Abstract
Mesenchymal stem cells (MSCs) show accelerated regeneration potential when these cells experience hypoxic stress. This “preconditioning” has shown promising results with respect to cardio-protection as it stimulates endogenous mechanisms resulting in multiple cellular responses. The current study was carried out to analyze the effect of hypoxia on the expression of certain growth factors in rat MSCs and cardiomyocytes (CMs). Both cell types were cultured and assessed separately for their responsiveness to hypoxia by an optimized dose of 2,4,-dinitrophenol (DNP). These cells were allowed to propagate under normal condition for either 2 or 24 h and then analyzed for the expression of growth factors by RT-PCR. Variable patterns of expression were observed which indicate that their expression depends on the time of re-oxygenation and extent of hypoxia. To see whether the growth factors released during hypoxia affect the fusion of MSCs with CMs, we performed co-culture studies in normal and conditioned medium. The conditioned medium is defined as the medium in which CMs were grown for re-oxygenation till the specified time period of either 2 or 24 h after hypoxia induction. The results showed that the fusion efficiency of cells was increased when the conditioned medium was used as compared to that in the normal medium. This may be due to the presence of certain growth factors released by the cells under hypoxic condition that promote cell survival and enhance their fusion or regenerating ability. This study would serve as another attempt in designing a therapeutic strategy in which conditioned MSCs can be used for ischemic diseases and provide more specific therapy for cardiac regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases are still the major cause of morbidity and mortality worldwide despite our continuous progress in the understanding of the molecular mechanisms associated with the advancement of these diseases and their treatment strategies [1, 2]. Heart has very limited regeneration capacity following myocardial infarction. This irreparable damage to cardiomyocytes occurs due to sudden deprivation of oxygen supply to the heart [3]. A variety of cells e.g. embryonic stem cells, hematopoietic stem cells, mesenchymal stem cells, cardiac stem cells, and endothelial progenitor cells have shown improved cardiac function in animal models [4, 5]. Mesenchymal stem cells (MSCs) are the adult pluripotent stem cells. They have the ability to differentiate into osteocytes, chondrocytes, adipocytes, fibroblasts, myobalsts, endothelial cells and cardiomyocytes [6]. This trans-differentiation property of MSCs makes these cells an important source for the regulation of several damaged tissues in tissue engineering processes [7].
It has been observed that MSCs show an accelerated regeneration potential when they experience hypoxic stress [8]. This phenomenon called “preconditioning” has shown promising results for cardio-protection as it stimulates endogenous mechanisms that result in multiple cellular responses. These responses include activation of specific cell surface receptors by growth factors and cytokines [2, 9]. A number of endogenous processes are initiated in response to tissue damage. Stem cell mobilization is the first step in the tissue regeneration process. Stem cell factor (SCF) and hypoxia inducing factor (HIF) mobilize stem cells towards the site of injury [10]. Hepatocyte growth factor (HGF) becomes up-regulated during tissue and organ damage and this may be involved in the recruitment of expanded MSCs to the damaged tissue [11]. Fibroblast growth factor (FGF) is involved in differentiation of resident cardiac precursor cells into functional cardiomyocytes [12]. IL-7 may promote a variety of indispensable functions e.g. regeneration ability of injured tissues by stimulating differentiation of resident stem-like progenitor cells [13].
The present study was conducted to analyze the effect of hypoxic stress on gene expression of some of the cardio-protective growth factors in mesenchymal stem cells (MSCs) and cardiomyocytes (CMs). Co-culture study of MSCs and CMs were performed in conditioned medium to observe whether fusion between these two types of cells can be enhanced by the presence of hypoxia induced growth factors in the conditioned medium. Various conditions of chemical hypoxia and re-oxygenation were used prior to co-culture to see if there is any change in the number of fused cells associated with the differences in the gene expression patterns of specific growth factors.
Materials and Methods
Animals
All animal procedures were carried out in accordance with the international guidelines for the care and use of laboratory animals and approval from the local ethical committee. Sprague–Dawley (SD) rats weighing 200–300 g were used throughout the study.
Cell Culture
MSCs
MSCs were isolated from tibia and femur of SD rats. Whole bone marrow was cultured in Dulbecco’s modified eagle’s medium (DMEM; GIBCO, Boston, USA) supplemented with 10 % fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin, 1 mM sodium pyruvate and 4 mM l-glutamine. Culture was maintained at 37 °C in a humidified atmosphere containing 5 % CO2. Non-adherent hematopoietic cells were removed from the adherent MSCs. Medium was changed after every 3 days. The cells were observed under microscope regularly for morphological examination and confluency. The cells were sub-cultured when they reached approximately 70 % confluence. The first sub-cultured population is termed passage-1 (P1) cells. MSCs of passages 1–2 (P1 or P2) were used throughout the study.
CMs
Cultures of neonatal cardiomyocytes were prepared from the ventricles of 1–2 days old SD rats [14]. Briefly, whole hearts were excised and immediately transferred into ice-cold phosphate-buffered saline (PBS), followed by sterile ice cold balanced salt solution (20 mM HEPES–NaOH; pH 7.6, 130 mM NaCl,1 mM NaH2PO4, 4 mM glucose, 3 mM KCl). The ventricles were excised and minced in 0.05 % trypsin–EDTA. Cells were digested in 0.25 % trypsin–EDTA at 37 °C for 2–4 min and centrifuged at 3,500 rpm for 10 min at 4 °C. The cell pellet was resuspended in a maintenance medium containing DMEM/F12 (1:1) supplemented with FBS (20 %), penicillin (100 U/ml), streptomycin (100 mg/ml) and bovine insulin (1 μg/ml).
Characterization of MSCs and CMs
Immuno-cytochemistry
Cultured MSCs were analyzed for the presence of surface markers by immunostaining with antibodies against integrin β1 (CD29), Thy-1 (CD90), homing associated cell adhesion molecule (CD44) and c-kit (CD117) while rat neonatal cardiomyocytes were analyzed for the presence of cardiac specific proteins using antibodies against actin, troponin T and GATA4. Cells were fixed in 4 % paraformaldehyde, and blocked in PBS containing 2 % BSA, 2 % normal goat serum and 0.2 % Nonidet P-40 followed by incubation with primary antibodies at 1:100 dilution in blocking solution overnight at 4 °C. This was followed by incubation with Alexa fluor 546 goat anti mouse secondary antibody (invitrogen) for 1 h at room temperature. The cells were counter-stained with DAPI and examined under fluorescent microscope. Quantification of positive cells was done by ImageJ, a public domain Java image processing program [15]. Different images and within each image several fields were selected and number of cells were counted in terms of DAPI staining. Out of these, positive cells were counted and percentage was calculated.
Flow cytometry
MSCs were also characterized for the presence of surface markers by flow cytometry. Cells were suspended in FACS solution (1 % BSA, 1 mM EDTA, 0.1 % sodium azide in PBS). After treatment with blocking solution (1 % BSA in PBS) for 2 min at room temperature, the cells were incubated with specific primary antibodies against CD29, CD44 and CD90 in 1:40 dilution for 30 min. Alexa fluor 546 goat anti mouse secondary antibody was then added to each tube and incubated on ice for 30 min. Finally, FACS solution was added to each tube. Cells were analyzed in flow cytometer. MSCs treated only with secondary antibody was used as control.
Analysis of Growth Factors Expressed in Response to Hypoxia
Hypoxia
2,4-dinitrophenol (DNP) was used to induce hypoxia chemically [16]. For optimization, different concentrations (0.05–2 mM) of DNP were used at two different time points i.e. 10 and 20 min. Cells were observed morphologically and the number of dead cells were counted. Cytotoxity measurement was also performed to quantitate number of dead cells by flow cytometry. After optimization, 0.25 mM concentration of DNP was used throughout the experiments for both time periods. Prior to DNP treatment, cells were washed twice with glucose free DMEM. Medium containing DNP was removed and cells were re-oxygenated for either 2 or 24 h by incubating them at 37 °C in humidified chamber with 5 % CO2. Untreated cells were used as control.
PCR
Total RNA from treated and control groups were isolated using RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. 1 μg of RNA was reverse transcribed using the Superscript RT Kit (invitrogen) and amplified using oligonucleotide primers corresponding to genes specific for stem cell factor (SCF), interleukin-7 (IL-7), IL-7 receptor (IL-7 R), hepatocyte growth factor (HGF), insulin-like growth factor (IGF), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF). Rat GAPDH gene was used as an internal standard. The primer sequences used in this study along with the expected product sizes and annealing temperatures are listed in Table 1. The products of reverse transcriptase reaction were denatured for 1 min at 94 °C, followed by 35 cycles of amplification: denaturation at 94 °C (1 min), annealing at 49–63 °C (1 min), and extension at 72 °C (1 min) and a final extension at 72 °C for 10 min. One-fifth of each PCR product was electrophoretically resolved on 1 % agarose gel.
Analysis of Fusion Efficiency of MSCs and CMs
Hypoxia
CMs were treated with 0.25 mM DNP for 20 min and re-oxygenated for either 2 or 24 h.
MSCs were grown in the normal medium as described earlier. Prior to co-culture, MSCs and CMs were treated with cell labeling dyes, PKH26 and PKH67 (sigma) respectively according to the manufacturer’s instructions. CMs were subjected to DNP treatment as described earlier. Co-culture studies were performed either in the normal or conditioned medium i.e. the medium used for the re-oxygenation of CMs after DNP treatment was kept and used for co-culture.
Co-culture analysis was done by fluorescent microscopy and flow cytometry. Different groups that were used for co-culture studies are outlined in Table 2.
Statistical Analysis
Data is presented as means ± standard error of means (SEM). Statistical analysis were performed by using one way ANOVA and bonferroni’s post hoc test for multiple comparisons. Value of p < 0.05 was considered statistically significant.
Results
Characterization of MSCs and CMs
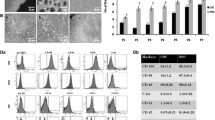
Immunocytochemistry analysis of rat bone marrow MSCs showed that almost all of the cultured cells expressed CD29, CD44, CD90 and ckit cell surface markers (Fig. 1). Flow cytometry analysis of MSCs showed that the expression was ≥80 % for CD29, CD44 and CD90 (Fig. 2). CMs showed positive expression of cardiac specific proteins, actin, troponin T and GATA4 (Fig. 3).
Immunocytochemical analysis of rat mesenchymal stem cells (MSCs) at passage 1 (P1): a Cells were treated with specific primary antibodies against ckit, CD29, CD44, CD90 and CD34 and Alexa fluor 546 secondary antibody while control was treated only with the secondary antibody; b Quantification of the number of positive cells treated with each of these specific antibodies using ImageJ software
Flow cytometry analysis of rat bone marrow derived MSCs: MSCs were positive for a CD29, b CD 44 and c CD90. MSCs were treated with anti rat CD29, CD44 and CD90 and Alexa fluor 546 goat anti mouse secondary antibody. ≥80 % of cells was positive for these surface markers. MSCs treated only with secondary antibody was used as control. Data was interpreted using Cell Quest software. FSC was selected as the threshold parameter and threshold was set to a value of 52 to eliminate small debris. A total of 10,000 events were acquired from each sample to calculate the percentages. Experiments were repeated three times for statistical analysis
Immunocytochemical analysis of neonatal rat cardiomyocytes (CMs) at passage 1 (P1): a Cells were treated with specific primary antibodies against actin, troponin T, and GATA4 and Alexa fluor 546 secondary antibody while control was treated only with the secondary antibody; b Quantification of the number of positive cells treated with each of these specific antibodies using ImageJ software
Growth factor expression analysis by RT-PCR
An optimized concentration (0.25 mM) of DNP at 10 and 20 min was selected to induce hypoxic stress. Different concentrations (0.05–2 mM) of DNP were used for optimization. Cells were examined morphologically for number of dead cells. At 0.05 and 0.1 mM concentrations, no change in the morphology of MSCs was observed. At 0.25 and 0.5 mM concentrations, cells were slightly shrunken but regained normal morphology after re-oxygenation while concentrations of 1 and 2 mM were toxic to cells and almost all cells became dead. There was no significant difference in the number of apoptotic cells in case of normal and hypoxic cells after 24 h of re-oxygenation (results not shown). The mRNA expression patterns of SCF, IL-7, IL-7 R, HGF, IGF, TGF-β and VEGF were analyzed in case of both MSCs and CMs by RT-PCR after exposing cells to DNP for 10 and 20 min and re-oxygenating them for either 2 or 24 h.
CMs
After 2 h of re-oxygenation, no significant change in the mRNA expression of any of the growth factors was observed when CMs were exposed to 10 min hypoxia whereas, after 20 min of DNP treatment, the expression of IGF was increased and that of IL-7 R was decreased significantly (Fig. 4a). After 24 h of re-oxygenation, CMs exposed to 10 min hypoxia showed significant increase in the mRNA expression of IL-7. The mRNA expression of VEGF was decreased when CMs were exposed to both 10 and 20 min hypoxia (Fig. 4b).
RT-PCR gene expression analysis of growth factors released in response to hypoxia in CMs: Expression levels of SCF, IL-7, IL-7 R, HGF, IGF, TGFβ, VEGF and GAPDH after hypoxia induction and a 2 h and b 24 h of re-oxygenation. Lane 1: growth factor expression in untreated or normal MSCs and hypoxic MSCs after 10 and 20 min respectively. Bar diagram showing densitometry analysis of growth factors from each group
MSCs
MSCs exposed to 10 min hypoxia and 2 h re-oxygenation showed significant down-regulation in the mRNA expression of IL-7 while in case of 20 min hypoxia, they showed significant down-regulation of IL-7, IL-7 R and HGF (Fig. 5a). In case of 24 h re-oxygenation, MSCs exposed to 10 min hypoxia showed significant increase in the mRNA expression of IL-7 while significant up regulation of SCF, IL-7, and IL-7 R was observed after 20 min of hypoxia (Fig. 5b).
RT-PCR gene expression analysis of growth factors released in response to hypoxia in MSCs: Expression levels of SCF, IL-7, IL-7 R, HGF, IGF, TGFβ, VEGF and GAPDH after either 10 or 20 min DNP exposure for hypoxia induction and a 2 h and b 24 h of re-oxygenation. Lane 1: growth factor expression in untreated or normal MSCs and hypoxic MSCs after 10 and 20 min respectively. Bar diagram showing densitometry analysis of growth factors from each group
Co-culture studies
The 5-day co-culture of MSCs and hypoxic CMs have shown a significant increase in the number of fused cells when the conditioned medium was used and the time of re-oxygenation was increased from 2 to 24 h (Fig. 6a). The 15-day co-culture of MSCs and hypoxic CMs have also shown a significant increase in the number of fused cells both in case of normal and conditioned medium. This increase in the number of fused cells was observed both in case of 2 and 24 h re-oxygenation (Fig. 6b). In all cases, the number of fused cells increased when the co-culture period was increased from 5 to 15 days except in case of normal (control) cells and in case where normal medium was used and reo-oxygenation time period was 24 h after hypoxia (Fig. 6c–e). We have observed several double labeled cells and elongated cell projections in co-culture through fluorescent microscopy which are indicative of cell fusion (Fig. 7). In some cases, we also found elongated cell projections protruding from different cells indicating that the cells are interacting with each other. This most probably would result in direct cell to cell connections in due course of time.
Flow cytometry graphs showing co-culture of MSCs (labeled with PKH26) and CMs (labeled with PKH67): The co-culture was analyzed after a 5 days and b 15 days. The study includes anlaysis of normal MSCs and normal CMs (control), normal MSCs and hypoxic CMs after 2 h re-oxygenation in normal medium (Normal 2 h RO), normal MSCs and hypoxic CMs after 2 h re-oxygenation in conditioned medium (conditioned 2 h RO), normal MSCs and hypoxic CMs after 24 h re-oxygenation in normal medium (normal 24 h RO), normal MSCs and hypoxic CMs after 24 h re-oxygenation in conditioned medium (conditioned 24 h RO). Bar diagrams showing statistical comparison within groups in c 5 and d 15 days and e the overall comparison in all of the above mentioned groups between 5 and 15 days of co-culture. Data was interpreted using Cell Quest software. FSC was selected as the threshold parameter and threshold was set to a value of 52 to eliminate small debris. Dot plot with quadrant was made and FACS analysis was done on the basis of percentages. A total of 10,000 events were acquired to calculate the percentages. Two parameters displayed simultaneously in a plot; one parameter FL1 530 (green fluorescence detector) was displayed on the y-axis and the other parameter FL2 585 (red fluorescence detector) was displayed on the x-axis. Merged cells which possess both red and green fluorescence appeared in upper right region of 2-D plot. Experiments were repeated three times. Data is presented as means ± standard error of the means (SEM) and calculated using Microsoft Excel. Statistical significance (*p < 0.05; **p < 0.01 and ***p < 0.001) was determined by analysis of variance (ANOVA) and bonferroni’s post hoc test for multiple comparisons using SPSS software. (Color figure online)
Co-culture analysis of MSCs (labeled with PKH26) and CMs (labeled with PKH67) by fluorescent microscopy: a Normal MSCs and normal CMs; b 5 days co-culture: MSCs and hypoxic CMs (2 h) in normal medium; c 5 days co-culture: MSCs and hypoxic CMs (2 h) in conditioned medium; d 15 days co-culture: MSCs and hypoxic CMs (2 h) in normal medium; e 15 days co-culture: MSCs and hypoxic CMs (2 h) in conditioned medium; f 5 days co-culture: MSCs and hypoxic CMs (24 h) in normal medium; g 5 days co-culture: MSCs and hypoxic CMs (24 h) in conditioned medium; h 15 days co-culture: MSCs and hypoxic CMs (24 h) in normal medium; i 15 days co-culture: MSCs and hypoxic CMs (24 h) in conditioned medium
Discussion
Growth factors are released by cells in response to various stimuli. A number of studies have been conducted to analyze the up-regulation of various growth factors in response to oxygen deprivation [17–21]. In this study, we used 2,4-dinitrophenol (DNP) to induce hypoxia chemically. DNP is a known metabolic inhibitor used with success to induce metabolic stress in different cell types [22, 23]. It decreases intracellular ATP production and induces chemical hypoxia [24]. Growth factors released in response to hypoxia were analyzed by RT-PCR. These growth factors could play important role during the process of myocardial infarction. The concentration of the DNP was optimized to obtain controlled hypoxia in which the cells were not completely dead but revived shortly after re-oxygenation process. We used two different time points to see if there is any effect of the extent of hypoxia on these growth factors. Earlier we have reported preliminary studies done for optimization [25]. Here we report the analyses of the mRNA expressions for SCF, IL-7, IL-7 R HGF, IGF, TGF-β and VEGF in control and DNP treated MSCs and CMs. In all cases, the re-oxygenation was maintained for either 2 or 24 h. We observed mixed pattern of expression in case of SCF, IL-7, IL-7R, HGF, IGF and VEGF. In some cases up-regulation was observed only after 20 min exposure to DNP indicating that the expression was dependent on the extent of hypoxia. Expression pattern was also dependent on time of re-oxygenation; in some cases significant changes in the expression was observed only when re-oxygenation time was 24 h. Whereas analysis was done only at two re-oxygenation time points i.e. 2 and 24 h, it is highly likely that the expression was induced at some mid-points as well. In this study, change in SCF and IL-7 expressions was noteworthy; SCF was up-regulated in case of MSCs whereas IL-7 was up-regulated both in case of MSCs and CMs. The response of growth factors varies with the extent of hypoxia and time of re-oxygenation and if crucially monitored, they can be beneficially used against many ischaemic conditions along with stem cells in cellular therapies.
Cell fusion is often observed in co-culture studies of different cells in vitro [26–28]. It may lead to trans-differentiation in vivo when stem cells are introduced in the infarcted myocardium. It has been shown in previous studies that MSCs can be differentiated into various cell types including endothelial cells and cardiomyocytes as well as bone, fat, cartilage, muscle, epithelium, and neural cells both in vitro and in vivo [29–33]. To see the effect of preconditioning on fusion between MSCs and CMs, we co-cultured these cells in the normal and conditioned medium. As the conditioned medium is the one which is taken after the re-oxygenation of CMs for either 2 or 24 h, this medium is expected to be rich in growth factors. Only CMs were used for hypoxia treatment so as to mimic the ischemic condition of myocardial infarction. At 5 days of co-culture, there was a gradual increase in the number of fused cells in case of conditioned medium as compared to the normal medium. Significant increase in the percentage of fused cells was observed in case of conditioned medium with 24 h re-oxygenation as compared to that of 2 h re-oxygenation. This indicates that when cells were re-oxygenated for 2 h, there might be lesser number of growth factors present in the conditioned medium or some of the specific growth factors that promote fusion or trans-differentiation of cells were not present in the medium during that time. Similarly at 15 days of co-culture, there was also a gradual increase in the number of fused cells where conditioned medium was used. The percentage of fused cells was significantly increased in case of conditioned medium with both 2 and 24 h of re-oxygenation. In all cases, fused cells were more in case of 15 days as compared to 5 days of co-culture except where normal medium was used and CMs used for co-culture were earlier exposed to 24 h of re-oxygenation. The possible reason may be that the normal medium does not consist of the required growth factors. The time period of release of growth factors should be between 2 and 24 h therefore CMs exposed to 2 h of re-oxygenation in co-culture showed significant increase in the number of fused cells even in the normal medium. It can therefore be assumed that the growth factors were still being released in the medium during the co-culture. Through microscopy it was observed that elongated cell projections were present protruding from various cells in co-culture indicating that the cells are interacting with each other. This most probably would result in direct cell to cell connections in due course of time. It can be inferred from these results that fusion efficiency of MSCs and CMs can be enhanced by the addition of certain growth factors released during hypoxia and this efficiency continues to increase when the time of co-culture advances.
Growth factors released during hypoxia can promote the fusion of transplanted MSCs with the host cardiomyocytes. However, we still currently do not fully understand the exact mechanism by which the interaction of these two types of cells takes place and exactly which molecules increase the fusion rate. It has been shown by clinical studies that regeneration of myocardium restores cardiac function after stem cell transplantation [34, 35]. However, fusion of normal cells does have their limitations in terms of survival as the fate of the fused cells is unknown.
Conclusion
The present study provides evidence that cell to cell fusion can be increased by means of conditioned medium obtained after hypoxia-re-oxygenation that may comprise certain growth factors. The fused cells may proliferate and contribute to the regeneration of damaged myocardium. As the mechanisms whereby adult stem cells repair the damaged myocardium are still unclear, it is important to determine the contribution of various factors in the fusion process so that a rational basis for the use of these cells for the therapy for damaged heart can be determined. The results of the present study suggest that various growth factors are expressed at different levels. They may be able to increase the fusogenic ability of cells in co-culture. This study would aid in designing a therapeutic strategy in which conditioned MSCs or MSCs over-expressed with certain growth factors can be used for ischemic diseases.
Abbreviations
- SCF:
-
Stem cell factor
- TGF-β:
-
Transforming growth factor-β
- VEGF:
-
Vascular endothelial growth factor
- IL-7:
-
Interleukin-7
- HGF:
-
Hepatocyte growth factor
- IGF:
-
Insulin-like growth factor
- MSCs:
-
Mesenchymal stem cells
- CM:
-
Cardiomyocytes
- DNP:
-
2,4-Dinitrophenol
References
Massie BM (2011) Novel targets for the treatment of heart failure: perspectives from a heart failure clinician and trialist. J Mol Cell Cardiol 51:438–440
Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P (2009) Pharmacological preconditioning of mesenchymal stem cells with Trimetazidine protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharm Exp Ther 329:543–550
Srivastava D, Ivey KN (2006) Potential of stem-cell-based therapies for heart disease. Nature 441:1097–1099
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S (2001) Neovascularization of ischemic myocardium by human bone marrow derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7:430–436
Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P (2001) Bone marrow cells regenerate infarcted myocardium. Nature 410:701–705
Brehm M, Zeus T, Strauer BE (2002) Stem cells-clinical application and perspectives. Herz 27:611–620
Ioannidou E (2006) Therapeutic modulation of growth factors and cytokines in regenerative medicine. Curr Pharm Des 12:2397–2408
Ma T, Grayson WL, Frohlich M, Vunjak-Novakovic G (2009) Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog 25:32–42
Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K (2006) Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol 291:H886–H893
Garin G, Mathews M, Berk BC (2005) Tissue resident bone marrow-derived progenitor cells: key players in hypoxia-induced angiogenesis. Circ Res 97:955–957
Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A (2006) Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 24:1254–1264
Kanellakis P, Slater NJ, Du XJ, Bobik A, Curtis DJ (2006) Granulocytes colony-stimulating factor and stem cell factor improve endogenous repair after myocardial infarction. Cardiovasc Res 70:117–125
Chen Y, Shao JZ, Xiang LX, Dong XJ, Zhang GR (2008) Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biol 40:815–820
Sreejit P, Kumar S, Verma RS (2008) An improved protocol for primary culture of cardiomyocyte from neonatal mice. In Vitro Cell Dev Biol Anim 44:45–50
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Jovanović S, Du Q, Sukhodub A, Jovanović A (2009) M-LDH physically associated with sarcolemmal K ATP channels mediates cytoprotection in heart embryonic H9C2 cells. Int J Biochem Cell Biol 41:2295–2301
Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR (2008) Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol 294:C675–C682
Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ (2006) Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20:661–669
Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S et al (2004) Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109:1543–1549
Lee SH, Lee YJ, Song CH, Ahn YK, Han HJ (2010) Role of FAK phosphorylation in hypoxia-induced hMSCS migration: involvement of VEGF as well as MAPKS and eNOS pathways. Am J Physiol Cell Physiol 298:C847–C856
Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY (2007) STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol 42:1009–1015
Brady PA, Zhang S, Lopez JR, Jovanović A, Alekseev AE, Terzic A (1996) Dual effect of glyburide, an antagonist of KATP channels, on metabolic inhibition-induced Ca2+ loading in cardiomyocytes. Eur J Pharmacol 308:343–349
Jovanović A, Jovanović S, Lorenz E, Terzic A (1998) Recombinant cardiac ATP-sensitive K+ channel subunits confer resistance towards chemical hypoxia-reoxygenation injury. Circulation 98:1548–1555
Han J, Kim E, Ho WK, Earm YE (1996) Blockade of the ATP-sensitive potassium channel by taurine in rabbit ventricular myocytes. J Mol Cell Cardiol 28:2043–2050
Haneef K, Naeem N, Iqbal H, Jamall S, Kabir N, Salim A (2010) Gene expression pattern in rat bone marrow mesenchymal stem cells in response to hypoxia. Pak J Biochem Mol Biol 43:90–93
Ishikawa F, Shimazu H, Shultz LD, Fukata M, Nakamura R, Lyons B et al (2006) Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. FASEB J 20:950–952
Lacza Z, Horvath E, Busija DW (2003) Neural stem cell transplantation in cold lesion: a novel approach for the investigation of brain trauma and repair. Brain Res Protoc 11:145–154
Nygren JM, Jovinge S, Breitbach M, Säwén P, Röll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE (2004) Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med 10:494–501
Herzog EL, Chai L, Krause DS (2003) Plasticity of marrow-derived stem cells. Blood 102:3483–3493
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49
Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y (2006) Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Throm 13:77–81
Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG (2008) Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transpl 17:303–311
Xu J, Liu X, Jiang Y, Chu L, Hao H, Liua Z, Verfaillie C, Zweier J, Gupta K, Liu Z (2008) MAPK/ERK signaling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J Cell Mol Med 12:2395–2406
Cselenyák A, Pankotai E, Horváth EM, Kiss L, Lacza Z (2010) Mesencymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 11:29–38
Xu J, Liu X, Chen J, Zacharek A, Cui X, Savant-Bhonsale S, Chopp M, Liu Z (2010) Cell-cell interaction promotes rat marrow stromal cell differentiation into endothelial cell via activation of TACE/TNFalpha signaling. Cell Transpl 19:43–53
Acknowledgments
The financial support for this study was provided by the Higher Education Commission, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haneef, K., Naeem, N., Khan, I. et al. Conditioned medium enhances the fusion capability of rat bone marrow mesenchymal stem cells and cardiomyocytes. Mol Biol Rep 41, 3099–3112 (2014). https://doi.org/10.1007/s11033-014-3170-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3170-1