Abstract
Both transgenic as well as traditional breeding approaches have not been completely successful in inducting resistance against geminiviruses in crop plants. This demands the utilization of non-viral, non-plant compounds possessing antiviral characteristics as an alternate and effective strategy for developing durable resistance against geminiviruses. In recent years, several antiviral molecules have been developed for the treatment of plant virus infections. These molecular antiviral compounds target various geminiviral-DNA and -protein via interacting with them or by cleaving viral RNA fragments. Applications of these proteins such as GroEL, g5g and VirE2 have also provided a convincing evidence of resistance against geminiviruses. Taking advantage of this information, we can generate robust resistance against geminiviruses in diverse crop plants. In this context, the present review provides epigrammatic information on these antiviral compounds and their mode of action in modulating virus infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Geminiviruses are small circular single-stranded DNA (ssDNA) viruses which cause severe losses in economically important crops all over the world [16]. Being non-enveloped viruses with distinct geminate morphology, the family is named ‘Geminiviridae’. They have circular ssDNA components (either mono or bi-partite) of ~2.7 kb encapsidated within twinned quasi-icosahedral particles. These viruses are transmitted through insect vectors such as whiteflies, leafhopper, and treehopper. Members of geminivirus family are grouped into seven genera based on their genome organization (ICTV 2012, http://ictvonline.org/virusTaxonomy.asp; [30]). The genomic components have regions and motifs to control the viral gene replication and expression. It has some conserved iterons and a putative stem-loop structure with the highly conserved nucleotide ‘TAATATTAC’ which participates in the initiation of rolling circle replication [19]. They have few but efficient proteins for their genome replication, movement, encapsidation and host RNAi suppressors. Two additional DNA molecules referred to as alpha- and beta-satellites have shown to be associated with a genus of geminivirus called begomoviruses. They repeatedly subsist in disease complex due to its high mutation rates which allow them to acclimatize quickly against unfavorable environments. This is a major problem for the plant virologist which makes them ineffective in their combat strategies against geminiviruses. Upon virus infection not all plants can effectively activate their defense components, such as small RNA mediated resistance or R-gene mediated resistance.
The application of genetic engineering provides a novel source to integrate new virus resistance traits into the desirable cultivars. Conventional methods to control the virus transmitting arthropods via cultural strategies and pesticide applications against vectors have been unsuccessful at mitigating the impact of geminiviruses. Thus, planting resistant genotypes is the most reliable and effective means to manage geminivirus diseases. Due to availability of very few resistant sources, non-conventional methods have been used to confer virus resistance via introducing virus-derived genes such as coat protein (CP), replicase associated proteins, nuclear and cellular movement proteins, defective particles along with non-coding counterpart into susceptible plants. Apart from these approaches, usage of non-coding viral RNAs [i.e. smallRNAs, antisense RNA and double-stranded (ds) RNAs homologous] have also shown to be a potential method for providing virus resistance in bean [5], blackgram [23] cassava [38], tobacco [4] and tomato [9, 25, 40]. In spite of all these approaches, very few promising examples of crops are available till date which have been released for cultivation and have shown resistance against broad spectrum viruses. In this context, the present review summarizes the application of non-virus, non-plant compounds in generating virus resistant plants through transgene-based approaches.
Emergence of relevant techniques to fight against geminiviruses
In this decade, several relevant techniques have emerged to combat geminiviruses. Various RNAi strategies, transgene-mediated approaches and host-gene mediated genetic engineering strategies along with traditional breeding have been applied to reduce the geminivirus infection [14, 24]. Pathogen derived resistance approaches, which are based on post transcriptional gene silencing (PTGS)/RNA silencing, have been employed against diverse plant viruses [22, 38]. Introduction of the hairpin (hp) RNA constructs is the most resourceful way to generate virus-specific dsRNAs in transgenic plants [17]. Furthermore, various host factors have been identified to play an important role in the geminivirus–plant interactions [30], but transgenic plants overexpressing these host factors have not been proved much effective against geminiviruses.
Using the above discussed approach, effective resistance against Maize streak virus (MSV) has been developed in maize [32]. Collapse in degree of geminivirus resistance in various transgenic plants has also been reported. Failure or limited effectiveness of the RNAi based strategies has been highlighted in the case of MSV [33] and Mungbean yellow mosaic virus [34]. In these cases, full-length or truncated antisense Rep gene constructs were found to be ineffective in providing resistance. Limitations of these approaches have also been shown. The main drawback of RNAi was the ‘off target’ silencing of unintended transcript. Another disadvantage was that the efficiency of silencing depends on base-pairing between target and antisense RNAs and thus, broad-spectrum virus resistance is difficult to achieve. Plant derived toxins such as dianthin [12] and BARNASE–BARSTAR genes [39] have also been utilized to accomplish resistance in Nicotiana and cassava against African cassava mosaic virus. Although in these reports resistances against the viruses have been accomplished, the constitutive expression proved lethal to the plants. Another disadvantage is that even in the absence of the viral trans-activating proteins, leaky expression was observed.
Since the effort of all these approaches for providing resistance against geminivirus infections has not been completely successful or limited, thus effective and alternate strategies need to be developed to confer a significant level of resistance. Viruses have shown to encounter the antiviral molecules through their ability to mutate the genome. It is also essential to target conserved elements of geminiviruses with new molecular antiviral approaches. These modern approaches which tend to be more promising than other strategies are summarized in subsequent sections.
Modern approaches implying molecular antiviral compounds to control geminivirus replication
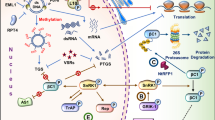
In recent years, various molecular approaches have been developed to generate geminivirus resistant plants by application of diverse antiviral compounds (Fig. 1; Table 1). For this, molecules not only from plant/or viruses, but antiviral compounds from diverse animal or pathogens need to be applied in the present scenario.
Schematic representation of antiviral molecules and their mode of action against geminiviruses. Protein–protein, protein–DNA binding activity of various anti-viral molecules to interfere the virus replication, movement and encapsidation has been depicted. Binding of ssDNA with g5p, hinder the interaction of ssDNA-nuclear shuttle proteins (NSP) and restricts cellular movement of ssDNA. AZPs interact with the viral intergenic region and obstruct the viral DNA replication. Similarly, Agrobacterium virE2 have affinity towards ssDNA, thus interfere with the replication process of geminivirus. GroELs competes with the NSP to restrict the cell to cell movement. Ribozymes targets the viral transcripts which help in reducing the further translation of the infectious viral proteins. Antibodies have binding affinity with the geminivirus proteins, which hampers the specific functions of viruses. Dotted blue line highlighted the virus genomic parts targeted for the binding of antiviral compounds. AZPs artificial zinc finger proteins, CW cell wall, NSP nuclear shuttle protein, CP coat protein, MP movement protein, NM nuclear membrane, Trp transcription activator protein. (Color figure online)
Artificial zinc finger protein approach to prevent replication
An artificial zinc finger protein (AZP) has successfully been applied against various geminiviruses to achieve resistance [13, 18, 31]. The strategy behind the application of AZPs was to exploit their affinity towards the Rep dsDNA binding site of geminiviruses (Fig. 1). Transgenic Arabidopsis plants expressing AZPs showed resistance to Beet severe curly top virus infection [31]. This approach has been consistent in tomato expressing AZPs against the Tomato yellow leaf curl virus (TYLCV), [13]. Although these strategies have resulted in significant resistance, their applicability was limited due to sequence variability in the origin of replication between the geminiviruses. To overcome this problem, AZPs were generated from a conserved sequence motif of begomoviruses by Chen et al. [6], which revealed that AZPs technology may be used as source of durable resistance against multiple begomoviruses. An advantage of this approach is that the DNA viruses do not encode genes for the proficient enzyme to inactivate AZPs via chemical modification. Even if the chance of mutation exists, mutation at the binding sites of DNA as well as the mutation in viral proteins must occur simultaneously, which indeed is unfavorable for viruses.
The promising role of milk derivatives
DNA binding properties of some proteins have been utilized to prevent the geminivirus infection. One of the most fascinating examples of such compound is milk and its derivatives [8, 21]. This may be due to these chemical properties such as in vitro binding ability with viral DNA by hydrophobic interactions along with transcription and translation inhibition activity (Fig. 1). Recent study has revealed the antiviral role of esterified whey protein fractions (a-lactalbumin, b-lactoglobulin, and lactoferrin) against TYLCV infection in tomato [1]. This study provides an idea of generating genetically modified plants harboring these protein products which may show altered viral replication. However, the transgenic developed by these strategies may need further examination of its effectiveness against the geminiviruses infection and stability to withstand its phenotype.
Role of ribozyme mediated strategy in hindering viral replication
Ribozymes were shown to inhibit gene expression by cleaving the target RNAs. As the different species of Geminiviridae family share a common mode of rolling circle replication [11], targeting viral replication machinery might be useful as a broad and durable control strategy in defending geminivirus infection. In the first report on this strategy, it was revealed that the hammerhead ribozyme was targeted to the mRNA of replication initiator protein (Rep) of Mungbean yellow mosaic India virus (MYMIV), ultimately causing a significant decrease in MYMIV replication (Fig. 1) [7]. Following this, targeting other necessary geminiviral proteins can provide possibilities for superior virus resistance in plants. Although, due to high rate of mutation of the viral genome, efficacy of the strategy reduces the possibility of the virus resistance in successive generation. Thus, the selection of the conserved targeted region as well as its broad-spectrum application should be the main motive for generation of plants with durable geminivirus resistance.
Peptide aptamers mediated resistance against geminiviruses
Peptide aptamers are recombinant, small amino acid sequence of protein inserted into a protein scaffold [10]. These tiny molecules can dislocate various protein–protein and protein–DNA interactions. This property of aptamers had been utilized to hamper the function of viral proteins (Fig. 1) [15, 27]. Peptide aptamers binding to Tomato golden mosaic virus-Rep interferes with viral replication in plant cells, thus providing a new tool for studying Rep function besides serving as the foundation for the progress of crop improvement with broad-spectrum resistance against DNA viruses [15]. Similar study was also performed in TYLCV and Tomato mottle virus infection, in which peptide aptamers interacting with Rep proteins were targeted. This study also revealed that two of the peptide aptamers interacted with the Rep proteins from viruses representing three major Geminiviridae genera, providing broad spectrum resistance [26].
Recent approaches involving molecular antiviral compounds to control geminivirus movement
Involvement of bacterial proteins in providing tolerance against geminiviruses
During plant–bacterial interactions, diverse host protein interacts with the bacterial proteins. These interactions have an adverse effect on plants, but some characteristic features of these non-plant proteins can be utilized for defense against geminivirus. For example, VirE2 from Agrobacterium, which showed interaction with various plant proteins, was identified as a potential antiviral protein as it possess nuclear targeted ssDNA binding activity (Fig. 1) [35]. In their study, they have shown that MYMIV infection was suppressed in the transgenic Nicotiana overexpressing virE2 gene. Another study showed the effectiveness of the E. coli g5p protein in providing tolerance against ToLCV. In this study, g5p protein which can bind to ssDNA has been proposed to obstruct the interaction between viral DNA and nuclear shuttle proteins (Fig. 1) [20]. This resulted in the inhibition of viral DNA movement, thus providing resistance in Nicotiana benthamiana. Once ingested by their insect vector, geminiviruses are translocated from the insect gut to the salivary glands for excretion with saliva. As a preventive measure, the viruses interact with the GroEL proteins of the endosymbiotic bacteria present in the vector to avoid damage through hemolymphatic action. These chaperonin family proteins, GroELs, are involved in protein post-translational folding and subunit assembly. Intervention in this interaction was shown to result in loss of infectivity [37]. In a study, it was revealed that, these GroEL expressed in the plant phloem trapped the TYLCV particles. Further, it was demonstrated that whitefly derived-GroEL expressing tomato plants have provided moderate resistance to TYLCV [2, 3].
Although some successful results were recently documented, these bacterial protein mediated resistance still needs to be investigated for conferring broad spectrum tolerance. Moreover, due to equal affinity of the antiviral molecule and the virus protein towards the binding on target DNA, artificially introduced protein needs to be applied in higher level for effective inhibition of DNA replication.
Application of antibodies mediated technology to target geminivirus encapsidation
With the help of modern molecular biotechnological advances, it is now attainable to develop virus resistant crops. In this regard, antibody-based resistance strategy provides a novel platform to develop transgenic plants resistant to viruses. In this approach, antibodies or antibody fragments corresponding to the target viral proteins are expressed in the plant to hinder the necessary viral infection. The first successful application of recombinant antibody-mediated resistance approach was against Artichoke mottled crinkle virus in which the expression of a cytosolic single-chain variable fragment (scFv) against CP was used [36]. With this discovery, the antibody-mediated approach was also applied in case of geminiviruses to develop resistance in plants. The recombinant antibodies successfully accomplished TYLCV resistance in N. benthamiana [28, 29] can be used as an antibody-mediated resistance approach across the plant to generate resistance. This strategy appears to be more precise as it depends on the affinity and specificity of the antibody towards the target protein.
Conclusions and future prospects
Over the past 10 years, many new antiviral molecules have been discovered as a consequence of examining the nature of geminiviral components such as DNA, RNA and proteins. The major problem with this approach which needs to be addressed is that the majority of antiviral compounds known to manage the spread of geminiviruses are restricted. Viruses have shown to encounter the antiviral molecules through their ability to mutate the genome. It is also essential to target conserved elements of geminiviruses with new molecular antiviral approaches. The selection pressure of these compounds may generate mutants, thus virus can become resistant to these compounds. This may also lead to the emergence of new species or species of geminiviruses with enhanced virulence.
Biotechnological advances have now conferred importance towards these antiviral proteins and the identification of novel compounds that generate resistance in numerous ways. The majority of efforts to exploit this understanding to engineer enhanced virus resistance in plants have been successful. However, field evaluation has not been done so far, especially to examine their effects on crop yield which is one of the prime target areas of plant protection against virus. It is also important to investigate the toxic nature of any non-viral protein to provide safer production system. The initiation of high-throughput technologies such as protein–protein interaction, bioinformatics-aided drug design and docking technologies to study the molecular interaction of geminiviral proteins with novel compounds could be useful for identifying potential molecular antiviral agents. Invention of novel molecular antiviral compounds must continue, which may possibly establish to be a much improved alternative of RNAi. In the present scenario, the combined strategies such as application of multiple virus resistance approaches along with the molecular antiviral methods could be more effective to target various check points of geminivirus replication and movement. However, these hypotheses need to be applied and tested before leading to any conclusion. Thus, the advancement of safe, efficient, and economical antiviral compounds have been amongst the main concern, as numerous plant viral infection are still incurable.
References
Abdelbacki AM, Taha SH, Sitohy MZ et al (2010) Inhibition of tomato yellow leaf curl virus (TYLCV) using whey proteins. Virol J 3:7–26
Akad F, Dotan N, Czosnek H (2004) Trapping of Tomato yellow leaf curl virus (TYLCV) and other plant viruses with a GroEL homologue from the whitefly Bemisia tabaci. Arch Virol 149:1481–1497
Akad F, Eybishtz A, Edelbaum D et al (2007) Making a friend from a foe: expressing a GroEL gene from the whitefly Bemisia tabaci in the phloem of tomato plants confers resistance to tomato yellow leaf curl virus. Arch Virol 152:1323–1339
Asad S, Haris WA, Bashir A et al (2003) Transgenic tobacco expressing geminiviral RNAs are resistant to the serious viral pathogen causing cotton leaf curl disease. Arch Virol 148:2341–2352
Bonfim K, Faria JC, Nogueira EOPL et al (2007) RNAi-mediated resistance to Bean golden mosaic virus in genetically engineered common bean (Phaseolus vulgaris). Mol Plant-Microbe Interact 20:717–726
Chen W, Qian Y, Wu X et al (2014) Inhibiting replication of begomoviruses using artificial zinc finger nucleases that target viral-conserved nucleotide motif. Virus Genes 48:494–501
Chilakamarthi U (2007) Mukherjee SK, Deb JK (2007) Intervention of geminiviral replication in yeast by ribozyme mediated downregulation of its Rep protein. FEBS Lett 581:2675–2683
Chobert JM, Sitohy M, Billaudel S et al (2007) Anticytomegaloviral activity of esterified milk proteins and L-polylysines. J Mol Microbiol Biotechnol 13:255–258
Fuentes A, Ramos P, Fiallo E et al (2006) Intron-hairpin RNA derived from replication associated protein C1 gene confers immunity to tomato yellow leaf curl virus infection in transgenic tomato plants. Transgenic Res 15:291–304
Geyer CR, Brent R (2000) Selection of genetic agents from random peptide aptamer expression libraries. Methods Enzymol 328:171–208
Heyraud F, Matzeit V, Kammann M et al (1993) The conserved nonanucleotide motif of the geminivirus stem loop sequence promotes replicational release of virus molecules from redundant copies. Biochimie 75:605–615
Hong Y, Saunders K, Hartley MR, Stanley J (1996) Resistance to geminivirus infection by virus-induced expression of dianthin in transgenic plants. Virology 220:119–127
Koshino-Kimura Y, Takenaka K, Domoto F, Aoyama Y, Sera T (2008) Generation of plants resistant to tomato yellow leaf curl virus by using artificial zinc-finger proteins. Nucleic Acids Symp Ser 52:189–190
Kumar A, Sarin NB (2013) RNAi: a promising approach to develop transgenic plants against geminiviruses and insects. J Plant Physiol Pathol 1:1
Lopez-Ochoa L, Ramirez-Prado J, Hanley-Bowdoin L (2006) Peptide aptamers that bind to a geminivirus replication protein interfere with viral replication in plant cells. J Virol 80:5841–5853
Mansoor S, Briddon RW, Zafar Y, Stanley J (2003) Geminivirus disease complexes: an emerging threat. Trends Plant Sci 8:128–134
Mitter N, Dietzgen RG (2012) Use of hairpin RNA constructs for engineering plant virus resistance. Methods Mol Biol 894:191–208
Mori T, Takenaka K, Domoto F, Aoyama Y, Sera T (2013) Inhibition of binding of tomato yellow leaf curl virus rep to its replication origin by artificial zinc-finger protein. Mol Biotechnol 54:198–203
Orozco BM, Hanley-Bowdoin L (1996) A DNA structure is required for geminivirus replication origin function. J Virol 70:148–158
Padidam M, Beachy RN, Fauquet CM (1999) A phage single-Stranded DNA (ssDNA) binding protein complements ssDNA accumulation of a geminivirus and interferes with viral movement. J Virol 73:1609–1616
Pan Y, Lee A, Wan J et al (2006) Antiviral properties of milk proteins and peptides. Int Dairy J 16:1252–1261
Patil BL, Ogwok E, Wagaba H, Mohammed IU, Yadav JS et al (2011) RNAi-mediated resistance to diverse isolates belonging to two virus species involved in Cassava brown streak disease. Mol Plant Pathol 12:31–41
Pooggin M, Shivaprasad PV, Veluthambi K, Hohn T (2003) RNAi targeting of DNA virus in plants. Nat Biotechnol 21:131–132
Prins M, Laimer M, Noris E, Schubert J, Wassenegger M, Tepfer M (2008) Strategies for antiviral resistance in transgenic plants. Mol Plant Pathol 9:73–83
Ramesh SV, Mishra AK, Praveen S (2007) Hairpin RNA-mediated strategies for silencing of tomato leaf curl virus AC1 and AC4 genes for effective resistance in plants. Oligonucleotides 17:251–257
Reyes MI, Nash TE, Dallas MM, Ascencio-Ibáñez JT, Hanley-Bowdoin L (2013) Peptide aptamers that bind to geminivirus replication proteins confer a resistance phenotype to tomato yellow leaf curl virus and tomato mottle virus infection in tomato. J Virol 87:9691–9706
Rudolph C, Schreier PH, Uhrig JF (2003) Peptide-mediated broad-spectrum plant resistance to tospoviruses. Proc Natl Acad Sci USA 100:4429–4434
Safarnejad MR, Fischer R, Commandeur U (2009) Recombinant-antibody-mediated resistance against tomato yellow leaf curl virus in Nicotiana benthamiana. Arch Virol 154:457–467
Safarnejad MR, Jouzani GS, Tabatabaei M et al (2011) Antibody-mediated resistance against plant pathogens. Biotechnol Adv 29:961–971
Sahu PP, Sharma N, Puranik S, Muthamilarasan M, Prasad M (2014) Involvement of host regulatory pathways during geminivirus infection: a novel platform for generating durable resistance. Funct Integr Genomics 14:47–58
Sera T (2005) Inhibition of virus DNA replication by artificial zinc finger proteins. J Virol 79:2614–2619
Shepherd DN, Mangwende T, Martin DP et al (2007) Maize streak virus-resistant transgenic maize: a first for Africa. Plant Biotechnol J 5:759–767
Shepherd DN, Mangwende T, Martin DP, Bezuidenhout M, Thomson JA, Rybicki EP (2007) Inhibition of maize streak virus (MSV) replication by transient and transgenic expression of MSV replication-associated protein mutants. J Gen Virol 88:325–336
Shivaprasad P, Thillaichidambaram P, Balaji V, Veluthambi K (2006) Expression of full-length and truncated Rep genes from Mungbean yellow mosaic virus-Vigna inhibits viral replication in transgenic tobacco. Virus Genes 33:365–374
Sunitha S, Marian D, Hohn B, Veluthambi K (2011) Antibegomoviral activity of the agrobacterial virulence protein VirE2. Virus Genes 43:445–453
Tavladoraki P, Benvenuto E, Trinca S (1993) Transgenic plants expressing a functional single-chain Fv antibody are specifically protected from virus attack. Nature 366:469–472
van den Heuvel JFJM, Verbeek M, van der Wilk F (1994) Endosymbiotic bacteria associated with circulative transmission of potato leaf roll virus by Myzus persicae. J Gen Virol 75:2559–2565
Vanderschuren H, Akbergenov R, Pooggin MM, Hohn T, Gruissem W et al (2007) Transgenic cassava resistance to African cassava mosaic virus is enhanced by viral DNA-A bidirectional promoter-derived siRNAs. Plant Mol Biol 64:549–557
Zhang P, Fu¨tterer J, Frey P, Potrykus I, Puonti-Kaerlas J, Gruissem W (2003) Engineering virus-induced ACMV resistance by mimicking a hypersensitive reaction in transgenic cassava plants. In: Vasil IK (ed) Plant biotechnology 2002 and beyond: proceedings of the 10th IAPTC&B congress. Kluwer Academic Publishers, Dordrecht, pp 143–146
Zrachya A, Kumar PP, Ramakrishnan U et al (2007) Production of siRNA targeted against TYLCV coat protein transcripts leads to silencing of its expression and resistance to the virus. Transgenic Res 16:385–398
Acknowledgments
Authors work in this area is supported by the core grant of National Institute of Plant Genome Research, New Delhi, India. Authors also thank Ms. Jananee Jaishankar for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahu, P.P., Prasad, M. Application of molecular antiviral compounds: novel approach for durable resistance against geminiviruses. Mol Biol Rep 42, 1157–1162 (2015). https://doi.org/10.1007/s11033-015-3852-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-015-3852-3