in mice (Fig. 5c).
d-Ribose–l-cysteine attenuates unpredictable chronic mild stress-induced changes in the brain concentrations of TNF-α (a), IL-6 (b) and caspase-3 (c) in mice. Bar signifies the mean ± SEM of 6 animals/group. *P < 0.05 relative to vehicle group; #P < 0.05 relative to UCMS control (One-way ANOVA followed by Bonferroni post-hoc test). VEH Vehicle, UCMS unpredictable chronic mild stress, DRLC d-ribose–l-cysteine
Histomorphological features and neuronal densitometric counts of stressed mice
Photomicrographs of the hippocampus (CA3), prefrontal cortex and amygdala of mice subjected to UCMS are presented in Fig. 6a–c. Non-stress control animals displayed moderate normal structural organizations of neuronal cells, with the cells appearing compact. The UCMS-induced histomorphological distortions of the hippocampal (CA3) (Fig. 6a), prefrontal cortex (Fig. 6b) and amygdala (Fig. 6c) were much improved in DRLC (25–100 mg/kg, p.o) groups. As presented in Fig. 6d, mice exposed to UCMS had profound decline in the number of viable neuronal cells in these brain areas relative to non-stress control. However, DRLC (25, 50 and 100 mg/kg, p.o) or ginseng (50 mg/kg, p.o.) attenuated the UCMS-induced neuronal cell loss as shown by increased neuronal densitometric counts in the three brain regions (Fig. 6d).
a Photomicrograph of the hippocampal region of mice subjected to unpredictable chronic mild stress. A Non-stress control, B Stress control, C DRLC (25 mg/kg), D DRLC (50 mg/kg), E DRLC (100 mg/kg) and F ginseng (50 mg/kg) ×400. Arrows indicate neuronal cells. b Photomicrograph of the prefrontal cortex of mice subjected to unpredictable chronic mild stress. A Non-stress control, B Stress control, C DRLC (25 mg/kg), D DRLC (50 mg/kg), E DRLC (100 mg/kg) and F ginseng (50 mg/kg) ×400. Arrows indicate neuronal cells. c Photomicrograph of the amygdala of the mice exposed to unpredictable chronic mild stress. A Non-stress control, B Stress control, C DRLC (25 mg/kg), D DRLC (50 mg/kg), E DRLC (100 mg/kg) and F ginseng (50 mg/kg) ×400. Arrows indicate neuronal cells. d d-Ribose–l-cysteine attenuates UCM-induced histomorphological alteration and loss of viable neuronal cells (densitometic counts) in stressed mice. Bar signifies the mean ± SEM of 3 animals/group. *P < 0.05 relative to vehicle group; #P < 0.05 relative to UCMS control (Two-way ANOVA followed by Bonferroni post-hoc test). VEH Vehicle, UCMS unpredictable chronic mild stress, DRLC d-Ribose–l-cysteine, CA3 cornu ammonis 3
Discussion
The adaptogenic-like activity of DRLC and the putative mechanisms underlying its action were investigated in mice exposed to UCMS. The UCMS replicates the patterns human experience stresses on daily basis and is widely used for the exposition of the interplays between the psychopathological consequences and the deregulated biochemical signaling pathways associated with chronic stress [7,8,9,10]. The findings from this study indicate that oral administration of DRLC reversed memory impairment, anxiogenic and depressive-like behaviors in UCMS-mice. The increase in adrenal weight, blood glucose and corticosterone concentrations in mice subjected to UCMS were attenuated by DRLC. It further restores brain MDA, GSH and antioxidant enzymes status compromised by UCMS in mice. DRLC exhibited inhibitory activity against UCMS-induced upsurge in brain levels of nitrite and pro-inflammatory cytokines in mice. The deregulated brain AChE and caspase-3 activities induced by UCMS were also normalized by DRLC treatment. The histomorphological studies showed that DRLC displayed neuroprotective effect as depicted by decreased neuronal distortions and neurodegeneration in the hippocampal CA3 region, prefrontal cortex and amygdala of mice subjected to UCMS.
Several reports indicate that persistent stress causes behavioral derangements [1, 3, 10, 25]. It has been shown clinically that repeated uncontrollable stress plays important role in ageing-related cognitive disorders, and a key player in the pathogenesis of AD [3]. Persistent stress or traumatic life event has been found to impair the ability of susceptible individuals to form new, retain and to recover stored memory with increasing tendencies of anxiogenic and depressive episodes [3, 25, 26]. The UCMS model which closely replicates the pattern of human stress exposures have been extensively documented to cause cognitive impairment via loss of cortical dendrites in rodents [3, 27]. Our study revealed that mice subjugated to UCMS had impaired spatial and non-spatial memories, and anxiety-like behaviors. This result is similar to earlier reports that have also shown that rodents subjected to chronic stress exhibited deficits in spatial/non-spatial memories, increased anxiety and elevated brain AChE activity, which were reversed by treatments with adaptogens [2, 10]. In this study, the glutathione booster, DRLC reversed UCMS-induced memory deficits, anxiety and enhanced cholinergic pathway through inhibition of AChE activity in mice. Besides, our recent studies also showed that DRLC improves memory performance in rodents exposed to neurotoxicants such as LPS [17]. Overall, these findings suggest that DRLC might attenuate memory dysfunctions compromised by chronic stress and environmental neurotoxicants.
The HPA axis is the primary neuroimmune system implicated in the release of cortisol with the consequential activation of oxidative, inflammatory and other molecular signaling pathways that orchestrate stress-induced organ pathologies [1, 2, 4, 8]. Among the early effects of stress is the activation of adrenal glands to produce cortisol, which in turns increased blood glucose levels through gluconeogenesis, lipogenesis, and reduced cellular glucose uptake [1,2,3]. The released cortisol is meant to help maintain cellular homeostasis, but when in excess, damages various organs of the body particularly the brain and the adrenal glands; the two main frontliners in the battle against stress. Excess cortisol has been shown to cause adrenal hypertrophy [1,2,3, 10]. Thus, adrenal gland enlargement, increased glucose and cortisol concentrations are widely considered as major indicators of chronic stress states [1,2,3, 10]. Our results also showed that UCMS increased adrenal gland weight, blood glucose and serum corticosterone concentrations in mice, which were reversed by DRLC treatment. Previous studies have reported that compounds including ginseng with adaptogenic-like property attenuated adrenal hypertrophy, hyperglycemia and cortisol release in rodents exposed to chronic stress [1,2,3, 10]. Consequently, the ability of DRLC to normalize UCMS-induced adrenal hypertrophy and deregulated glucose and corticosterone levels suggest an adaptogenic-like activity.
Cortisol-mediated increased formation of neuronal damaging molecules such as ROS/NOS have been implicated in the psychopathalogical sequlae caused by chronic stress [2, 4]. In addition, injured neuronal cells release cytokines, which additionally heightened tissue injury, free radical generation followed by gradual neuronal death that characterized neurological deficits especially loss of cognition and depression [17, 28]. Several studies have shown that physical and psychological stressors induce depletion of antioxidant machineries [26, 29]. The first line of defense against oxidative stress entails activation of antioxidant defenses mechanisms, which swings into action to protect cellular constituents against injurious effects of oxidant molecules [2, 4]. Thus, the loss of neuronal antioxidant protective machineries plays a crucial role in mediating neuroinflammation and increased vulnerability to neurodegeneration [4]. Protracted inflammation in combination with oxidative stress have been identified as primary mediators of brain damage and hallmarks of senescence, a condition related to reduced adaptation [4, 30]. In fact, age-related diseases have been reported as complications arising from senescence occasioned by diminished malleability to stress [4, 30]. Our findings also showed that chronic stress increased oxidative stress characterized by elevated MDA and nitrite contents complemented by decreased antioxidant defense systems (GSH, SOD and catalase) in mouse brain. Our data also showed increased pro-inflammatory cytokines (TNF-α and IL-6) in the brains of mice subjected to UCMS paradigms, which is congruent with the non-specific effects of stress in rodent’s whole brain [10]. However, DRLC in a similar fashion to ginseng reduces the UCMS-induced increases in oxidative stress and pro-inflammatory cytokines, which might have contributed to its adatogenic-like activity. It is well reported in literature that adaptogens produce non-specific resistance to stress [31] by decreasing the levels of mediators of oxidative stress and chronic inflammation [2, 4, 5].
The histopathological consequences of prolonged stress on neuronal integrity and functioning have been thoroughly studied over the years especially in relation to cognitive disorders. The UCMS has been shown to cause cognitive impairment, emotional flattening through degeneration of neurons and dendritic spines of the hippocampal areas in rodents through the upstream cortisol-related mechanisms [2,3,4, 27]. Indeed, alterations of dendritic spines in the CA3 and decreased synaptogenesis in the sub-granular zone of the hippocampus, prefrontal cortex and amygdala have been reported to cause poor formation and storage of new memories in rodents [27]. Our results revealed that UCMS-induced neuronal derangements in the hippocampus (CA3), prefrontal cortex and amygdala of mice were attenuated by DRLC suggesting neuroprotective activity. The neuroprotective and cognitive effects exhibited by DRLC herein may be connected to its well-known ability to enhance GSH levels in neuronal cells [12, 13, 17]. Thus, the development of safer GSH precursor molecule such as DRLC with the capability to readily cross the blood brain barrier [13] and confer neuroprotection against injurious oxidative and inflammatory insults in chronic stress holds potential therapeutic benefits that need to be explored.
Executioner caspases are popularly known in the mediation of cell death during apoptosis [31, 32]. Of these caspases, caspase-3 plays the role of cleaving the mainstream of caspase substrates that are needed for initiation of apoptosis occasioned by stimulus factors such as stress [32, 33]. Caspase 3 is well recognized as an important rate-limiting apoptotic factor in the mediation of neuronal cell death [32]. Surprisingly, our data showed that exposures of mice to UCMS protocols resulted in decreased caspase-3 activity in mouse brain. Although, several studies have shown that caspase-3 also plays essential role in dendritic pruning, neuroprotection, neurogenesis and synaptogenesis [31,32,33,34]. Moreover, in vitro studies further indicated that caspase-3 facilitates neuroprotective activity via a bidirectional modulation of pro and anti-apoptotic pathways in cultured neuronal cells when they are mildly stimulated [32,33,34,35]. In line with this, in another study elsewhere, stress was shown to down-regulate caspase-3 activity accompanied by increased neuronal cell death [34, 35]. Therefore, boosting caspase-3 activity under this condition might confer neuroprotection and might serves as a striking additional approach to enhance resilience of an organism to stress. Adaptogens have also been found to exhibit neuroprotection by inhibiting program cell death during apoptosis [2, 5]. The findings that DRLC increases brain activity of caspase-3 in mice might also be contributing to its adaptogenic-like property in decreasing neuronal cell distortions induced by UCMS. Nevertheless, further studies using TUNEL test is necessary in order to delineate the adaptogenic-like effects of DRLC on apoptotic-like mechanisms in mice exposed to chronic stress. Meanwhile, further extensive investigations are necessary to establish the reason (s) for the lack of dose-dependency in some of the effects exhibited by DRLC in this study.
In conclusion, our study showed that DRLC exhibits adaptogenic-like activity by enhancing antioxidant defense mechanisms and positively modulated the neuronal cells damaging molecules in mice exposed to UCMS. These findings suggest that DRLC might be beneficial in ameliorating stress-induced psychopathologies relevant to anxiety, memory and aging-related disorders.
Abbreviations
- GSH:
-
Glutathione
- DRLC:
-
d-Ribose–l-cysteine
- UCMS:
-
Unpredictable chronic mild stress
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide-dismutase
- TNF-α:
-
Tumor necrosis factor-alpha
- IL-6:
-
Interleukin-6
- AChE:
-
Acetyl-cholinesterase
- HPA:
-
Hypothalamic–pituitary–adrenal
- IOAA:
-
Index of open arm avoidance
References
Panossian A, Wikman G (2010) Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress-protective activity. Pharmaceuticals 3:188–224
Panossian A (2017) Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Ann NY Sci 1401:49–67
McEwen BS, Gray JD, Nasca C (2015) Recognizing resilience: learning from the effects of stress on the brain. Neurobiol Stress 1:1–11
Panossian A, Seo E, Efferth T (2018) Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 50:257–284
Wiegant FAC, Limandjaja G, de Poot SAH (2008) Plant adaptogens activate cellular adaptive mechanisms by causing mild damage. In: Lukyanova L, Takeda N, Singal PK (eds) Adaptation biology and medicine. Narosa Publishing House Pvt Ltd, New Delhi, pp 319–332
Hovhannisyan AS, Nylander M, Panossian AG (2015) Efficacy of adaptogenic supplements on adapting to stress: a randomized, controlled trial. J Athlet Enhanc 4:4
Chakravarty S, Reddy BR, Sudhakar SR, Saxena S, Das T, Meghah V, BrahmendraSwamy CV, Kumar A, Idris MM (2013) Chronic unpredictable stress (CUS)-induced anxiety and related mood disorders in a Zebra fish model: altered brain proteome profile implicates mitochondrial dysfunction. PLoS ONE 8:63302
Willner P, Mitchell PJ (2002) The validity of animal models of predisposition to depression. Behav Pharmacol 13:169–188
Anisman H, Matheson K (2005) Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev 29:525–546
Umukoro S, Aluko OM, Eduviere AT, Owoeye O (2016) Evaluation of adaptogenic-like property of methyl jasmonate in mice exposed to unpredictable chronic mild stress. Brian Res Bull 121:105–114
Roberts JC, Francetic DJ (1991) Mechanisms of chemoprotection by ribo-cysteine, a thiazolidineprodrug of L-cysteine. Med Chem Resp 1:213–219
KaderT PCM, Williams MJ, Gieseg SP, McCormick SP (2014) Ribose-cysteine increases glutathione-based antioxidant status and reduces LDL in human lipoprotein(a) mice. Atherosclerosis 237:725–733
Aoyama K, Nakaki T (2013) Impaired glutathione synthesis in neurodegeneration. Int J Mol Sci 14:21021–21044
Falana B, Adeleke O, Orenolu M, Osinubi A, Oyewopo A (2017) Effect of D-ribose-L-cysteine on aluminum induced testicular damage in male Sprague-Dawley rats. JBRA Assisted Reproduction 21:94–100
Omran H, Illien S, MacCarter D (2003) D-Ribose improves diastolic function and quality of life in congestive heart failure patients: a prospective feasibility study. Eur J Heart Fail 5:615–619
Teitelbaum JE, Johnson C, St. Cyr J (2006) The use of d-ribose in chronic fatigue syndrome and fibromyalgia: a pilot study. J Altern Complemen Med 12:857–862
Emokpae O, Ben-Azu B, Ajayi AM, Umukoro S (2020) D-Ribose-l-cysteine attenuates lipopolysaccharide-induced memory deficits through inhibition of oxidative stress, release of proinflammatory cytokines, and nuclear factor-kappa B expression in mice. Naunyn-Schmied Arch Pharm 393:909–925
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathionereductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta 582:67–78
Okhawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1995:351–358
Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Goth LA (1991) Simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151
Green LC, Tannenbaum SR, Goldman P (1981) Nitrate synthesis in the germ free and conventional rat. Science 212:56–58
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum protein by means of biuret reaction. J Biol Chem 177:751
Ellman GL, Courtney KD, Andre JV, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Alò R, Mele M, Fazzari G, Avolio E, Canonaco M (2015) Exposure to sub-chronic unpredictable stress accounts for antidepressant-like effects in hamsters treated with BDNFandCNQX. Brain Res Bull 118:65–77
Kuhlmann S, Piel M, Wolf OT (2005) Impaired memory retrieval after psychological stress in healthy young men. J Neurosci 25:2977–2982
Alfarez DN, Joëls M, Krugers HJ (2003) Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci 17:1928–1934
Tonnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzh Dis 57:1105–1121
Ben-Azu B, Emokpae O, Ajayi AM, Jarikre TA, Orhode V, Aderibigbe AO, Umukoro S, Iwalewa EO (2020) Repeated psychosocial stress causes glutamic acid decarboxylase isoform-67, oxidative-Nox-2 changes and neuroinflammation in mice: Prevention bytreatment with a neuroactiveflavonoid, morin. Brain Res 1744:146917
Stranahan AM, Mattson MP (2012) Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci 13:209–216
Slee EA, Adrain C, Martin SJ (2001) Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276:7320–7326
Amelio MD, Cavallucci V, Cecconi F (2010) Neuronal caspase-3 signaling: not only cell death. Cell Death Differ 17:1104–1114
Dash PK, Blum S, Moore AN (2000) Caspase activity plays an essential role in long-term memory. NeuroReport 11:2811–2816
Khalil H, Peltzer N, Walicki J, Yang J, Dubuis G, Gardiol N, Held W, Bigliardi P, Marsland B, Liaudet L, Widmann C (2012) Caspase-3 protects stressed organs against cell death. Mol Cell Biol 32:4523–4533
Fernando P, Brunette S, Megeney LA (2005) Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. FASEB J 19:1671–1673
Acknowledgement
Authors thank the technical staff of the Department of Pharmacology and Therapeutics as well as Dr T. Aina of the Department of Veterinary Pathology of the University of Ibadan for their assistance in the biochemical and histological studies during the course of the research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare that they have no conflict of interest.
Ethical approval
The experimental procedures were approved by the University of Ibadan Animal Care and Use Research Ethics Committee (UI-ACURE/18/0086) and performed in accordance with the care and use of Laboratory Animals of the NIH Guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okoh, L., Ajayi, A.M., Ben-Azu, B. et al. d-Ribose–l-cysteine exhibits adaptogenic-like activity through inhibition of oxido-inflammatory responses and increased neuronal caspase-3 activity in mice exposed to unpredictable chronic mild stress. Mol Biol Rep 47, 7709–7722 (2020). https://doi.org/10.1007/s11033-020-05845-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05845-1