Abstract
The goal of this study was to determine the protective role of ellagic acid (EA) against CCl4-induced muscle injury in rats. In this study, 36 Wistar albino rats (n = 36, 8 weeks old) were used. The rats were divided into 4 groups and 9 rats were included each group. Groups: (i) control Group: standard diet; (ii) EA Group: standard diet + EA group; (iii) CCl4 group: standard diet + CCl4 group; (iv) EA + CCl4 group: standard diet + EA + CCl4. The animals were decapitated after 8 weeks, and their muscle tissues were received and investigated. In the muscle tissue, TNF-α, COX-2, Nrf-2, NF-kB, caspase-3 and bcl-2 expression levels were analyzed by the western blotting technique, lipid peroxidation was detected by MDA (malondialdehyde), and catalase and GSH levels were determined by a spectrophotometer. In our findings, in comparison to the CCl4 group, in the EA + CCl4 group, the Nrf-2 and caspase-3 protein expression levels, GSH and catalase activities increased, while the NF-kB, bcl-2, TNF-α and COX-2 protein expression levels and MDA levels decreased. These results suggest that EA reduces muscle tissue damage rate in rats and that EA may also be used as a potential drug to protect against muscle tissue damage in the future.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We think that the result of this study will bring important knowledge to the literature and we did not find any studies on the contents of this article. A muscle functions as an organ that regulates and secretes metabolism. Muscle and bone interact not only mechanically but also with secreted biochemical signaling molecules [1]. Carbon tetrachloride (CCl4) is a strong carcinogenic agent that leads to production of reactive free radicals and initiates cell damage. CCl4 has been shown to be a pulmonary toxic compound that causes deterioration of the alveolar septa by accumulation of countless neutrophils, fibroblasts and macrophages in blood vessels [2]. It is also a potent hepatotoxic agent commonly used to induce liver degeneration in vivo with oxidative stress [3]. Besides the liver, oxidative damage has been determined in the kidneys, brain, lung, testes and muscles. CCl4 induces oxidative stress through free radical formation which causes tissue damage [4]. Under normal conditions, many internal and external stress factors constantly change the cellular balance. Antioxidants play an important role in protecting cells against these factors [5]. In recent studies, EA, which is known as a polyphenol compound with its antioxidant properties, has been found to play a protective role against lipid peroxidation and damage to skeletal muscles. It was expressed that the antioxidant effect of EA against skeletal muscle ischemia and reperfusion injury [6] inhibits vascular smooth muscle cell proliferation and prevents atheroma formation in streptozotocin-induced diabetic rats [7]. It was also found to significantly inhibit the phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 induced by oxidized LDL [8]. Caspase-3 is a member of the caspase family. It has a central role on the apoptotic pathway [9].
Caspases, which are localized in the cytosol in the cell, are divided into two as initiator and effector caspases and are also called cysteine proteases. In apoptosis, they break down peptides especially from aspartate residues. The main task of DNA polymerase enzyme activity is to block the apoptosis of the cell to prevent cell damage [10]. Bcl-2 is an internal mitochondrial membrane protein. It is also one of the pro-apoptotic, anti-apoptotic or anti-autophagic proteins. It serves anti-apoptotic function [11]. The nuclear factor erythroid 2-associated factor 2 (Nrf-2) is an essential regulator for antioxidation. Its main function is to improve the expression of anti-oxidative genes such as HO-1 [12]. NF-kB is a nuclear transcription factor that arranges the expression of multiple genes that are critical for cellular apoptosis, proliferation, tumorigenesis, inflammation and various autoimmune diseases. It is a dimeric subunit complex of the NF-kB/Rel family [13]. It is a family of DNA-binding proteins that play a role in transcriptional regulation of many gene products. Activation of NF-kB may be induced in response to lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α), interleukins, radiation and other stimulating agents [14]. Cyclooxygenase (COX) enzymes have two isoforms: COX-1 and COX-2. It is known that the COX-2 enzyme catalyzes conversion of arachidonic acid into prostaglandins, which play an important role in proliferation of cancer cells [15]. TNF-α is an inflammatory cytokine and has a role in initiation and growth of cancer. TNF-α modulates various aspects of cancer cell phenotypes such as cell proliferation, migration, invasion and metastatic potential [16].
Materials and methods
Preparation and application of carbon tetrachloride
CCl4 was applied to the rats twice a week for 8 weeks intraperitoneally (1,5 ml/kg) syringed with olive oil at a rate of 1:3 [17].
Ellagic acid preparation and application to rats
100 mg of EA (A15722) was dissolved in 10 ml of DMSO (dimethyl sulfoxide) [18], after EA from this stock solution was administered to the animals intraperitoneally (10 mg/kg body weight) five times a week. The study continued for 8 weeks [19, 20].
In vivo experimental design
All animals were housed, cared and used experimentally according to the ‘Guide for the Care and Use of Experimental Animals’ approved by the Institutional Animal Ethics Committee, Firat University, Elazig (Registration Number: 27.02.2019/2019-04). 36 male Wistar albino (n = 36, 8 weeks) rats were used in this study, and the rats were distributed into four groups. The groups were:
- Group (i):
Control: feed with only standard diet,
- Group (ii):
CEA: standard diet + EA group (10 mg/kg, ip),
- Group (iii):
CCl4: standard diet + CCl4 group (1,5 ml/kg, ip),
- Group (IV):
EA + CCl4: standard diet + CCl4 (1,5 ml/kg, ip) + EA (10 mg/kg, ip).
Feed and water were given ad libitum for the process of the study. When work was finished, the rats were sacrificed and the muscle tissue was received and kept at − 80 °C until analysis.
Muscle tissue MDA analysis
0,5 gr muscle tissue samples were sliced into small sections after which they were homogenized in 4.5 ml, 1.15% KCl solution MDA analysis was carried out on this homogenate according to the method described by Ohkawa et al. [21] and some minor changes were made on this method [21]. The results were recorded as nmol/g muscle tissue MDA [17, 22].
Muscle tissue homogenization and SDS-PAGE and western blotting
Muscle tissue samples were cut into small sections and lysed in a lysis buffer (0.5 M Tris [pH 8], EDTA, β-Mercaptoethanol, Phenyl methyl sulfonyl fluoride [PMSF]) in a glass homogenizer. The cut tissue samples were centrifuged at 15,000 rpm for 45 min. The supernatant was collected and kept at − 80 °C until usage [17]. The protein samples of the muscle tissues were run on 12% gel via the SDS-PAGE method and transported to a nitrocellulose membrane. The samples were then incubated overnight at 4 °C with specific primary antibodies against TNF-α, Nrf-2, NF-kB, COX-2, caspase-3 and bcl-2 (dilution: 1/500 for all antibodies). Later, the samples were incubated by the HRP‐conjugated secondary antibody (1/1000) for 60 min at room temperature, the nitrocellulose membrane was tested with DAB staining and the protein levels measured with an imaging program [17, 23, 24].
Catalase activity assay
Hydrogen peroxide was added into the 1/15 M Na-phosphate buffer (Na2HPO4–NaH2PO4, pH 7) solution. 1 ml of this solution was incubated, the supernatant was then added at different concentrations and then, the absorbance changes were read [17].
GSH analysis
GSH measurement was made according to the method reported by Yigit et al. [25]. This protocol describes a procedure for determining glutathione (GSH) concentrations in different cells and tissues. The absorbance was read on a spectrophotometer at 412 nm against distilled water [25].
Statistical analysis
All results of this study were analyzed by the SPSS 22 package program. One-Way ANOVA, Post Hoc Tukey, Games Howell, Duncan and LSD tests were performed to detect intragroup differences. In terms of the reliability of the statistics, the analyses were conducted by at least three replications.
Results
MDA analysis
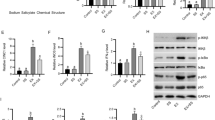
Table 1 and Fig. 1 show that the MDA amount decreased in the EA and EA + CCl4 groups in comparison to the CCl4 groups (p < 0.05).
Catalase activity results
Table 2 and Fig. 2 show that the catalase activity of the EA + CCl4 group was observed higher than the CCl4 group.
GSH analysis
In our findings, the GSH amount of the EA + CCl4 group was observed higher than the CCl4 group (Table 3 and Fig. 3).
TNF-α, Nrf-2, NF-kB, COX-2, Caspase-3 and Bcl-2 protein expression levels
Figure 4a reveals that the lowest rate of caspase-3 was calculated in the CCl4 group, whereas the highest rates were in the control and EA groups (p < 0.05). Figure 4b shows that the bcl-2 protein expression levels of the CCl4 group was determined higher in comparison to the EA and EA + CCl4 groups (p < 0.05). Figure 4c points out that the Nrf-2 protein expression in the CCl4 group was obtained as lower than the EA and EA + CCl4 groups (p < 0.05). Accordingly, it may be stated that EA increases these biomarkers’ protein synthesis. When the protein expression level of NF-kB was evaluated as shown in Fig. 4d, a statistically significant distinction was obtained between the groups (p < 0.05). The NF-kB amount in the EA + CCl4 group was detected to be lower than that of the CCl4 group. As seen in Fig. 4e, f, the highest COX-2 amount was obtained in the CCl4 group, but with EA application, the COX-2 expression level decreased in the EA + CCl4 group. The TNF-α amount in the EA + CCl4 group was determined to be lower than that of the CCl4 group (Fig. 4g).
Discussion
The aim of our study was to investigate the protective role of ellagic acid (EA) on caspase-3, bcl-2, Nrf-2, NF-kB, TNF-α and COX-2 protein expressions in case of CCl4-induced muscle injury in rats. In apoptosis, which is a controlled mechanism for physiological cell death, several proteins play an essential role [26]. Activation or inhibition of these proteins leads to not only apoptosis but also a non-apoptotic differentiation process [27, 28]. Our results showed that high expression of caspase-3 and Nrf-2 and low expression of bcl-2, NF-kB, TNF-α and COX-2 are associated with decreased muscle tissue damage. If the synthesis of caspase-3 and Nrf-2 protein is increased by the effect of ellagic acid in the cell, if the synthesis of NF-kB, TNF-α, COX-2 and bcl-2 protein is decreased by the effect of ellagic acid in the cell, then we may state that ellagic acid reduces muscle tissue by directing damaged cells to apoptosis. When we look at caspase-3 and Nrf-2 protein expressions in especially the EA + CCl4 group, we may easily see these proteins’ over expressions in comparison to the CCl4 group. We think that, despite the suppression of carbon tetrachloride on expressions of this protein, ellagic acid was able to induce the synthesis of these proteins by inhibiting CCl4 inhibition. Similarly, when we look at the bcl-2, NF-kB, TNF-α and COX-2 protein expressions, we may see that ellagic acid can reduce the synthesis of these proteins in the EA + CCl4 group despite CCl4. Moreover, our results showed that a high amount of MDA can damage DNA. In this context, when we look at our results, especially after EA application, the MDA level decreased in the EA + CCl4 group in comparison to the CCl4 group. These results showed that ellagic acid protects muscle tissue DNA against CCl4 damage. Additionally, high glutathione and increased catalase enzyme activity indicate that ellagic acid protects muscle tissue against CCl4 damage. We consider that the results of this study will contribute essential knowledge for future academic studies and according to our knowledge, the effect of ellagic acid on expression of these proteins (caspase-3, bcl-2, Nrf-2, NF-kB, TNF-α and COX-2) in muscle tissue damage was studied for the first time in this article. According to many in vitro and in vivo studies, EA has an anti-tumorigenic antiproliferative drug action [29]. Lee et al. researched the effect of tocotrienol-rich fraction (TRF) on skeletal muscle damage in diabetic mice. In the TRF groups, caspase-3 and Nrf-2 expressions increased, but bcl-2, NF-kB and TNF-α expressions decreased [30]. Similarly, when we look at our results, after EA supplementation, the caspase-3 and Nrf-2 expressions increased, but the bcl-2, NF-kB and TNF-α expressions decreased in the EA + CCl4 group in comparison to the CCl4 group. These results show that ellagic acid induces Nrf-2 and caspase-3 biomarker synthesis in CCl4-induced muscle damage and inhibits the synthesis of the NF-kB, TNF-α and COX-2 biomarkers, resulting in apoptosis of the damaged cells. Li et al. explored the effects of hypoxia-induced pulmonary artery smooth muscle proliferation and they concluded that NF-kB expression levels decreased in the hypoxia-treated groups [31]. Gok investigated the protective effect of EA in rats with liver injury with CCl4. They reported that MDA levels decreased in the EA + CCl4 groups with EA application in comparison to CCl4 groups. Additionally, they also observed that EA treatment increased caspase-3 expression and decreased bcl-2 expression in comparison to the CCl4 group [32]. According to our results with EA application, the MDA levels decreased in the EA + CCl4 group in comparison to the CCl4 group. Aslan investigated the protective effect of tomato powder (TP) in rats with colorectal cancer to azoxymethane. When compared to the azoxymethane (AO) group, TP increased caspase-3 expression and decreased Cox-2 expression levels in the TP + AO group [10]. Rani et al. stated that EA inhibits atherosclerosis and intracellular ROS formation in streptozotocin-induced diabetic rats [7]. Yu et al. investigated the anti-oxidative and protective effects of linarin, a natural compound, on myocardial (heart muscle) cells. In linarin groups, they stated that MDA and ROS levels decreased. Additionally, they also observed that linarin treatment increased Nrf-2 expression and reduced NF-kB and Bcl-2 expression [12]. Akdemir et al. investigated the antioxidant effects of EA and hesperidin (HEPP) against skeletal muscle ischemia/reperfusion injury (I/R). In EA + HES groups, CAT activity increased, but MDA levels decreased in comparison to the case in ischemia and I/R groups. Additionally, they also reported that EA and HEPP with antioxidant properties had cell-protective effects against lipid peroxidation [6]. In our study, after EA application, the catalase activity increased in the EA + CCl4 group, while the MDA levels decreased. These results show that ellagic acid increases catalase enzyme activity and reduces muscle tissue damage.
Hayes et al. investigated the effect of lutein, sesamol, ellagic acid and olive leaf extract on lipid oxidation and oxymyoglobin oxidation. They concluded that lutein, sesamol, ellagic acid and olive leaf extract decreased MDA levels [33]. Ekinci et al. researched the potential benefits of quercetin on skeletal muscle ischemia–reperfusion injury, and they reported that MDA levels decreased, but CAT activity significantly increased in the quercetin-treated groups [34]. Hadi et al. studied the effects of green tea on oxidative stress and muscle damage. They reported that the levels of MDA significantly decreased in green tea groups [35]. Onal et al. examined the effects of hypothermia and ozone (O3) on skeletal muscle ischemia–reperfusion injury in rats. They reported that hypothermia and ozone down-regulated the tourniquet-induced ischemic reperfusion injury in the musculoskeletal system by decreasing the levels of oxidative and nitrosative stress parameters and increasing antioxidant enzymes. Additionally, they also concluded that CAT activity was increased in the hypothermia and ozone groups and the MDA levels decreased significantly [36].
Yang et al. investigated the protective effects of kaempferol-3′-sulfonate on hydrogen peroxide introduced into vascular smooth muscle cells. In the H2O2-treated group in comparison to the control group, GSH levels decreased and MDA levels significantly increased. Furthermore, they found that kaempferol-3′-sulfonate increased caspase-3 expression and decreased Bcl-2 expression in comparison to H2O2 [37]. Seigner et al. studied the anti-inflammatory effects of the plant Symphytum officinale on muscle and joint pain. They concluded that there was a significant decrease in NF-kB and COX-2 expression levels in the plant-given group [38]. When our results were evaluated, the bcl-2 and COX-2 decreased in the EA + CCl4 group in comparison to the CCl4 group and thus, we may easily state that EA has a preventive effect on muscle damage. Hsu et al. investigated the effects of ginseng steroids on the oxidative stress and antioxidant capacity of rat skeletal muscles. They reported that the GSH levels and CAT activities significantly increased in the ginseng steroids groups [39]. Guan et al. examined the antioxidant, anti-inflammatory and antitumor effects of celastrol on the skeletal muscle in diabetic rats and in the celastrol-given group, they concluded that the levels of MDA decreased and the level of GSH increased [40]. According to our results, the GSH levels increased, but MDA decreased after EA supplementation in the EA + CCl4 group in comparison to the CCl4 group. Wang et al. reported that, in lung injury caused by skeletal muscle ischemia and reperfusion in rats, COX-2 expression levels decreased in the skeletal reperfusion group in comparison to the NS-398 (COX-2 inhibitor) group [41]. Cheng et al. investigated the protective effect of lutein against ischemia/reperfusion injury in rat skeletal muscles. They also concluded that Nrf-2 expression levels significantly increased, while NF-kB and COX-2 expression levels significantly decreased in the lutein-treated groups in comparison to the skeletal reperfusion group [42].
Aslan et al. researched the preventive effects of EA in rats with liver damage induced by CCl4. They found that NF-kB and Bcl-2 expression levels decreased, but Nrf-2 and caspase-3 expression levels increased in the EA treatment group in comparison to the CCl4 group [17]. Hseu et al. searched the role of EA in human keratinocyte (HaCaT) cells on oxidative stress and apoptosis and they revealed that EA administration reduced ROS production and MDA levels. Additionally, they concluded that caspase-3 expression levels increased and Bcl-2 expression levels significantly decreased in the EA-treated groups [43]. Basham et al. investigated the effects of curcumin supplementation on oxidative stress, inflammation, muscle damage and muscle pain caused by exercise. According to their results, oxidative stress and inflammation decreased in the curcumin group. Moreover, they also concluded that there was a significant decrease in TNF-α expression levels and MDA levels in the curcumin groups [44]. El-Shitany et al. stated that nanoparticle ellagic acid protected against cisplatin-induced hepatotoxicity in rats [45]. Ahire and Mishra revealed that oral supplementation of EA significantly inhibited the induction of micronuclei aberrations against gamma radiation (1.5–3 Gy) and EA has a preventive effect on some cancer cells [46]. Accordingly, our results indicated that, with EA application, the TNF-α protein expression decreased in the EA + CCl4 group in comparison to the CCl4 group. These results show that ellagic acid decreases TNF-α protein expression and reduces muscle tissue damage.
Conclusion
Especially when the results obtained from this study were evaluated, it was concluded that ellagic acid had a significant effect on the mechanism of apoptosis. It protects the muscle tissue against CCl4 damage, induces caspase-3 and Nrf-2 protein signaling pathways, suppresses Bcl-2, NF-kB, TNF-α and COX-2 protein signaling pathways and directs damaged cells to apoptosis. Thus, it is seen that it causes destruction of damaged cells in a controlled manner. Additionally, it is seen that EA reduces MDA levels and prevents damage to the DNA of healthy cells. In particular, it is seen that EA contributes significantly to reduction of muscle tissue damage by increasing catalase enzyme activity and glutathione levels. When all these results are evaluated, we conclude that EA may have similar effects when ingested by humans and animals.
Abbreviations
- AO:
-
Azoxymethane
- CAT:
-
Catalase activity
- CCl4 :
-
Carbon tetrachloride
- EA:
-
Ellagic acid
- EDTA:
-
Ethylenediaminetetraacetic acid
- ETs:
-
Ellagitannins
- FUDAM:
-
Firat University Experimental Animals Research Institute
- HaCaT:
-
Human keratinocyte cell
- HEPP:
-
Hesperidin
- I/R:
-
Ischemia/reperfusion
- Ip:
-
Intraperitoneal
- MDA:
-
Malondialdehyde
- NF-Kb:
-
Nuclear factor kappa B
- O3 :
-
Ozone
- PAGE:
-
Polyacrylamide gel electrophoresis
- PMSF:
-
Phenylmethylsulphonyl fluoride
- PR1P:
-
Prominin-1
- RGC:
-
Retinal ganglion cell
- ROS:
-
Reactive oxygen species
- TGF-β1:
-
Growth factor-β1
- TNF-α:
-
Tumor necrosis factor-α
- TP:
-
Tomato powder
- TRF:
-
Tocotrienol-rich fraction
- COX-2:
-
Cyclooxygenase -2
- SDS:
-
Sodium dodecyl sulphate
- HBMVECs:
-
Microvascular endothelial cells
- VPF:
-
Vascular permeability factor
References
Bonewald L (2019) Use it or lose it to age: a review of bone and muscle communication. Bone 120:212–218
Ahmad B, Khan MR, Shah NA (2015) Amelioration of carbon tetrachlorideinduced pulmonary toxicity with oxalis corniculata. Toxicol Ind Health 31:1243–1251
Lavrentiadou SN, Tsantarliotou MP, Zervos IA, Nikolaidis E, Georgiadis MP, Taitzoglou IA (2013) CCl4 induces tissue-type plasminogen activator in rat braın: Protective effects of oregano, rosemary or vitamin E. Food Chem Toxicol 61:196–202
Taslidere E, Taslidere M, Elbe H, Cetin A, Ates B (2014) Protective effects of melatonin and quercetin on experimental lung injury induced by carbon tetrachloride in rats. Exp Lung Res 40:59–65
Aksit H, Aksit D, Bildik A, Kara H, Yavuz O, Seyrek K (2015) Deneysel karaciğer intoksikasyonunda n-asetil sistein’in glutatyon metabolizması ve lipid peroksidasyonuna etkileri. Ankara Üniv Vet Fak Derg 62:1–5
Akdemir F, Gulcin I, Karagoz B, Soslu R, Alwasel S (2016) A comparative study on the antioxidant effects of hesperidin and ellagic acid against skeletal muscle ischemia/reperfusion injury. J Enzym Inhib Med Chem 31:114–118
Rani UP, Kesavan R, Ganugula R, Avaneesh T, Kumar UP, Reddy G et al (2013) Ellagic acid inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and prevents atheroma formation ın streptozotocin-induced diabetic rats. J Nutr Biochem 24:1830–1839
Chang W, Yu Y, Chiang Su, Tseng C (2008) Ellagic acid suppresses oxidised low-density lipoprotein-induced aortic smooth muscle cell proliferation: Studies on the activation of extracellular signal-regulated kinase 1/2 and proliferating cell nuclear antigen expression. Br J Nutr 99:709–714
Mishra S, Vinayak M (2015) Role of ellagic acid in regulation of apoptosis by modulating novel and atypical PKC in lymphoma bearing mice. BMC Complem Altern Med 15:2–8
Aslan A (2011) The effect of lycopene on the cyclooxygenase (cox-2), caspase-3, caspase-9, bax, bcl-2, p53 protein expression and DNA damage in rats with azoxhymethane-induced colorectal cancer. PhD Thesis, Firat University Institute of Natural and Applied Sciences Elazığ
Akyol Z, Gürkan A, Arisan ED, Yerlikaya P, Unsal N (2016) DENSpm overcame Bcl-2 mediated resistance against paclitaxel treatment in MCF-7 breast cancer cells via activating polyamine catabolic machinery. Biomed Pharmacother 84:2029–2041
Yu Q, Li X, Cao X (2017) Linarin could protect myocardial tissue from the injury of ıschemia-reperfusion through activating Nrf-2. Biomed Pharmacother 90:1–7
Cai Y, Shen Y, Gao L, Chen M, Xiao M, Huang Z et al (2016) Karyopherin alpha 2 promotes the inflammatory response in rat pancreatic acinar cells via facilitating NF-κB activation. Dig Dis Sci 61:747–757
Li N, Song Y, Zhao W, Han T, Lin S, Ramirez O et al (2016) Small interfering RNA targeting NF-κB attenuates lipopolysaccharide-induced acute lung injury in rats. BMC Physiol 16:7
Celik S, Albayrak AT, Akyuz S, Ozel AE (2019) Synthesis, molecular docking and ADMET study of ıonic liquid as anticancer ınhibitors of DNA and COX-2, TOPII enzymes. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2019.1604263
Oku T, Shimada K, Kenmotsu H, Ando Y, Kurisaka C, Sano R et al (2018) Stimulation of peritoneal mesothelial cells to secrete matrix metalloproteinase-9 (MMP-9) by TNF-α: A Role in the ınvasion of gastric carcinoma cells. Int J Mol Sci 19:3961
Aslan A, Gok O, Erman O, Kuloglu T (2018) Ellagic Acid impedes carbontetrachloride-induced liver damage in rats through suppression of NF-kB, Bcl-2 and regulating Nrf-2 and caspase pathway. Biomed Pharmacother 105:662–669
Grossi MR, Berni A, Pepe G, Filippi S, Meschini R, Papeschi C et al (2014) Evaluation of the effects of ellagic acid (EA) on 7,12-dimethylbenz (α) anthracene (DMBA) ınduced micronuclei in mammalian cells in vitro and in vivo. Toxicol Lett 224:240–245
Kaya I (2012) Effect of ellagic acid on plasma paraoxonase, sialic acid and oxidative stress parameters in mice with chronic fluorosis. PhD Thesıs, Kafkas University Health Sciences Institute Kars
Gu L, Deng WS, Liu Y, Jiang CH, Sun LC, Sun XF et al (2014) Ellagic acid protects lipopolysaccharide/D-galactosamine-induced acute hepatic ınjury in mice. Int Immunopharmacol 22:341–345
Ohkawa H, Ohishi N, Yagi K (1979) Assay for Lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Aslan A, Can MI (2014) Milk thistle impedes the development of carbontetrachloride-induced liver damage in rats through suppression of bcl-2 and regulating caspase pathway. Life Sci 117:13–18
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Aslan A, Can MI, Kuloglu T, Baspinar S (2016) Milk thistle may induce apoptosis in development of carbontetrachloride-induced liver DNA damage in rats. Prog Nutr 18:146–151
Yiğit M (2015) Effects of astaxanthin on antioxidant parameters (GSH, GSH-PX, MDA), Ca2+ signaling and apoptosis levels in arpe-19 cells on cigarette smoke-related hydrokuinon induced oxidative stress model. Medical Thesis, Süleyman Demirel University Health Sciences Institute Isparta
Nagata S (2018) Apoptosis and clearance of apoptotic cells. Annu Rev Immunol 36:489–517
Shimizu K, Sawasaki T (2013) Nek5, a novel substrate for caspase-3, promotes skeletal muscle differentiation by up-regulating caspase activity. FEBS Lett. 587(14):2219–2225
Aslan A, Boydak D, Can MI, Kuloglu T (2015) Nigella sativa improves the carbon tetrachloride-induced lung damage in rats through repression of erk/akt pathway. Bangladesh J Pharmacol 10:654–659
Mady FM, Shaker MA (2017) Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int J Nanomed 12:7405
Lee H, Lim Y (2018) Tocotrienol-rich fraction supplementation reduces hyperglycemia-induced skeletal muscle damage through regulation of insulin signaling and oxidative stress in type 2 diabetic mice. J Nutr Biochem 57:77–85
Li Y, Liu S, Zhang Y, Gao Q, Sun W, Fu L et al (2018) Histone demethylase JARID1B regulates proliferation and migration of pulmonary arterial smooth muscle cells in mice with chronic hypoxia-induced pulmonary hypertension via nuclear factor-kappa B (NFkB). Cardiovasc Pathol 37:8–14
Gok O (2017) The effect of ellagic acid on some apoptotic proteins expression in liver of rats experimentally to made up damage with carbon tetrachloride. Master's Thesis, Firat University Institute of Natural and Applied Sciences Elazığ
Hayes JE, Stepanyan V, Allen P, O’Grady MN, O’Brien NM, Kerry JP (2009) The effect of lutein, sesamol, ellagic acid and olive leaf extract on lipid oxidation and oxymyoglobin oxidation in bovine and porcine muscle model systems. Meat Sci 83:201–208
Ekinci Akdemir FN, Gülçin I, Karagöz B, Soslu R (2016) Quercetin protects rat skeletal muscle from ischemia reperfusion injury. J Enzyme Inhib Med Chem 31:162–166
Hadi A, Pourmasoumi M, Kafeshani M, Karimian J, Maracy MR, Entezari MH (2017) The effect of green tea and sour tea (Hibiscus sabdariffa L.) supplementation on oxidative stress and muscle damage in athletes. J Diet Suppl 14:346–357
Onal O, Yetisir F, Sarer A, Zeybek ND, Onal CO, Yurekli B et al (2015) Prophylactic ozone administration reduces intestinal mucosa injury induced by intestinal ischemia-reperfusion in the rat. Mediat Inflamm. https://doi.org/10.1155/2015/792016
Yang X, Wang Q, Wang C, Qin X, Huang Y, Zeng R (2016) Synthesis and protective effect of kaempferol-3'- sulfonate on hydrogen peroxide induced injury in vascular smooth muscle cell. Chem Biol Drug Des 87:841
Seigner J, Junker-Samek M, Plaza A, D‘Urso G, Masullo M, Piacente S (2019) A symphytum officinale root extract exerts anti-inflammatory properties by affecting two distinct steps of NF-κB signaling. Front Pharmacol 10:1–12
Hsu MF, Yu SH, Korivi M, Jean WH, Lee SD, Huang CY (2017) Hormetic property of ginseng steroids on anti-oxidant status against exercise challenge in rat skeletal muscle. Antioxidants 6:36
Guan Y, Cui ZJ, Sun B, Han LP, Li CJ, Chen LM (2016) Celastrol attenuates oxidative stress in the skeletal muscle of diabetic rats by regulating the AMPK-PGC1α-SIRT3 signaling pathway. Int J Mol Med 37:1229–1238
Wang L, Shan Y, Ye Y, Jin L, Zhuo Q, Xiong X et al (2016) COX-2 inhibition attenuates lung injury induced by skeletal muscle ischemia reperfusion in rats. Int Immunopharmacol 31:116–122
Cheng F, Zhang Q, Yan FF, Wan JF, Lin CS (2015) Lutein protects against ischemia/reperfusion injury in rat skeletal muscle by modulating oxidative stress and inflammation. Immunopharmacol Immunotoxicol 37:329–334
Hseu YC, Chou CW, Kumar KS, Fu KT, Wang HM, Hsu LS et al (2012) Ellagic acid protects human keratinocyte (hacat) cells against uva-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol 50:1245–1255
Basham SA, Waldman HS, Krings BM, Lamberth J, Smith JW, McAllister MJ (2019) Effect of curcumin supplementation on exercise-ınduced oxidative stress, ınflammation, muscle damage, and muscle soreness. J Diet Suppl. https://doi.org/10.1080/19390211.2019.1604604
El-Shitany NAE, Abbas AT, Ali SS, Eid B, Harakeh S, Neamatallah T et al (2019) Nanoparticles ellagic acid protects against cisplatin-induced hepatotoxicity in rats without ınhibiting its cytotoxic activity. Int J Pharmacol 15:465–477
Ahire V, Mishra KP (2017) Ellagic acid as a potential anti-cancer drug. Int J Radiol Radiat Ther 3:234–235
Funding
The article was supported by Firat University Scientific Research Projects Unit (FUBAP) with the project code FF.16.42. Some results of this article were presented orally at the 1st International Mardin Artuklu Multidisciplinary Studies Congress (19–21 April 2019) in Mardin, Turkey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Ethical approval
All applicable international, national and institutional guidelines for the care and use of experimental animals were followed. The animal section of this study was performed by the approval of the Animal Experiments Ethics Committee of Firat University with the approval number of 27.02.2019 /2019-04.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aslan, A., Beyaz, S., Gok, O. et al. The effect of ellagic acid on caspase-3/bcl-2/Nrf-2/NF-kB/TNF-α /COX-2 gene expression product apoptosis pathway: a new approach for muscle damage therapy. Mol Biol Rep 47, 2573–2582 (2020). https://doi.org/10.1007/s11033-020-05340-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05340-7