Abstract
Shelterin complex and its associated molecules are imperative for proper functioning and maintenance of human telomeres. These molecules in association with human telomerase have been found altered in most cancers including multiple myeloma thereby proposed them as suitable therapeutic targets. Further, due to aggressive and recurring behavior of myeloma novel, efficacious and safe therapeutic agents for disease prevention are primary requirements for treatment of this disease. This maiden attempt evaluated the anti-proliferative properties of tanshinone I (TanI) alone or in combination with lenalidomide (Len) on myeloma cancer cell lines (RPMI8226 and U226). Further, after drug treatment levels of telomerase activity (TA) and molecular expression (mRNA & protein) of shelterin complex and its associated molecules have also been investigated. Results demonstrated that, TanI significantly inhibited proliferation of myeloma cells in dose and time dependent manner as observed through cytotoxicity assay. Additionally, induction of apoptosis by TanI and in combination with Len was observed in myeloma cells through propidium iodide (PI) staining, annexin V-FITC/PI staining, TUNEL and caspase-3/7 activity assays. Further, drug treatment significantly decreased (p < 0.01) TA and molecular expression of ACD, TERF2IP and TANK1 in comparison to vehicle control (0.1% DMSO) myeloma cells. Thus, this maiden in-vitro study provided initial evidences of therapeutic potential of TanI alone or in combination with chemotherapeutic agent Len as novel anticancer agents in myeloma cells which need further evaluation in future. Lastly, down-regulation of TA and decreased expression of these molecules underscores their potential as plausible therapeutic targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy which involves excessive proliferation of atypical plasma cells infiltrating exclusively in the bone marrow microenvironment. They are further characterized by secretion of atypical monoclonal proteins in blood and urine, renal dysfunction, anemia etc. MM is represented by 13% of hematological malignancies and 1% of total neoplastic diseases [1, 2]. Despite several advances in treatment of myeloma, majority patients relapse after successful remission of the disease [3]. Therefore, this further prompts us to continue our search for novel therapeutics.
Telomeres are nucleoprotein entities, which safeguards chromosomal ends from nucleolytic degradation, end to end fusion and repair/recombination mechanisms [4]. In human, length of this telomeric DNA is maintained by human telomerase reverse transcriptase (htert), a ribonucleoprotein, which sequentially adds TTAGGG telomeric repeats to chromosomal ends [5]. Normal somatic cells display minimal levels of telomerase activity (TA) causing telomere shortening with progressive cell divisions, thus leading to cellular senescence [6], whereas majority cancers (> 85% of cancers) demonstrate very high TA due to reactivation of telomerase which provides them uncontrolled proliferative potential by preventing telomere shortening with every cell division [7]. Few studies stated that higher TA correlated significantly with lower progression free survival [8,9,10]. One of our published reports in MM showed that high TA correlated positively with disease severity and can be used in better prediction of disease progression [10]. Further, due to shorter telomere length of malignant cells than normal somatic cells, they are more prone to earlier apoptosis following telomerase inhibition. Multiple approaches such as G-quadruplex interacting agents, siRNAs targeting hTERT have shown significant apoptosis and/or senescence in variety of human immortal and cancer cells including MM, in-vitro studies [8, 9]. Thus, this property of human telomerase reverse transcriptase holds great potential to be used as suitable therapeutic target.
Further, telomeres are wrapped around by group of proteins essentially known as Shelterin complex. The core of complex is made up of six proteins namely telomeric repeat binding factor 1 and 2 (TERF1, TERF2), protection of telomeres 1 (POT1), adrenocortical dysplasia homolog (ACD), telomeric repeat-binding factor 2-interacting protein 1 (TERF2IP) and TERF1 & TERF2 interacting nuclear factor 2 (TIN2) which in association with other proteins; Tankyrase 1 (TANK1) and PIN2/TERF1 interacting, telomerase inhibitor 1 (PINX1); prevents telomeres from undergoing recombination, repair and end-to-end fusion, thus facilitates telomere length maintenance and its regulation [11]. In our previous study, we have shown alteration in expression levels of shelterin complex and its associated molecules in MM patients with special emphasis on TERF2 [10]. Further, a crucial molecule ACD plays critical role in telomere length maintenance by recruiting telomerase at telomeres and acts as positive regulator of telomere length [12]. Few studies reported that molecular expression of ACD was significantly higher in HepG2 resistant cells and its overexpression in HCT116 colorectal cancer cells increased radio-resistance whereas its down-regulation promotes apoptosis [13, 14]. Next, TERF2IP is a crucial molecule reported to play significant role in regulating TERF2-mediated DNA damage response in gastric cancer cells treated by etoposide [15]. In addition, two shelterin complex associated molecules, TANK1 which is an ADP ribosylation protein, promotes elongation of telomere length by telomerase [16] and PINX1, negatively regulates telomere length by preventing telomerase from elongating telomere length [17]. Both molecules play significant roles in regulating tumorigenesis [16, 17]. Thus, these studies emphasized importance of these molecules in tumor causation and progression. In addition to telomerase, significant involvement of shelterin complex and its associated molecules in cancer can be exploited as suitable therapeutic targets.
Recent advancements in development of novel agents such as proteasome inhibitor bortezomib (BTZ) and immunomodulator lenalidomide (Len) have significantly improved the outcome of disease. Still prognosis is not in favor of patients and complete remission is out of reach [18]. Moreover, these drugs show significant side-effects which weakens the overall quality-of-life of patients [19]. Therefore, identification of novel therapeutic agents which decreases toxicity caused due to these drugs and will improve the clinical outcome of MM is required.
Natural products are rich resources for identifying new lead compounds as anti-cancer agents [20, 21]. Among various types of molecules with anti-cancer activities, terpenes and terpenoids have been well documented. Further, tanshinone I (TanI) an important component of Chinese herb Salvia miltiorrhiza was earlier used for treatment of cardiovascular diseases and has recently demonstrated significant anti proliferative and cytotoxic potential in different cancer types [22, 23]. Additionally few studies unraveled the potential of TanI along with other tanshinones in decreasing telomerase expression in cancer cells thus provided impetus to investigate its effect in myeloma cancer cells [24, 25]. Further, we also investigated the effect of combination of TanI and a chemotherapeutic drug Len on anti-proliferative properties of myeloma cells.
This maiden attempt aimed to study the anti-proliferative effects of TanI, Len alone and their combination on myeloma cells. Further, effects of TanI and its combination with Len on levels of TA and molecular expression of above discussed molecules have also been investigated. This study will make an attempt to establish an association between apoptosis induction with alteration of TA and molecular expression of shelterin complex molecules in myeloma cells.
Materials and methods
Chemicals and reagents
TanI and Len were purchased from AdooQ biosciences (Irvine, CA). Stock solutions were made in DMSO and stored at − 20 °C until used. MTT (3-(4, 5-dimethyl thizol-2-yl)-2, 5-diphenyl tetrazolium bromide), dimethyl sulfoxide (DMSO), RPMI-1640 medium, JC1, RNase, and Propidium Iodide (PI) were purchased from Sigma-Aldrich Co. (St. Louis, MO). Annexin-V FITC/PI apoptosis detection kit from BioLegend (San Diego, CA) and Caspase-3/7 activity kit from Promega (Madison, WI) were procured. Primary and secondary antibodies were purchased from Santa Cruz Biotechnologies (Dallas, TX) and Abcam (Cambridge, UK). All other chemicals were of culture grade and commercially available.
Cell culture
RPMI8226 and U266 cell lines were obtained from ATCC (American type culture collection, Manassas, VA). Cells were grown in RPMI-1640 medium containing 2.0 mM l-glutamine, 10.0 mM HEPES, 1.0 mM sodium pyruvate, 4.5 g/L glucose, and 1.5 g/L sodium bicarbonate at 37 °C humidified incubator with 5.0% CO2. Media was complemented with 10% and 15% fetal bovine serum (Himedia, Maharashtra, India) for RPMI8226 and U266 cell lines respectively and 1× Pen-Strep (Thermo Fisher Scientific, Waltham, MA) was added to medium to inhibit microbial growth.
Cytotoxicity assessment using MTT
Myeloma cells (RPMI8226 and U266) were seeded in quadruplets in 96-well plate at a density of 1.0 × 105 cells/mL and 1.5 × 105 cells/mL respectively and treated with different concentrations of TanI (0.625, 1.25, 2.5, 5 µM) and Len (15.125, 31.25, 62.5, 125, 250 µM) for 24 h, 48 h and 72 h duration. After incubation is over, 10.0 µL of MTT (5.0 mg/mL) was added and incubated at 37 °C for 4 h. Crystals were dissolved in 100 µL of DMSO and read at 595 nm wavelength using spectrophotometer. Percentage survival was calculated as function of concentration keeping untreated cells as 100% viable.
Cell cycle analysis
Cell cycle distribution was analyzed by flow cytometry using PI staining. Briefly, 1.0 × 105 cells/mL and 1.5 × 105 cells/mL of RPMI8226 and U266 respectively of myeloma cells were synchronized in RPMI-1640 medium supplemented with 1% FBS for 12 h and then treated with different concentrations of TanI, Len and their combination for 24 h in complete medium (RPMI 1640 medium complemented with 10% FBS). After incubation, cells were washed twice and fixed with 70% ethanol overnight at – 20 °C, then stained with 200 µL of PI (100 µg/mL) for 20 min in darkness. Stained cells were acquired using BD FACSCanto (Franklin lakes, NJ) flow cytometer and analyzed with BD FACSDiva software.
Annexin-V/propidium iodide assay
Similarly in this assay about 1.0 × 105 cells/mL and 1.5 × 105 cells/mL of RPMI8226 and U266 respectively of myeloma cells were synchronized in RPMI-1640 medium supplemented with 1% FBS for 12 h and then after drug treatment, cells were harvested, washed twice with ice-cold 1× PBS and stained with Annexin-V FITC/PI dyes in Annexin V-FITC buffer and incubated for 30 min in darkness. The percentage of apoptotic cells were acquired using BD FACSCanto flow cytometer and analyzed by BD FACSDiva Software.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Furthermore in TUNEL assay, approximately 1.0 × 105 cells/mL and 1.5 × 105 cells/mL of RPMI8226 and U266 respectively of myeloma cells were synchronized in RPMI-1640 medium supplemented with 1% FBS for 12 h and then treated with drugs for 24 h. After incubation is over cells were harvested, fixed and permeabilised with 0.1% Triton X-100 in ice cold PBS. Cells were stained with FITC-tagged terminal deoxynucleotidyl transferase enzyme and incubated for 30 min in dark. Negative and positive controls were used with treatment groups. In here, positive control means both myeloma cells (RPMI8226 & U266) populations were treated with DNase I recombinant enzyme (30 U/mL; Fermentas, Thermo Fisher Scientific, Waltham, MA) for 10 min at room temperature and then stained as per the manufacturer’s instruction for further analysis. Cells were acquired and then analyzed in BD FACSDiva software.
Caspase-3/7 activity assay
Caspase-3/7 activity was detected using a luminometry based assay. In brief, 1.0 × 105 cells/mL and 1.5 × 105 cells/mL of RPMI8226 & U266 myeloma cells respectively were plated in 96-well plate and treated with different concentrations of TanI, Len and their combination for 24 h. After incubation, equal volume of premixed Apo-Glo™ assay reagent was added to each well and incubated in dark at 25 °C for 1 h. Samples were treated in triplicates. Luminescence was measured using luminometer (BioTek, Winooski, VT). Their activity was calculated and expressed in AU (arbitrary units).
Telomerase activity (TA)
After treatment is over, cells were harvested, lysed using lysis buffer and quantified using Bradford reagent with minor modifications [26]. TA was performed and analyzed using TeloTAAGGG Telomerase PCR ELISAPLUS (Roche, Basel, Switzerland) kit following protocol described in our previously published research article [10].
Real time PCR
Briefly, myeloma cells were drug treated for 24 h and then harvested for RNA isolation. Rest of experiments beginning from primers selection to real time PCR assay were performed and analyzed in accordance to protocols mentioned in our previous paper [10].
Western blot
After treatment of myeloma cells for defined duration, rest of experiments were performed and analyzed in accordance to protocols explained in our previous paper [10]. Experiments were repeated three times using β-Actin as loading control.
Statistical analysis
Unless otherwise indicated, all experiments were repeated at least three times and the differences were determined by two tailed Student’s t test. When statistical differences between more than 2 groups were analyzed, one-way ANOVA followed by Dunnett’s test was performed. Results were presented as mean ± standard deviation (SD) of three independent experiments. p value < 0.05 was considered as statistically significant.
Results
Anti-proliferative effects of TanI and Len on myeloma cells
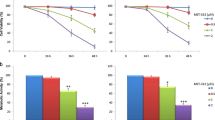
To determine efficacies of TanI and Len as anti-proliferative agents, we assessed the effects of TanI and Len on viability of myeloma cells using MTT dye for 24 h, 48 h and 72 h duration. TanI demonstrated significant decrease (p < 0.05) in survival of these myeloma cells in both time and dose dependent manner in comparison to vehicle (0.1% DMSO) treated cells at all time points. Inhibitory concentration (IC50) of TanI was determined as 2.0 µM for RPMI8226 and 2.5 µM for U266 cells at 24 h. Len is an immuno-modulatory drug that did not show any cytotoxicity on myeloma cells at all time points. Hence, we have used 10.0 µM of Len for all future experiments. Therefore, these results demonstrated that TanI significantly inhibited growth of myeloma cells while Len did not show significant effects (Fig. 1).
Determination of IC50 of TanI and Len on myeloma cell lines: Different concentrations of TanI and Len were given to RPMI8226, U266 myeloma cells for 24 h, 48 h and 72 h in 96 well plates. After incubation is over, MTT dye was added and IC50 was calculated from the graph plotted for percentage (%) survival versus drug concentration in both myeloma cells
Effects of TanI, Len alone or in combination on cell cycle analyses in myeloma cells
Effects of TanI, Len and their combination on cell cycle progression were studied in RPMI8226 and U266 myeloma cell lines using PI staining. As shown in Fig. 2, after treatment for 24 h in RPMI8226 myeloma cells, significant apoptosis was observed at both doses; TanI 1.7 µM (20.6 ± 3.0%; p < 0.05) and TanI 2.0 µM (32.6 ± 3.1%; p < 0.05), in comparison to vehicle treated cells, whereas combination of TanI 2.0 µM ± Len 10.0 µM (35.9 ± 5.4%; p < 0.05) demonstrated significantly increased apoptosis. Similarly, in U266 myeloma cells, significant increase in cell death was observed only at TanI 2.5 µM (15.0 ± 2.1%; p < 0.05) dose in comparison to vehicle treated cells. Len alone did not show significant change in apoptosis i.e. sub G0/G1 phase of cell cycle. Thus, our results revealed that TanI dose-dependently increased the percentage of cells in sub-G0/G1 phase in treatment group cells. Combination of TanI and Len did not show much difference in cells population in sub-G0/G1 phase compared to TanI treatment alone. Further, this dramatic increase in percentage of cells in sub G0/G1 phase prompted us to check apoptosis in these cells.
Effects of TanI and Len alone or their combination treatment on cell cycle analysis of myeloma cells. a, c Represents histograms; b, d bar graphs showing cell cycle analyses in RPMI8226 and U266 myeloma cells respectively after treatment with TanI and Len alone or their combination for 24 h and analyzed using propidium iodide staining assay. Values are presented as mean ± SD of three independent experiments. *p < 0.05 versus vehicle (0.1% DMSO) treated cells
Combination of TanI and Len induced apoptosis in myeloma cells as detected by AnnexinV-FITC/PI and TUNEL assay
Similarly to cell cycle assay, significantly increased (p < 0.01) percentage of apoptotic cells were seen with increasing dose of TanI in comparison to vehicle treated myeloma cells. In RPMI8226 cells at 1.7 µM of TanI (24.4 ± 1.2%; p < 0.05) and 2.0 µM TanI (40.0 ± 3.9%; p < 0.05) percentage of apoptotic cells were observed after 24 h drug treatment. Similarly, in U266 cells at 2.0 µM TanI (10.4 ± 3.2%; p < 0.05) and at 2.5 µM TanI (20.5 ± 3.9%; p < 0.05) percentage of apoptotic cells were observed after 24 h. Additionally, combination of TanI and Len further increased the percentage of apoptotic cells in both myeloma cells (RPMI8226: 46.1 ± 3.1%; U266: 30.4 ± 5.6%) (Fig. 3).
Effects of TanI and Len alone or their combination treatment on apoptosis of myeloma cells and analyzed using Annexin V/PI assay. a, c Representative scatter plots; b, d bar graphs showing percentage of RPMI8226 and U266 myeloma cells respectively undergoing apoptosis after treatment with TanI and Len alone or their combination for 24 h. Number of apoptotic cells was determined after FITC labeled annexin V and PI staining by FACS analysis. Values are presented as mean ± SD of three independent experiments. *p < 0.05 versus vehicle (0.1% DMSO) treated cells
Further, to prove apoptotic effects of TanI and Len on myeloma cells, we studied apoptosis through TUNEL assay. These results demonstrated that, both single and combination of drug treatment, significantly increased (p < 0.05) the percentage of TUNEL-FITC positive cells (as observed through mean fluorescence intensity) in comparison to vehicle treated cells (Fig. 4). These TUNEL positive cells demonstrated presence of DNA fragmentation and indicated population of cells undergoing apoptosis. Taken together, combination treatment approach induced apoptosis more potentially than single drug approach in myeloma cells. Hence, these results provided evidences that mechanism of cells undergoing death was predominantly apoptosis.
Histograms and bar graphs showing the representative images of TUNEL assay performed on myeloma cells. a, c Representative histograms; b, d bar graphs showing percentage of RPMI8226 and U266 myeloma cells respectively undergoing apoptosis after treatment with TanI and Len alone or their combination for 24 h. Values are presented as mean ± SD of three independent experiments. *p < 0.05 versus vehicle (0.1% DMSO) treated cells
Effects of TanI, Len and their combination on Caspase-3/7 activity in myeloma cells
To further substantiate the effects of drug treatment on apoptosis of myeloma cells, caspase-3/7 activity was studied in a luminescence based assay. Results revealed that significantly increased (p < 0.05) caspase-3/7 activity was observed with increasing dose of TanI in RPMI8226 (TanI 1.7 µM: 9236 ± 521.2; TanI 2.0 µM: 13,769.5 ± 940.3) in comparison to vehicle cells (5078 ± 128.3). Similar results were observed with U266, (TanI 2.0 µM: 6679 ± 446.4; TanI 2.5 µM: 7551 ± 365.3) in comparison to vehicle treatment (2095 ± 117.3). Further, Len treatment alone could not significantly changed caspase-3/7 activity while combination treatment of TanI and Len increased (RPMI8226 TanI 2.0 µM + Len 10.0 µM: 14197 ± 621.2; U266 TanI 2.5 µM + Len 10.0 µM: 7823 ± 199.2) their activity compared to when TanI used as single drug regime in both myeloma cells (Fig. 5a, b).
Determination of caspase-3/7 enzymatic activity and telomerase activity after treatment of myeloma cells with TanI, Len alone or in combination for 24 h. a, b Bar graphs showed the caspase-3/7 activity in RPMI8226 and U266 myeloma cells respectively. c, d Bar graphs showed telomerase activity (TA) in both myeloma cells (RPMI8226 and U266) respectively. Caspase-3/7 activity was represented in RLU. Experiment was performed in triplicates and mean ± SD was calculated from the triplicates. RLU relative luminescence unit; p < 0.05 versus vehicle (0.1% DMSO) treated cells
Inhibition of telomerase activity by TanI, Len and their combination in myeloma cells
TA measured in myeloma cells after drug treatment revealed that with increasing dose of TanI significantly reduced (p < 0.05) TA from 100% (vehicle treated) to 85% at 1.7 µM TanI and 72% at 2.0 µM TanI in RPMI8226 cells, whereas in U266 cells, TA was reduced to 83% and 75% at 2.0 µM and 2.5 µM TanI respectively in comparison to vehicle treated cells. Further, Len alone did not show significant change in TA in both myeloma cells, whereas combination of TanI and Len, displayed significant decrease (p < 0.05) in TA (RPMI8226: 65%, U266: 68%) in both myeloma cells (Fig. 5c, d). Thus combination of drug was much more potent in decreasing TA compared to TanI or Len alone.
Effects of TanI, Len and their combination on molecular expression (mRNA & protein) of shelterin complex & its associated molecules in myeloma cells
Shelterin complex plays significant role in regulating access of telomerase on telomere, thereby helps in maintaining and regulating telomere length in cancer cells. Thus, we further checked the effects of these drugs alone or in combination on mRNA and protein expression of shelterin complex along with its associated molecules in both myeloma cells. It was observed that both TanI alone or in combination with Len demonstrated significantly decreased (p < 0.05) relative mRNA and protein expression of ACD, TERF2IP and TANK1 genes in RPMI8226 cells in comparison to vehicle treated cells. Similarly in U266 cells, only ACD and TANK1 showed significant decrease (p < 0.05) in mRNA and protein expression in comparison to vehicle control. Additionally, significantly increased (p < 0.05) relative mRNA expression of PINX1 was observed in both cell lines with increasing dose of TanI and combination of TanI and Len respectively. The representative image of relative mRNA and protein expression of all studied molecules after drug treatment have been shown in Figs. 6a, b and 7a–f respectively.
Relative mRNA expression determination of shelterin complex and its associated molecules using real time PCR analysis. Effects of TanI, Len alone or their combination treatment for 24 h on relative mRNA expression in a RPMI8226 and b U266 myeloma cells was studied. GAPDH was used for gene expression normalization. Values are presented as mean ± SD of three replicates. *p < 0.05 versus vehicle (0.1% DMSO) treated cells. TERF1 and TERF2 telomere repeat binding factor 1 and 2, POT1 protection of telomere 1, ACD adrenocortical dysplasia homolog, TERF2IP telomeric repeat-binding factor 2-interacting protein 1, TIN2 TERF1 & 2 interacting protein 2, TANK1 tankyrase 1 and PINX1 PIN2/TERF1 interacting telomerase inhibitor 1
Effects of TanI, Len alone or their combination of drug treatment on protein expression of shelterin complex and its associated molecules in myeloma cells. Representative images of western blots in a, b for RPMI8226; d, e for U266 myeloma cells and c, f showed bar graphs demonstrating relative fold change in expression of proteins in RPMI8226 & U266 myeloma cells respectively. Cell lysate containing equal amounts of total cell protein were resolved on 10% SDS–PAGE and processed downstream for western blotting using anti-TERF1, anti-TERF2, anti-POT1, anti-TIN2, anti-ACD, anti-TERF2IP, anti-TANK-1, anti-PINX1, and anti-β-Actin antibodies. Relative fold intensity was calculated using densitometry analysis and β-Actin was used as internal control to normalize for equal loading. Values are presented as mean ± SD of three independent experiments. *p < 0.05 versus vehicle treated cells. TERF1 and TERF2 telomere repeat binding factor 1 and 2, POT1 protection of telomeres 1, ACD adrenocortical dysplasia homolog, TERF2IP telomeric repeat-binding factor 2-interacting protein 1, TIN2 TERF1 & 2 interacting protein 2, TANK1 tankyrase 1, PINX1 PIN2/TERF1 interacting telomerase inhibitor 1 and kDa kilodalton
Discussion
In the present study we have revealed that TanI, a component of Chinese herb, Salvia miltiorrhiza, displayed significant anti-proliferative and cytotoxic potential on myeloma cancer cells through induction of apoptosis, in-vitro. Apoptotic properties of TanI on myeloma cells were visualized through different techniques including PI staining, TUNEL assay, caspase-3/7 activity and annexin V/PI staining assays. Further, this maiden study showed that TanI alone or in combination with Len significantly down-regulated TA and modulated expression of shelterin complex molecules thus indicated their involvement in apoptosis induction after drug treatment in myeloma cells.
Various mechanistic studies demonstrated that TanI inhibited growth of cancer cells either by cell cycle arrest or apoptosis induction [22, 23]. Our results are in concordance with previous reports demonstrating apoptosis induction as one of the molecular mechanisms in inhibiting myeloma cells growth [21, 27, 28]. Our study showed significantly increased caspase-3/7 activity and enhanced DNA fragmentation which is in accordance with previous studies performed on breast and prostate cancer cells [21, 29].
Telomerase reactivation has been observed in majority of cancer cell types and its role as potential therapeutic target has been extensively studied in the last decade [6, 7]. Several studies reported telomerase repression to apoptosis induction in both solid and hematological malignancies including MM [29,30,31]. Liu et al. showed that TanI down-regulated TA and up regulated levels of caspase-3 accompanied with significant apoptosis in monocytic leukemic cells [24]. Further Song et al. reported that TanII inhibited cell proliferation by decreasing TA in leukemic cells [32]. Additionally our previous study showed that TA correlated positively with disease progression [10]. Thus, these reports prompt us to investigate anti-proliferative effects of TanI on myeloma cells via modulation of TA. Present study displayed that TanI dose-dependently increased the apoptosis with simultaneous decrease in TA in both myeloma cells, thus suggesting involvement of TA axis in mediating cell death in myeloma cells. Further combination of TanI and Len significantly down-regulated the TA. Thus, our results are in concordance with previously published reports and suggest that reduction in TA might play crucial roles in apoptosis induction and further proposed its prospects as suitable therapeutic target.
Recent studies have mentioned significant involvement of shelterin complex along with its associated molecules in disease progression in multiple cancers including MM [10,11,12,13,14], thus we investigated effects of TanI alone and in combination with Len on their expression levels. Our results showed that TanI dose dependently decreased mRNA and protein expressions of ACD, TERF2IP and TANK1 in both myeloma cells with simultaneous increase in apoptosis. Other molecules showed insignificant change in their expression. Similarly studies on human laryngeal and colorectal carcinoma reported that silencing the expression of ACD leads to decreased cells proliferation and drug resistance in these cells [12, 13]. In addition to that, studies also illustrated that silencing TANK1 in lung adenocarcinoma cells inhibited their proliferation by inducing apoptosis and reducing their migration [15]. Thus, our results are in accordance with these reports and substantiated them as potential therapeutic targets.
Len is an immuno-modulatory drug that demonstrates its anti-myeloma effects through several mechanisms such as anti-angiogenic, pro-apoptotic, anti-proliferative and immuno-modulation [33]. Recent study on myeloma cells reported that combination of Len with arsenic trioxide significantly sensitizes these cells to apoptosis than Len alone by up-regulating cereblon expression [34]. Our results confirmed that combination of Len and TanI significantly induced cell death compared to Len alone. Also effects were visible through modulation in TA and expression levels of shelterin complex molecules.
In conclusion, present study demonstrated that TanI induces significant dose dependent anti-proliferative and cytotoxic effects in myeloma cells. In vitro results showed that combination of TanI with standard chemotherapeutic drug Len, potentiates cell death in myeloma cells suggesting crosstalk in signaling pathways which needs further evaluation. Further, apoptosis is the main pathway followed by TanI for inhibiting myeloma cells growth. Apoptotic effects of TanI alone or in combination with Len were accompanied with decreased in TA and molecular expression of ACD, TERF2IP and TANK1 in myeloma cells, thereby identified them as prospective therapeutic targets. This maiden attempt substantiated the therapeutic potential of TanI in myeloma cells which needs to be established by preclinical studies in future.
References
Munshi NC, Anderson KC (2013) New strategies in the treatment of multiple myeloma. Clin Cancer Res 19:3337–3344. https://doi.org/10.1158/1078-0432.CCR-12-188
Bianchi G, Richardson PG, Anderson KC (2015) Promising therapies in multiple myeloma. Blood 126:300–310. https://doi.org/10.1182/blood-2015-03-575365
Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, Haessler J, Feather J, Hoering A, Moreau P, LeLeu X (2012) Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia 26:149–157. https://doi.org/10.1038/leu.2011.196
Day JP, Marder BA, Morgan WF (1993) Telomeres and their possible role in chromosome stabilization. Environ Mol Mutagen 22:245–249. https://doi.org/10.1002/em.2850220411
Blackburn EH, Greider CW, Henderson E, Lee MS, Shampay J, Lentzen DS (1989) Recognition and elongation of telomeres by telomerase. Genome 31:553–560. https://doi.org/10.1139/g89-104
Shay JW (1997) Telomerase in human development and cancer. J Cell Physiol 173:266–270. https://doi.org/10.1002/(SICI)1097-4652(199711)173:2%3C266::AID-JCP33%3E3.0.CO;2-B
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015. https://doi.org/10.1126/science.7605428
Park HS, Choi J, See CJ, Kim JA, Park SN, Im MSK, Kim SM, Lee DS, Hwang SM (2017) Dysregulation of telomere lengths and telomerase activity in myelodysplastic syndrome. Ann Lab Med 37:195–203. https://doi.org/10.3343/alm.2017.37.3.195
Miyazaki Y, Yoshida N, Nozaki T, Inoue H, Kikuchi K, Kusamaet K (2015) Telomerase activity in the occurrence and progression of oral squamous cell carcinoma. J Oral Sci 57:295–303. https://doi.org/10.2334/josnusd.57.295
Kumar R, Khan R, Gupta N, Seth T, Sharma A, Kalaivani M, Sharma A (2018) Identifying the biomarker potential of telomerase activity and shelterin complex molecule, telomeric repeat binding factor 2 (TERF2), in multiple myeloma. Leuk Lymphoma 59:1677–1689. https://doi.org/10.1080/10428194.2017.1387915
De Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Gene Dev 19:2100–2110. https://doi.org/10.1101/gad.1346005
Zhong FL, Batista LFZ, Freund A, Pech MF, Venteicher AS, Artandi SE (2012) TPP1 OB-fold domain controls telomere maintenance by recruiting telomerase to chromosome ends. Cell 150:481–494. https://doi.org/10.1016/j.cell.2012.07.012
Tang T, Zhou FX, Lei H, Yu HJ, Xie CH, Zhou YF, Liu SQ (2009) Increased expression of telomere related proteins correlates with resistance to radiation in human laryngeal cancer cell lines. Oncol Rep 21:1505–1509. https://doi.org/10.3892/or00000381
Yang L, Wang W, Hu L, Yang X, Zhong J, Li Z, Yand H, Lei H, Yu H, Liao ZK, Zhou F, Xie C, Zhou Y (2013) Telomere-binding protein TPP1 modulates telomere homeostasis and confers radio resistance to human colorectal cancer cells. PLoS ONE 8:e81034. https://doi.org/10.1371/journal.pone.0081034
Li X, Liu W, Wang H, Yang L, Li Y, Wen H, Ning H, Wang J, Zhang L, Li J, Fan D (2015) Rap1 is indispensable for TRF2 function in etoposide-induced DNA damage response in gastric cancer cell line. Oncogenesis 4:e144, 1–5. https://doi.org/10.1038/oncsis.2015.1
Lu H, Lei Z, Lu Z, Lu Q, Lu C, Chen W, Wang C, Tang Q, Kong Q (2013) Silencing tankyrase and telomerase promotes A549 human lung adenocarcinoma cell apoptosis and inhibits proliferation. Oncol Rep 30:1745–1752. https://doi.org/10.3892/or.2013.2665
Wang S, Zhang H, Zhu J, Li C, Zhu J, Bowen S, Zhang B, Wang C (2017) PinX1 is a potential prognostic factor for non-small-cell lung cancer and inhibits cell proliferation and migration. Biomed Res Int 2017:1–9. https://doi.org/10.1155/2017/7956437
Turner JG, Dawson J, Emmons MF, Cubitt CL, Kauffman M, Shacham S, Hazlejurst LA, Sullivan DM (2013) CRM1 inhibition sensitizes drug resistant human myeloma cells to topoisomerase II and proteasome inhibitors both in vitro and ex vivo. J Cancer 4:614–625. https://doi.org/10.7150/jca.7080
Nooka AK, Kastritis E, Dimopoulos MA, Lonial S (2015) Treatment options for relapsed and refractory multiple myeloma. Blood 125:3085–3099. https://doi.org/10.1182/blood-2014-11-568923
Normile D (2003) The new face of traditional Chinese medicine. Science 299:188–190. https://doi.org/10.1126/science.299.5604.188
Wang M, Cao J, Zhu JY, Qiu J, Zhang Y, Shu B, Ou TM, Tan JH, Gu LQ, Huang ZS, Sheng Y, Li D (2017) Curcusone C induces telomeric DNA-damage response in cancer cells through inhibition of telomeric repeat factor 2. BBA 1865:1372–1382. https://doi.org/10.1016/j.bbapap.2017.08.022
Li Y, Gong Y, Li L, Abdolmaleky HM, Zhou JR (2013) Bioactive tanshinone I inhibits the growth of lung cancer in part via downregulation of Aurora A function. Mol Carcinogen 52:535–543. https://doi.org/10.1002/mc.21888
Lee CY, Sher HF, Chen HW, Liu CC, Chen CH, Lin CS, Yang PC, Tsay HS, Chen JJ (2008) Anticancer effects of tanshinone I in human non-small cell lung cancer. Mol Cancer Ther 7:3527–3538. https://doi.org/10.1158/1535-7163.MCT-07-2288
Liu XD, Fan RF, Zhang Y, Yang HZ, Fang ZG, Guan WB, Lin DJ, Xiao RZ, Huang RW, Huang HQ, Liu PQ (2010) Down-regulation of telomerase activity and activation of caspase-3 are responsible for tanshinone I-induced apoptosis in monocyte leukemia cells in vitro. Int J Mol Sci 11:2267–2280. https://doi.org/10.3390/ijms11062267
Soares J, Keppler BR, Wang X, Lee KH, Jarstfer MB (2011) Ortho-Quinone tanshinones directly inhibit telomerase through an oxidative mechanism mediated by hydrogen peroxide. Bioorg Med Chem Lett 21:7474–7478. https://doi.org/10.1016/j.bmcl.2011.09.112
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Liu JJ, Zhang Y, Lin DJ, Xiao RZ (2009) Tanshinone IIA inhibits leukemia THP-1 cell growth by induction of apoptosis. Oncol Rep 21:1075–1081. https://doi.org/10.3892/or_00000326
Shin EA, Sohn EJ, Won G, Choi JU, Jeong M, Kim B, Kim MJ, Kim SH (2014) Upregulation of microRNA135a-3p and death receptor 5 plays a critical role in Tanshinone I sensitized prostate cancer cells to TRAIL induced apoptosis. Oncotarget 5:5624–5636. https://doi.org/10.18632/oncotarget.2152
Bashash D, Delshad M, Safaroghli-Azar A, Safa M, Momeny M, Ghaffari SH (2017) Novel pan PI3K inhibitor-induced apoptosis in APL cells correlates with suppression of telomerase: an emerging mechanism of action of BKM120. Int J Biochem Cell Biol 91:1–8. https://doi.org/10.1016/j.biocel.2017.08.009
Weiss C, Uziel O, Wolach O, Nordenberg J, Beery E, Bulvick S, Kanfer G, Cohen O, Ram R, Bakhanashvili M, Magen-Nativ H (2012) Differential downregulation of telomerase activity by bortezomib in multiple myeloma cells-multiple regulatory pathways in vitro and ex vivo. Br J Cancer 107:1844–1852. https://doi.org/10.1038/bjc.2012.460
Shammas MA, Koley H, Bertheau RC, Neri P, Fulciniti M, Tassone P, Blotta S, Protopopov A, Mitsiades C, Batchu RB, Anderson KC (2008) Telomerase inhibitor GRN163L inhibits myeloma cell growth in vitro and in vivo. Leukemia 22:1410–1418. https://doi.org/10.1038/leu.2008.81
Song YYSL, Yuan SL, Yang YM, Wang XJ, Huang GQ (2005) Alteration of activities of telomerase in tanshinone IIA inducing apoptosis of the leukemia cells. Zhongguo Zhong Yao Za Zhi 30:207–211
Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, Prince HM (2010) Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 24:22–32. https://doi.org/10.1038/leu.2009.236
Jian Y, Gao W, Geng C, Zhou H, Leng Y, Li Y, Chen W (2017) Arsenic trioxide potentiates sensitivity of multiple myeloma cells to lenalidomide by upregulating cereblon expression levels. Oncol Lett 14:3243–3248. https://doi.org/10.3892/ol.2017.6502
Acknowledgements
Financial assistance to Mr. Raman Kumar as senior research fellow by Indian Council of Medical Research (ICMR), New Delhi, India is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any study with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kumar, R., Gupta, N., Himani et al. Novel combination of tanshinone I and lenalidomide induces chemo-sensitivity in myeloma cells by modulating telomerase activity and expression of shelterin complex and its associated molecules. Mol Biol Rep 45, 2429–2439 (2018). https://doi.org/10.1007/s11033-018-4409-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4409-z