Abstract
The green microalga, Tetraselmis suecica, is commonly used in scientific, industrial, and aquacultural purposes because of its high stress tolerance and ease of culture in wide spectrums of environments. We hypothesized that carotenoids help to protect Tetraselmis cells from environmental stress by regulating genes in biosynthetic pathways. Here, we determined three major carotenogenic genes, phytoene synthase (PSY), phytoene desaturase (PDS), and β-lycopene cyclase (LCY-B) in T. suecica, and examined the physiological parameters and gene expression responses when exposed to redox-active metals and non-redox-active metals. Phylogenetic analyses of each gene indicated that T. suecica clustered well with other green algae. Real-time PCR analysis showed that TsPSY, TsPDS, and TsLCY-B genes greatly responded to the redox-active metals in CuSO4 followed by CuCl2, but not to the non-redox-active metals. The redox-active metals strongly affected the physiology of the cells, as determined by cell counting, reactive oxygen species (ROS) imaging, and photosynthetic efficiency. This suggests that carotenoids protect the cells from oxidative damage caused by metals, thereby contributing to cell survival under various stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The green microalga Tetraselmis (Chlorodendrophyceae, Chlorophyta) is considered one of the best and most simple model organisms, owing to its ease of culture, and is widely used as a feed in the aquaculture industries [1]. Due to its high nutritional content, Tetraselmis is used as a feed for penaeid shrimps, mollusca larvae, Artemia, rotifers, and as a probiotic in fish [2]. Moreover, it has been recommended as a good source of single cell protein (SCP) [3, 4], and is rich in vitamin E, carotenoids, chlorophyll, and tocopherols [5]; hence it has been proposed as a food supplement in human and animal diets [6]. The pigment extract of Tetraselmis has been patented for its ability to enhance dermal pigmentation, reduce psoriasis lesions, and increase hair growth [6]. More recent studies reported that Tetraselmis extract reduces oxidative stress in human cells due to the presence of high levels of carotenoids [7], and has also been recently used for biofuel production [8, 9].
Most of the previous studies have reported that Tetraselmis sp. is a robust alga that survives in a wide range of pH, salinity, and temperature [9, 10]. In case of metals, Tetraselmis sp. had a much higher Kd value for Cu than other marine microalgae, which indicated that it adsorbed relatively more Cu to cell surfaces compared with other species [11, 12]. Hence, Tetraselmis sp. can tolerate a wide range of exposures to metals such as copper (Cu), nickel, and lead [13], and also has the capability to detoxify the metals that was briefly discussed by Nassiri et al. [14]. Recently, Tsai et al. [15] demonstrated that Tetraselmis can be adapted to grow even at 40 °C. This suggests that this microalga may have a great tolerance to a wide range of environmental stressors.
Tetraselmis cells may be able to survive with cellular defence mechanisms in such difficult environments because of two major antioxidant defence systems: (i) enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPx), glutathione reductase (GR), monodehydro ascorbate reductase (MDHAR), and dehydro ascorbate reductase (DHAR); and (ii) non-enzymatic antioxidants such as ascorbic acid (AA), reduced glutathione (GSH), α-tocopherol, carotenoids, flavonoids, and the osmolyte proline [16]. These two systems work together to scavenge ROS. Among these carotenoids are considered to be the first line of defence because of their capability to quench the singlet oxygen (1O2) and to scavenge other reactive species such as peroxyl radical (ROO·), hydroxyl radical (HO·), hypochlorous acid (HOCl), and anion peroxynitrite (ONOO–) [17, 18]. Previous environmental studies have shown that the microalgae can enhance carotenoid production when they are exposed to salt stress, intense light, UV-irradiation, nutrient deficiencies, and hormone treatment [19,20,21]. Therefore, microalgal carotenoids are capable of defending the cells from oxidative stress induced by adverse environmental stressors [18, 22, 23]. This implies that carotenoids protect the Tetraselmis cells against ROS production, which may cause the cells to become highly tolerant.
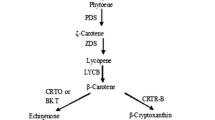
Biosynthetic pathways involved in carotenoid formation were elucidated in plants and algae (Fig. 1). In brief, the phytoene synthase (PSY) catalyses the condensation of two GGPP molecules into the first C40 carotenoid, phytoene, which is then desaturated into ζ-carotene by phytoene desaturase (PDS). Both PSY and PDS have also been proposed as regulatory points for carotenoid biosynthesis [24, 25]. Further desaturation and cyclization lead to the generation of β-carotene and other carotenoids. One key enzyme in this process is lycopene β-cyclase (LCY-B), which catalyses a two-step reaction that creates a β-ionone ring at each end of the lycopene molecule to produce astaxanthin, β-carotene and lutein [18, 26]. Hence, the expression of these genes may be important for the carotenoid biosynthetic pathway in microalgae, including Tetraselmis, for understanding the survival strategies after exposure to environmental stressors such as heavy metals (HMs). The exact mechanisms underlying the protective function of carotenoids in Tetraselmis after exposure to HMs, however, are still poorly understood. In addition, the types of protective mechanisms, such as physiological and genomic responses, have not been clearly revealed to date.

Modified from Sathasivam and Ki [5]. Asterisks represent the gene used for gene expression. (Color figure online)
Proposed carotenoid biosynthetic pathway in T. suecica. The enzymes involved in each enzymatic conversions reaction are showing upstream (red colour) and downstream (pink colour). IPP isopentyl diphosphate, IPI isopentyl pyrophosphate isomerase, DMAPP dimethylallyl diphosphate, GGPP geranylgeranyl pyrophosphate, GGPPS geranylgeranyl pyrophosphate synthase, PSY phytoene synthase, PDS phytoene desaturase ZDS-ζ-carotene desaturase, LCY-B lycopene β-cyclase, LCY-E lycopene ε-cyclase, BKT β-carotene ketolase, CrtR-B β-carotene hydroxylase.
In the present study, we evaluated the potential roles of carotenogenic genes of Tetraselmis suecica (T. suecica) in protecting the cell under stress from damage, in view of physiological and genomics perspectives. Particularly, we investigated the various physiological parameters, focusing on chlorophyll fluorescence characteristics, photosynthetic efficiency, ROS level, carotenoids production and its transcriptional changes of the pathway genes after exposure to redox-active metals and non-redox-active metals. In our previous studies, we found that T. suecica was highly tolerant to the HM contaminates [5, 13] and high thermal exposure (unpublished data). We hypothesized that this tolerance to HMs can occur through the expression of carotenoid biosynthetic pathway genes and its production. Elucidating the mechanism that contributes to HM tolerance may help to improve the efficiency of removing HMs from the aquatic environment.
Materials and methods
Growth and culture conditions
A strain (P-039) of T. suecica was obtained from the Korea Marine Microalgae Culture Center (Pukyong National University, Busan, Korea). The cells were cultured in an f/2 medium, using filtered seawater with additional macronutrients, vitamins and trace metals (CuSO4, ZnSO4, CoCl2, MnCl2, and NaMoO4) according to the method of Guillard and Ryther [27]. The cells were maintained at 20 °C, under a 12:12 h light: dark cycle with a photon flux density of ca. 65 µmol photons/m2/s.
Characterization and phylogenetic analysis of TsPSY, TsPDS and TsLCY-B amino acid sequence
Carotenoid gene sequences were obtained from the T. suecica EST data (741 K reads, 290 Mb) in our laboratory, where DNA sequences were determined by 454 pyrosequencing (GS-FLX Titanium; 454 Life Sciences, Roche, Branford, CT). Complete open reading frame (ORF) of each gene was further confirmed by polymerase chain reaction (PCR) and subsequent DNA sequencing. Molecular analyses were then performed using the deduced amino acid sequences. The sequence homology and predicted function for each T. suecica carotenoid genes were obtained by Basic Local Alignment Search Tool (BLAST) in the National Center for Biotechnology Information (NCBI) database [28]. The isoelectric point and theoretical molecular weight were determined by ProtParam software [29]. The SOPMA program and SignalP 4.0 Server were applied to predict secondary structures and analyze signal peptide, respectively [30, 31]. Phylogenetic trees of the TsPSY, TsPDS and TsLCY-B were separately constructed using the neighbor-joining (NJ) method in MEGA 7.0 [32]. The strength of the internal branches of the resulting trees was tested statistically by using bootstrap analysis with 1000 replications. For phylogenetic tree construction, heterokonts carotenogenic genes were used as outgroups.
Metal stress treatments
Exponential-phase T. suecica cultures were used for various stress induction experiments. As test toxic chemicals, we used redox-active metals (CuCl2, CuSO4 and K2Cr2O7) and non-redox-active metals (Al2SO4, CdCl2, CdSO4, NiSO4, PbCl2 and ZnSO4) because they are considered typical environmental contaminates. For exposure to toxic chemicals, we provided different concentrations of CuSO4 (1.0, 2.5, 5.0, 10.0, 25.0, and 50.0 mg/L) to the T. suecica cultures, considering the median effective concentration (EC50) of Cu (43.03 mg/L) according to Ebenezer and Ki [13]. For different time-course experiments, a concentration ten times less than the EC50 was subjected to treat the cells, i.e. 5.0 mg/L CuSO4 was used and the cells were harvested at different time intervals (6, 12, 24, 48, 72 h). For analysis of changes in gene expression with exposure to other HMs, the cultures were exposed to 5.0 mg/L of each HM and the cells were harvested after 24 h.
RNA extraction and cDNA synthesis
Cultured cells were harvested, immediately frozen in liquid nitrogen, and stored at − 80 °C until RNA extraction was performed. Total RNA was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA) and treated with DNAase (TaKaRa, Otsu, Japan). The concentration and purity of each RNA sample were measured using DU730 Life Science UV/vis spectrophotometer (Model V-730, Jasco, USA). The RNA samples with an absorption ratio ranging from 1.8 to 2.0 were used for subsequent experiments. For first-strand cDNA synthesis, the TOPscript™ RT DryMIX (dN6 plus) kit (Enzynomics, South Korea) was used according to the manufacturer’s protocol. Then, the first-strand cDNA templates were diluted 1:10 (v/v) with nuclease-free water for use in subsequent experiments.
Determination of TsPSY, TsPDS and TsLCY-B gene expression
Carotenoid gene expression profiles were measured by quantitative real-time (qRT) PCR. The primers used for qRT-PCR were as follows: TsPSY (forward: 5′-ACA CCA TTT CCA AGT TTC CG-3′; reverse: 5′-CTC GTC GTA GGT CTT GTA GCG-3′); TsPDS (forward: 5′-TGA ATG AGG ATG GGA GCG TG-3′; reverse: 5′-ACC GCT TCA TGA TGT CGC AG-3′); TsLCY-B (forward: 5′-GTA TGG CGT GAT GGT TGA GG-3′; reverse: 5′- GAG AAG GGC ATG ACG TAG AGG-3′). All qRT-PCR reactions were performed with the TOPreal™ qPCR 2X PreMIX SYBR Green Kit (TOP, Enzynomics Inc., Daejeon, Korea) in a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The qRT-PCR conditions were as follows: 4 min at 50 °C, 10 min at 95 °C, followed by 39 cycles of 10 s at 95 °C, 15 s at 65 °C, and 15 s at 72 °C. The specificity of amplification was verified through the creation and analysis of a melting curve from 65 to 95 °C. All the reactions were carried out in triplicates. The calculated mean value of the threshold cycle (CT) was used for evaluation of the relative fold change, following the method reported by Pfaffl [33]. The housekeeping gene α-tubulin (TUA) (forward: 5′-TAC GCG CCT GTT ATC TCT GC-3′; reverse: 5′-CGC ACT TGG CCA TCA TAG AG-3′) was used as the internal control [34].
Measurement of pigments and photosynthetic efficiency
Levels of chlorophyll a (Chl a) and carotenoids (CAR) were determined using a UV–Vis spectrophotometer (Model V-730, Jasco, USA). Specifically, 10 mL of the treated culture was filtered using Whatman GF/C filter paper and a vacuum pump filtration unit. The pigments were extracted from the filter paper in the dark using 90% acetone and their concentrations were estimated spectrophotometrically, following Sathasivam et al. [35]. In addition, for photosynthetic efficiency, culture samples (2 mL) exposed to various stressors were used to measure Chl a fluorescence with a Handy Plant Efficiency Analyser fluorometer (Handy PEA, Hansatech Instruments Ltd, Norfolk, UK). Measurements were taken following a dark-adaptation period of 20 min, which helped to oxidize the photosystem completely. The photosynthetic efficiency of all the samples was analyzed from the maximum quantum yield of PS II reaction centers; i.e., Fv/Fm = [Fm (maximal fluorescence yields) − Fo (minimal fluorescence yields)/Fm] [36].
Cell counting and ROS imaging
In all experiments, cell count was done under a light microscope (Carl Zeiss Axioskop; Oberkochen, Germany) by using hemocytometer (Neubauer, Hofheim, Germany).
Dihydroxyrhodamine 123 (DHR123-D1054; Sigma) staining was used to observe reactive oxygen species (ROS) production. Cells were treated with 1.0, 2.5 and 5.0 mg/L of CuSO4, whereas for other HMs 5.0 mg/L each metals were treated for 6, 24 and 72 h, were harvested. The harvested cells were stained with DHR123 for 1 h at a final concentration of 5.0 µg/L, harvested by centrifugation, and then washed twice with fresh f/2 medium. The cultures were resuspended in fresh f/2 medium and observed under a fluorescence microscope (Carl Zeiss Axioskop; Oberkochen, Germany) to determine the ROS production in single cells. The ROS combine with cellular components to oxidize DHR123 to rhodamine 123, which results in green fluorescence. The fluorescence intensity of ROS was quantified with ImageJ software (NIH; Bethesda, MD) using the fluorescence microscopy images.
Statistical analyses
The qRT-PCR results were analyzed using one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls Multiple Comparisons Test to determine the relative mRNA expression. These statistical analyses were performed using the Instant GraphPad software (GraphPad Software, Inc., CA). The difference was considered statistically at P < 0.05, and the data are presented as mean ± standard deviation (SD) values.
Results
Cloning and phylogenies of TsPSY, TsPDS, and TsLCY-B
Full open reading frames (ORFs) of TsPSY, TsPDS, and TsLCY-B (GenBank access Nos. MH457252, MH457251, and MH457250) were determined to be 1332, 1325, and 1038 bp in length, respectively (Supplementary Figs. 1, 2, 3). The TsPSY, TsPDS, and TsLCY-B ORFs encoded for proteins of 443, 440, and 345 amino acids with predicted molecular weights of 49.41, 48.23, and 38.09 kDa, and estimated isoelectric points of 5.89, 5.10, and 6.16, respectively. SignalP analyses showed that the maximal values of the original shearing site (C score), signal peptide (S score), and synthesized shearing site (Y score) were 0.130, 0.284, and 0.163 for TsPSY; 0.132, 0.162, and 0.116 for TsPDS; and 0.156, 0.126, and 0.127 for TsLCY-B, respectively. No signal peptides were detected in TsPSY, TsPDS, and TsLCY-B.
Neighbour-joining phylogenetic analyses revealed that all of the T. suecica carotenoid protein sequences were clustered to those of green algae (Fig. 2). In particular, in the tree of PSY, T. suecica formed a cluster with the green algae Coccomyxa subellipsoidea, whereas TsPDS formed a clade with the green alga Micromonas pusilla and Micromonas commoda. In addition, the clade attained by using the TsLCY-B sequence formed a separate branch from that of the other green algae, whereas heterokonts and land plants formed separate clades in all of the phylogenetic trees.
Phylogenetic tree of different types of T. suecica carotenoid pathway genes. a TsPSY; b TsPDS and c TsLCY-B. The tree was constructed in the MEGA 7 by using the neighbor-joining tree [32]. Bootstrap values derived from 1000 replicates are given at the respective nodes as percentages (values < 50% are not shown). T. suecica used in this study is marked in pink letter. Heterkonts were used as an outgroup. The list of accession number of the species are shown in Supplementary Table 1. (Color figure online)
Motif and structures of TsPSY, TsPDS, and TsLCY-B protein sequences
BLAST analyses showed that the TsPSY, TsPDS, and TsLCY-B ORF encoded proteins similar to PSY, PDS, and LCY-B, respectively, of different species recorded in the GenBank sequence database. Specifically, the TsPSY possessed a squalene and phytoene synthase signature motif at position 294–320 with 27 amino acids (LGTANQLTNILRDVGEDINERSRIYVP) (Supplementary Fig. 1). However, TsPDS and TsLCY-B did not possess any signature motif in their amino acid sequences (Supplementary Figs. 2, 3).
Upon comparisons of the predicted protein structures, we detected four conformational states, α-helix, β-turn, extended strand, and random coil, in the secondary structure of all types of T. suecica carotenoids (Fig. 3). The number of amino acids in each type was predicted to be 267 (60.27%), 15 (3.39%), 34 (7.67%), and 127 (28.67%) in TsPSY; 198 (45.00%), 22 (5.00%), 55 (12.50%), and 165 (37.50%) in TsPDS; and 133 (38.55%), 17 (4.93%), 57 (16.52%), and 138 (40.00%) in TsLCY-B, respectively. From these results, it is presumed that the α-helix and random coil were predominant in T. suecica carotenoids.
Effects of HMs on gene expressions of TsPSY, TsPDS, and TsLCY-B
As for genomic effects of redox-active metals, qRT-PCR analysis showed that the transcripts of TsPSY, TsPDS, and TsLCY-B were considerably induced by exposure to CuSO4, but their expression patterns were different according to the treatment concentrations (Fig. 4a). The TsPSY, TsPDS, and TsLCY-B were upregulated at 1.0 and 2.5 mg/L, and then significantly increased at 5.0 mg/L (P < 0.01). All of the tested carotenoid genes showed that highest fold changes (3.7-, 3.9-, and 3.9-fold changes) were obtained at 5 mg/L of CuSO4-exposed culture, respectively, whereas at 10.0, 25.0, and 50.0 mg/L of CuSO4 exposure, the expression level started to decrease gradually.
a Relative gene expression profile of TsPSY, TsPDS and TsLCY-B after 24 h exposure to 0 (control), 1.0, 2.5, 5.0, 10.0, 25.0 and 50.0 mg/L of CuSO4 and b Relative gene expression profiles of TsPSY, TsPDS and TsLCY-B exposed to 5 mg/L of CuSO4 and harvested at 0, 6, 12, 24, 48 and 72 h after treatments. Results are given as the means of triplicates ± SD. Dotted line represents 1.0-fold change. Significant differences between the treated and control groups are highlighted with *P < 0.05 and **P < 0.01
Dose-dependent experiments showed that T. suecica carotenoid gene expressions were significantly higher in cells treated with 5.0 mg/L CuSO4, when compared to 10.0, 25.0, and 50.0 mg/L CuSO4 (Fig. 4a), which was approximately 10-fold less than the EC50 of Cu (43.03 mg/L) to T. suecica [13]. Hence, we selected 5 mg/L of CuSO4 for further time course experiments. The qPCR analyses showed that the expression level of TsPSY and TsPDS were gradually increased until 24 h, and then gradually decreased at 48 and 72 h of exposure to CuSO4. However, the gene expression of TsLCY-B was induced with an increase up to 48 h, and then gradually decreased at 72 h (Fig. 4b).
To investigate the effect of other HMs on T. suecica, the gene expressions of TsPSY, TsPDS, and TsLCY-B were examined under specific conditions, such as treating the culture with 5.0 mg/L of redox-active and non-redox-active metals for 24 h. When compared to controls, the expression levels of TsPSY, TsPDS, and TsLCY-B were increased in the culture exposed to 5.0 mg/L of redox-active metal CuSO4, followed by CuCl2 (2.9-, 2.7-, and 3.0-fold changes), respectively (Fig. 5). In addition, the culture treated with non-redox-active metals showed a slight increase in the gene expression in the culture exposed to 5.0 mg/L of NiSO4, CdSO4, followed by ZnSO4. Overall, the other non-redox-active metals did not result in any significant changes in expressions of the carotenoid genes in T. suecica.
Relative gene expression profile of TsPSY, TsPDS and TsLCY-B after expose to 5.0 mg/L of redox-active and non-redox-active metals. Total RNA was extracted from T. suecica culture after 24 h treatment with 5 mg/L of each metals. Untreated T. suecica culture was used as the control (CK). Results are given as the means of triplicates ± SD. Dotted line represents 1.0-fold change. Significant differences between the treated and control groups are highlighted with *P < 0.05, **P < 0.01 and ***P < 0.001
Effect of HMs on Chl a and carotenoids content
Exposure of the redox-active metal CuSO4 to T. suecica induced a wide range of responses depending on the CuSO4 concentrations; whereas treatment with CuSO4 for 6 h at the initial experimental concentrations (i.e., 1.0 and 2.5 mg/L) had only slight effects on Chl a levels (Fig. 6a). Chl a was significantly lower in the cells exposed to relatively higher concentrations of CuSO4 (5.0, 10.0, 25.0, and 50.0 mg/L). In addition, Chl a levels were markedly lower after exposure to CuSO4 for 72 h, with a reduction of 39, 53, 68, 75, 81, and 85% at 1.0, 2.5, 5.0, 10.0, 25.0, and 50.0 mg/L of CuSO4 concentrations, respectively. Total carotenoid content was reached 0.80, 0.63, and 0.57 µg/mL after 24 h exposure to 5.0, 10.0, and 25.0 mg/L of CuSO4, respectively (Fig. 6b). Among the different times of exposure to redox-active and non-redox-active metals, Chl a was considerably affected in the culture exposed to CuSO4 followed by CuCl2, NiSO4, and CdSO4 (Fig. 6c). The total carotenoids were increased in the culture exposed to 24 h, and then progressively decreased in 48 and 72 h. The highest total carotenoid concentrations were obtained in the cultures exposed to the redox-active metals CuSO4 (0.8 µg/mL) and CuCl2 (0.7 µg/mL). In addition, in the cultures exposed to 24 h of non-redox-active metals (5.0 mg/L of each metal), the highest carotenoid content was obtained in the culture exposed to NiSO4 (0.65 µg/mL) followed by CdSO4 (0.6 µg/mL), whereas other non-redox-active metals did not show higher carotenoid production (Fig. 6d).
Exposure of T. suecica to redox and non-redox active metals at different time intervals. a Variation in chlorophyll a levels after expose to different concentration of CuSO4; b variation in total carotenoid levels after expose to different concentration of CuSO4; c variation in chlorophyll a levels after expose 5.0 mg/L of redox-active and non-redox-active metals and d variation in total carotenoid levels after expose to 5.0 mg/L of redox-active and non-redox-active metals. Untreated T. suecica culture was used as the control (CK). Results are given as the means of triplicates ± SD. Significant differences between the treated and control groups are highlighted with *P < 0.05 and **P < 0.01
Effect of HMs on photosystem and ROS
The cell count of the culture exposed to CuSO4 showed a gradual decrease with increasing CuSO4 doses and exposure time (data not shown). Fv/Fm and fluorescence kinetic curves (both Fo and Fm) of T. suecica was considerably affected by CuSO4 toxicity, and also declined with increasing CuSO4 concentrations and exposure time. In all concentrations of CuSO4, there was a slight decrease in fluorescence after exposure for 6 h, which was considerably decreased after exposure for 24 h (Fig. 7a).
a Variation in photosynthetic efficiency (Fv/Fm ratio) of T. suecica after 6 and 24 h exposure to different concentrations of CuSO4; b Variation in the fluorescence kinetic curves (both Fo and Fm) after 6 h exposure to 5.0 mg/L of redox-active and non-redox-active metals and c Variation in the fluorescence kinetic curves (both Fo and Fm) after 24 h exposure to 5.0 mg/L of redox-active and non-redox-active metals. Untreated T. suecica culture was used as the control (CK). Results are given as the means of triplicates ± SD. (Color figure online)
After 6 h of exposure, the fluorescence kinetic curves (both Fo and Fm) of T. suecica were not affected by all of the metals (Fig. 7b), whereas after 24 h exposure, some of the metals resulted in a decreasing pattern with exposure time (Fig. 7c). Among the HM exposures, the redox-active metals showed a gradually decrease with exposure time.
The relative chlorophyll auto-fluorescence (CAF) values decreased with CuSO4 doses and exposure time (Fig. 8a, b). On the other hand, ROS production was observed in T. suecica cells at 5.0 mg/L CuSO4 after the culture was exposed for 6, 24, and 72 h, whereas at 1.0 and 2.5 mg/L CuSO4, the ROS production was not observed until 24 h exposure, but slight ROS production was observed at 2.5 mg/L of CuSO4 at 72 h exposure (Fig. 8c, d). Additional experiments of exposure to the non-redox-active metals showed that CAFs had similar patterns to CuSO4 at low levels (Fig. 8e, Supplementary Fig. 4). However, ROS production by non-redox-active metals was also increased with dose and exposure time; though, the relative levels were much lower compared to those of CuSO4 (Fig. 8f, Supplementary Fig. 4). When compared to controls, red fluorescence (chlorophyll autofluorescence) decreased significantly, whereas green fluorescence (ROS production) slowly increased with increasing exposure time.
Exposure of T. suecica to redox-active and non-redox-active metals at different time intervals. a, b Relative chlorophyll autofluorescence (CAF) levels after expose to different concentration of CuSO4 at different time intervals (6, 24 and 72 h); c, d reactive oxygen species (ROS) production after expose to different concentration of CuSO4 at different time intervals (6, 24 and 72 h) and e, f relative CAF and ROS levels after expose to 5.0 mg/L of redox-active and non-redox-active metals at different intervals (6, 24 and 72 h). Untreated T. suecica culture was used as the control (CK). Relative ROS level was measured with ImageJ. Results are given as the means of triplicates ± SD. Dotted line represents the production of CAF and ROS level after 72 h exposure to 5.0 mg/L of CuSO4. Scale bar represent 10 µm. Significant differences between the treated and control groups are highlighted with *P < 0.05 and **P < 0.01. (Color figure online)
Discussion
Carotenoids are an imperative secondary metabolite synthesized by all photosynthetic and some non-photosynthetic organisms [37, 38]. In chlorophytes, the regulation of genes involved in carotenoid biosynthesis and its productivity under stress conditions have been extensively studied [20, 21]. However, the molecular roles and functions of carotenoids for the survival of T. suecica during unfavourable conditions remain unclear. In the present study, we have characterized three important carotenoid pathway genes of T. suecica and investigated their transcriptional expression responses after exposure to redox-active and non-redox-active metals.
As noted, carotenoids are the main pigments conferring excellent singlet oxygen quenching properties [39]. To test the effect of environmental contaminants on T. suecica, we employed some typical HMs (e.g., Cu, Al, Cd, Cr, Ni, Pb, and Zn) that commonly contaminate aquatic ecosystems. After exposure to various HMs, the expression patterns of TsPSY, TsPDS, and TsLCY-B were consistent, and these three genes were significantly upregulated (P < 0.01) after exposure to all of the Cu concentrations tested. Similar induced transcription expression of heat shock protein genes (HSP70 and HSP90) was reported in the marine microalgae Prorocentrum minimum [40], Ditylum brightwellii [41], Tetraselmis suecica [5], and Montastraea franksi [42] when they were exposed to the redox-active metal of Cu. Minhas et al. [43] reported that carotenoids were an important pigment for cell survival. In addition, Ahmed et al. [44] reported that total carotenoids, lutein, and β-carotene concentrations were significantly increased in T. suecica exposed to salicylic acid and methyl jasmonate. This result was well-documented with the carotenoid production in T. suecica after exposure to Cu. Taken together, these results suggest that redox-active metals cause adverse effects in T. suecica at the molecular and physiological levels, which result in the accumulation of carotenoids in the cells to overcome stress induced by HMs.
With regard to non-redox-active metals (Cd, Ni, Pb, and Zn), a slight increase was found in the gene expression of carotenoids, whereas Al2SO4 and K2Cr2O7 showed none or minimal changes. Similar results were obtained in T. suecica, of which HSP70 and HSP100 genes were significantly expressed (P < 0.01) in the cells exposed to redox-active metals when compared to those exposed to non-redox-active metals (unpublished data). In addition, a similar expression pattern was reported in the marine diatom Ditylum brightwellii, where significant HSP70 and HSP90 gene expressions were recorded in the cultures exposed to CuSO4 and NiSO4 compared to other chemicals [41]. However, most of the studies have reported that carotenoids are helpful to eliminate harmful non-radical and other radical species in the cells [45, 46], due to the nine or more conjugated double bonds in its structure. In the present study, higher expression levels of TsPSY, TsPDS, and TsLCY-B, even the production of carotenoids, were observed in HM-treated T. suecica. This demonstrated that the carotenogenic gene response was 10-fold more sensitive as compared to when the alga was exposed to the EC50 of T. suecica (43.03 mg/L) [13]. Therefore, these genes might be involved in HM homeostasis, making T. suecica a highly tolerant species; however, the level of expression depends on the concentration of HMs.
HMs could trigger ROS production through the disruption of electron transport, as they act as inhibitors of metallo-sensitive sites of photosystem II (PS II), which are particularly destructive aspects of oxidative stress [47]. A previous study reported that microalgae can accumulate significant amounts of carotenoids under stress conditions, in which cell division is retarded [48]. Likewise, the present study showed that a pronounced decrease in Chl a, Fv/Fm, and fluorescence kinetic results were detectable, whereas the ROS production, carotenoid production and its genes expression were increased in the cultures exposed to redox-active metals when compared to non-redox-active metals. Similar results were found in the green algae Closterium ehrenbergii after exposure to redox-active metals such as Cu [49, 50]. In addition, previous studies reported that the redox-active metal Cu has the most potent effects to inhibit oxygen evolution, accompanied by a significant decrease in the photosynthetic yield, which may reflect a specific interaction between Cu ions and TyrZ and TyrD on the D2 protein of PSII [51,52,53]. However, carotenoids have been found to function as a redox intermediate in an alternate pathway of electron transfer within the photosystem [54]. This shows that the carotenoid can act as scavengers to reduce or detoxify the ROS production in T. suecica, which inactivates and prevents them from contributing to photosynthesis.
The carotenoid biosynthetic pathway genes were gradually upregulated; an increase in the ROS production was observed, whereas the photosynthetic efficiency were adversely affected when the T. suecica was exposed to HMs conjugated with SO42− anion (CdSO4, CuSO4, NiSO4, and ZnSO4). Perhaps the carotenogenic gene expression may be affected by the types of metal and its conjugated anions. A similar result was found when D. brightwellii [41], P. minimum [55], and T. suecica (unpublished data) were exposed to metals conjugated with SO42− and Cl−. The metals conjugated with the former anion caused higher gene expression as compared to those exposed to Cl−-based chemicals [41]. From the previous results and our findings, an important point is noted: it seems that metal conjugates with anion (e.g., SO42−, Cl−) may be responsible for the specific induction of carotenoid biosynthetic pathway genes. This can be confirmed by the observation that metal ions that were conjugated with SO42− were able to regulate the transcription of TsPSY, TsPDS, and TsLCY-B; however, this was not the case for metals conjugated with Cl−. From another perspective, it can be investigated by considering the biological endpoints as one of the indicators for the bioavailability (or susceptibility) of metals in phytoplankton [56]. However, the Cu used in the present study was provided as a conjugated compound (CuSO4 and CuCl2), and thus, it was potentially differentially dissolved in the filtered seawater medium. In addition, HMs can react with the chelating agents, such as EDTA, present in the f/2 medium [41]. Similar principles can explain the behaviour of another divalent metal, cadmium. Thus, the bioavailability or susceptibility of metal salts may differently affect the toxicity and gene expression in T. suecica. Therefore, it seems that carotenogenic genes are differentially involved in protecting against various metal stressors.
From the overall results, T. suecica was notably more sensitive to the redox-active metal, Cu, as compared to the non-redox-active metals. This might be because redox-active metals can directly generate oxidative injury via the Haber–Weiss and Fenton reactions [57], whereas non-redox-active metals inflict oxidative stress via multiple indirect mechanisms [58, 59]. Antioxidant enzymes constitute the first line of defence against ROS; they are activated to neutralize toxic levels. Interestingly, from our expressed sequence tag (EST) data of T. suecica (TsEST), we identified 11 different types of antioxidant-related genes [5]. Among these, previous studies have reported that carotenoids prevent the free radical-dependent oxidation of proteins or DNA by capturing free radicals and by reducing the stress induced by ROS [18, 60]. Recent findings showed that extracts of T. suecica had strong antioxidant activities due to the presence of high levels of carotenoids [7]. In addition, in our EST library constructed from stressed T. suecica, all of the carotenoid biosynthetic pathway genes were identified, namely phytoene synthase, phytoene desaturase, ζ-carotene desaturase, lycopene ε-cyclase, lycopene β-cyclase, β-carotene hydroxylase, and zeaxanthin epoxidase [5]. Moreover, carotenoids may act as a carbon sink for the accumulation of excess carbon produced during photosynthesis under stress conditions [48]. In the present study, we also found that the carotenoid production and its gene expression (TsPSY, TsPDS, and TsLCY-B) were higher after exposure of T. suecica to most of the HMs. This indicated that significant amounts of carotenoids might accumulate in T. suecica cells to protect them during various stress conditions, such as during exposures to metals and heat [61].
Chelation of HMs may cause differential carotenogenic gene expression patterns in T. suecica. Because chelating agents act as high-affinity ligands of HMs, it is important to detoxify them and provide tolerance under HM stress. Microalga and other organisms preferentially synthesize metal-binding peptides, namely phytochelatins (PCs) and metallothioneins (MTs), which neutralize the toxic effect caused by HMs [62, 63]. However, in T. suecica, these two ligands were not found in our TsEST data [5]. From our results and previous findings, it was inferred that when T. suecica is exposed to non-redox-active metals, the carboxylic and amino acid pathways are activated to detoxify the HMs to less toxic or non-toxic metals [57, 64]. In addition, the non-redox-active metals induce oxidative stress through indirect mechanisms, such as interactions with the antioxidant defence system, disruption of the electron transport chain, or induction of lipid peroxidation [65]. This might also be one of the reasons that non-redox-active metals do not show any significant effect in T. suecica.
In conclusion, we found that the TsPSY, TsPDS, and TsLCY-B genes respond to metal stress and accumulate significant amounts of carotenoids after exposure to HMs; however, these genes are differentially expressed in response to various metal ions, such as metal cations or anions, or their combination, as well as redox-active and non-redox-active HMs. Moreover, physiological results clearly demonstrated that T. suecica was tolerant to various HMs as compared to other algae. T. suecica may possess internal detoxification mechanisms of HMs in its cells [13, 66], thus conferring tolerance to HMs. Regarding this, the carotenoid synthesized under different redox-active and non-redox-active HMs act as a defence mechanism for the algae to adapt to unfavourable environmental conditions and hazardous exposures. This partially explained why T. suecica was tolerant to HMs, making it a potential species for the bioremediation of HMs from hazardous environments while also being beneficial for the production of bioactive compounds such as carotenoids, which can be exploited in aquaculture.
References
El-Kassas HY, El-Sheekh MM (2016) Induction of the synthesis of bioactive compounds of the marine alga Tetraselmis tetrathele (West) Butcher grown under salinity stress. Egypt J Aquat Res 42:385–391. https://doi.org/10.1016/j.ejar.2016.10.006
Irianto A, Austin B (2002) Probiotics in aquaculture. J Fish Dis 25:633–642. https://doi.org/10.1046/j.1365-2761.2002.00422.x
Fabregas J, Abalde J, Herrero C, Cabezas B, Veiga M (1984) Growth of the marine microalga Tetraselmis suecica in batch cultures with different salinities and nutrient concentrations. Aquaculture 42:207–215. https://doi.org/10.1016/0044-8486(84)90101-7
Fabregas J, Herrero C (1985) Marine microalgae as a potential source of single cell protein (SCP). Appl Microbiol Biotechnol 23:110–113. https://doi.org/10.1007/BF01982726
Sathasivam R, Guo R, Wang H, Lim WA, Ki JS (2018) Expressed sequence tag library of the marine green algae Tetraselmis suecica: a focus on stress related genes for marine pollutions. J Appl Phycol 30:2387–2402. https://doi.org/10.1007/s10811-018-1445-y
Carballo-Cardenas EC, Tuan PM, Janssen M, Wijffels RH (2003) Vitamin E (alpha-tocopherol) production by the marine microalgae Dunaliella tertiolecta and Tetraselmis suecica in batch cultivation. Biomol Eng 20:139–147. https://doi.org/10.1016/S1389-0344(03)00040-6
Sansone C, Galasso C, Orefice I, Nuzzo G, Luongo E, Cutignano A, Romano G, Brunet C, Fontana A, Esposito F, Lanora A (2017) The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Sci Rep 7:41215. https://doi.org/10.1038/srep41215
Montero MF, Aristizabal M, Reina GG (2011) Isolation of high-lipid content strains of the marine microalga Tetraselmis suecica for biodiesel production by flow cytometry and single cell sorting. J Appl Phycol 23:1053–1057. https://doi.org/10.1007/s10811-010-9623-6
Patidar SK, Kim SH, Kim JH, Park J, Park BS, Han MS (2018) Pelagibaca bermudensis promotes biofuel competence of Tetraselmis striata in a broad range of abiotic stressors: dynamics of quorum–sensing precursors and strategic improvement in lipid productivity. Biotechnol Biofuels 11:102. https://doi.org/10.1186/s13068-018-1097-9
Park J, Park BS, Wang P, Patidar SK, Kim JH, Kim SH, Han MS (2017) Phycospheric native bacteria Pelagibaca bermudensis and Stappia sp. ameliorate biomass productivity of Tetraselmis striata (KCTC1432BP) in co-cultivation system through mutualistic interaction. Front Plant Sci 8:289. https://doi.org/10.3389/fpls.2017.00289
Levy JL, Stauber JL, Jolley DF (2007) Sensitivity of marine microalgae to copper: the effect of biotic factors on copper adsorption and toxicity. Sci Total Environ 387:141–154. https://doi.org/10.1016/j.scitotenv.2007.07.016
Levy JL, Angel BM, Stauber JL, Poon WL, Simpson SL, Cheng SH, Jolley DF (2008) Uptake and internalisation of copper by three marine microalgae: comparison of copper sensitive and copper tolerant species. Aquat Toxicol 89:82–93. https://doi.org/10.1016/j.aquatox.2008.06.003
Ebenezer V, Ki JS (2013) Quantification of the sub-lethal toxicity of metals and endocrine-disrupting chemicals to the marine green microalga Tetraselmis suecica. Fish Aquatic Sci 16:187–194. https://doi.org/10.5657/FAS.2013.0187
Nassiri Y, Ginsburger-Vogel T, Mansot JL, Wery J (1996) Effects of heavy metals on Tetraselmis suecica: Ultrastructural and energy-dispersive X-ray spectroscopic studies. Biol Cell 86:151–160. https://doi.org/10.1016/0248-4900(96)84779-4
Tsai HP, Chuang LT, Chen CN (2016) Production of long chain omega-3 fatty acids and carotenoids in tropical areas by a new heat-tolerant microalga Tetraselmis sp. DS3. Food Chem 192:682–690. https://doi.org/10.1016/j.foodchem.2015.07.071
Chokshi K, Pancha I, Ghosh A, Mishra S (2017) Nitrogen starvation–induced cellular crosstalk of ROS–scavenging antioxidants and phytohormone enhanced the biofuel potential of green microalga Acutodesmus dimorphus. Biotechnol Biofuels 10:60. https://doi.org/10.1186/s13068-017-0747-7
Rodrigues E, Mariutti LRB, Mercadante AZ (2012) Scavenging capacity of marine carotenoids against reactive oxygen and nitrogen species in a membrane-mimicking system. Mar Drugs 10:1784–1798. https://doi.org/10.3390/md10081784
Sathasivam R, Ki JS (2018) A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar Drugs 16:26. https://doi.org/10.3390/md16010026
Sathasivam R, Kermanee P, Roytrakul S, Juntawong N (2012) Isolation and molecular identification of β-carotene producing strains of Dunaliella salina and Dunaliella bardawil. from salt soil samples by using species-specific primers and internal transcribed spacer (ITS) primers. Afr J Biotechnol 11:16677–16687. https://doi.org/10.5897/AJB12.063
Sathasivam R, Praiboon J, Chirapart A, Trakulnaleamsai S, Kermanee P, Roytrakul S, Juntawong N (2014) Screening, phenotypic and genotypic identification of β-carotene producing strains of Dunaliella salina from Thailand. Indian J Geo-Mar Sci 43:2198–2216
Sathasivam R, Pongpadung P, Praiboon J, Chirapart A, Trakulnaleamsai S, Roytrakul S, Juntawong N (2018) Optimizing NaCl and KNO3 concentrations for high β-carotene production in photobioreactor by Dunaliella salina KU11 isolated from saline soil sample. Chiang Mai J Sci 45:106–115
Osundeko O, Dean AP, Davies H, Pittman JK (2014) Acclimation of microalgae to wastewater environments involves increased oxidative stress tolerance activity. Plant Cell Physiol 55:1848–1857. https://doi.org/10.1093/pcp/pcu113
Zuluaga M, Gueguen V, Pavon-Djavid G, Letourneur D (2017) Carotenoids from microalgae to block oxidative stress. BioImpacts 7:1–3. https://doi.org/10.15171/bi.2017.01
Chamovitz D, Sandmann G, Hirschberg J (1993) Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J Biol Chem 268:17348–17353
Fraser PD, Truesdale MR, Bird CR, Schuch W, Bramley PM (1994) Carotenoid biosynthesis during tomato fruit development (evidence for tissue-specific gene expression). Plant Physiol 105:405–413. https://doi.org/10.1104/pp.105.1.405
Cunningham JFX (2002) Regulation of carotenoid synthesis and accumulation in plants. Pure Appl Chem 74:1409–1417. https://doi.org/10.1351/pac200274081409
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can J Microbiol 8:229–239. https://doi.org/10.1139/m62-029
Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788. https://doi.org/10.1093/nar/gkg563
Geourjon C, Deleage G (1995) SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci 11:681–684. https://doi.org/10.1093/bioinformatics/11.6.681
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. https://doi.org/10.1038/nmeth.1701
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 9:e45. https://doi.org/10.1093/nar/29.9.e45
Guo R, Ki JS (2012) Evaluation and validation of internal control genes for studying gene expression in the dinoflagellate Prorocentrum minimum using real-time PCR. Eur J Protistol 48:199–206. https://doi.org/10.1016/j.ejop.2011.11.001
Sathasivam R, Ebenezer V, Guo R, Ki JS (2016) Physiological and biochemical responses of the freshwater green algae Closterium ehrenbergii to the common disinfectant chlorine. Ecotox Environ Safe 133:501–508. https://doi.org/10.1016/j.ecoenv.2016.08.004
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. https://doi.org/10.1146/annurev.arplant.59.032607.092759
Domonkos I, Kis M, Gombos Z, Ughy B (2013) Carotenoids, versatile components of oxygenic photosynthesis. Prog Lipid Res 52:539–561. https://doi.org/10.1016/j.plipres.2013.07.001
Young JB, Kim BY, Jung IL, Park DH (2012) Metabolic roles of carotenoid produced by non-photosynthetic bacterium Gordonia alkanivorans SKF120101. J Microbiol Biotechnol 22:1471–1477. https://doi.org/10.4014/jmb.1207.07038
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Guo R, Ebenezer V, Ki JS (2012) Transcriptional responses of heat shock protein 70 (Hsp70) to thermal, bisphenol A, and copper stresses in the dinoflagellate Prorocentrum minimum. Chemosphere 89:512–520. https://doi.org/10.1016/j.chemosphere.2012.05.014
Guo R, Lee M-A, Ki JS (2013) Different transcriptional responses of heat shock protein 70/90 in the marine diatom Ditylum brightwellii exposed to metal compounds and endocrine-disrupting chemicals. Chemosphere 92:535–543. https://doi.org/10.1016/j.chemosphere.2013.03.052
Venn AA, Quinn J, Jones R, Bodnar A (2009) P-glycoprotein (multi-xenobiotic resistance) and heat shock protein gene expression in the reef coral Montastraea franksi in response to environmental toxicants. Aquat Toxicol 93:188–195. https://doi.org/10.1016/j.aquatox.2009.05.003
Minhas AK, Hodgson P, Barrow CJ, Adholeya A (2016) A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front Microbiol 7:546. https://doi.org/10.3389/fmicb.2016.00546
Ahmed F, Fanning K, Netzel M, Schenk PM (2015) Induced carotenoid accumulation in Dunaliella salina and Tetraselmis suecica by plant hormones and UV-C radiation. Appl Microbiol Biotechnol 99:9407–9416. https://doi.org/10.1007/s00253-015-6792-x
Li Y, Sommerfeld M, Chen F, Hu Q (2008) Consumption of oxygen by astaxanthin biosynthesis: a protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae). J Plant Physiol 165:1783–1797. https://doi.org/10.1016/j.jplph.2007.12.007
Lemoine Y, Schoefs B (2010) Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth Res 106:155–177. https://doi.org/10.1007/s11120-010-9583-3
Clijsters H, Van Assche F (1985) Inhibition of photosynthesis by heavy metals. Photosynth Res 7:31–40. https://doi.org/10.1007/BF00032920
Einali A, Mazang-Ghasemi S, Valizadeh J, Noorozifar M (2017) Metabolic responses and β-carotene production by the unicellular green alga Dunaliella salina exposed to leaf extracts. Acta Bot Bras 31:180–190. https://doi.org/10.1590/0102-33062017abb0002
Wang H, Sathasivam R, Ki JS (2017) Physiological effects of copper on the freshwater alga Closterium ehrenbergii Meneghini (Conjugatophyceae) and its potential use in toxicity assessments. Algae 32:131–137. https://doi.org/10.4490/algae.2017.32.5.24
Wang H, Ebenezer V, Ki JS (2018) Photosynthetic and biochemical responses of the freshwater green algae Closterium ehrenbergii Meneghini (Conjugatophyceae) exposed to the metal coppers and its implication for toxicity testing. J Microbiol 56:426–434. https://doi.org/10.1007/s12275-018-8081-8
Sabat SC (1996) Copper ion inhibition of electron transport activity in sodium chloride washed photosystem II particle is partially prevented by calcium ion. Z Naturforsch 51:179 – 184. https://doi.org/10.1515/znc-1996-3-408
Maksymiec W, Baszynski T (1999) The role of Ca2+ ions in modulating changes induced in bean plants by an excess of Cu2+ ions. Chlorophyll fluorescence measurements. Physiol Plant 105:562–568. https://doi.org/10.1034/j.1399-3054.1999.105323.x
Guo R, Ebenezer V, Wang H, Ki JS (2017) Chlorine affects photosystem II and modulates the transcriptional levels of photosynthesis-related genes in the dinoflagellate Prorocentrum minimum. J Appl Phycol 29:153–163. https://doi.org/10.1007/s10811-016-0955-8
Tracewell CA, Vrettos JS, Bautista JA, Frank HA, Brudvig GW (2001) Carotenoid photooxidation in photosystem II. Arch Biochem Biophys 385:61–69. https://doi.org/10.1006/abbi.2000.2150
Guo R, Ki JS (2013) Characterization of a novel catalase-peroxidase (KATG) gene from the dinoflagellate Prorocentrum minimum. J Phycol 49:1011–1016. https://doi.org/10.1111/jpy.12094
Campbell PG, Errécalde O, Fortin C, Hiriart-Baer VP, Vigneault B (2002) Metal bioavailability to phytoplankton–applicability of the biotic ligand model. Comp Biochem Physiol C Toxicol Pharmacol 133:189–206. https://doi.org/10.1016/S1532-0456(02)00104-7
Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50. https://doi.org/10.1016/j.tplants.2008.10.007
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208. https://doi.org/10.2174/0929867053764635
Bielen A, Remans T, Vangronsveld J, Cuypers A (2013) The influence of metal stress on the availability and redox state of ascorbate, and possible interference with its cellular functions. Int J Mol Sci 14:6382–6413. https://doi.org/10.3390/ijms14036382
Gong M, Bassi A (2016) Carotenoids from microalgae: a review of recent developments. Biotechnol Adv 34:1396–1412. https://doi.org/10.1016/j.biotechadv.2016.10.005
Sathasivam R, Radhakrishnan R, Hashem A, Abd_Allahd EF (2019) Microalgae metabolites: a rich source for food and medicine. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2017.11.003
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182. https://doi.org/10.1146/annurev.arplant.53.100301.135154
Perales-Vela HV, Pena-Castro JM, Canizares-Villanueva RO (2006) Heavy metal detoxification in eukaryotic microalgae. Chemosphere 64:1–10. https://doi.org/10.1016/j.chemosphere.2005.11.024
Yang XE, Jin XF, Feng Y, Islam E (2005) Molecular mechanisms and genetic basis of heavy metal tolerance/hyperaccumulation in plants. J Integr Plant Biol 47:1025–1035. https://doi.org/10.1111/j.1744-7909.2005.00144.x
Keunen E, Remans T, Bohler S, Vangronsveld J, Cuypers A (2011) Metal-induced oxidative stress and plant mitochondria. Int J Mol Sci 12:6894–6918. https://doi.org/10.3390/ijms12106894
Pérez-Rama M, Alonso JA, Lopez CH, Vaamonde ET (2002) Cadmium removal by living cells of the marine microalga Tetraselmis suecica. Bioresour Technol 84:265–270. https://doi.org/10.1016/S0960-8524(02)00045-7
Acknowledgements
We thank Dr. H. Wang for critical comments on the early version of manuscript. This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (2015M1A5A1041805 and 2016R1D1A1A09920198), and by a grant from the National Institute of Fisheries Science (R2018043) funded to J.-S. Ki.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies conducted on human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sathasivam, R., Ki, JS. Differential transcriptional responses of carotenoid biosynthesis genes in the marine green alga Tetraselmis suecica exposed to redox and non-redox active metals. Mol Biol Rep 46, 1167–1179 (2019). https://doi.org/10.1007/s11033-018-04583-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-04583-9