Abstract
The p22phox protein subunit is essential for NADPH oxidase activity. The prevalence of C242T variants of p22phox gene was studied in 101 healthy Egyptian controls and 104 acute myocardial infarction (AMI) Egyptian patients. Contribution of oxidative stress, represented by serum oxidized-LDL (ox-LDL), in development of AMI was also examined and correlated with C242T gene variants. Genotyping and ox-LDL were assessed by PCR–RFLP and ELISA. Results showed that wild type CC genotype is prevalent in 27 % of controls; CT and TT are in 72 and 1 %. In patients, the distribution was 40.2, 59.8 and 0 % for CC, CT and TT; respectively, showing a significant difference (p = 0.0259). Serum ox-LDL levels were higher in patients than controls (p ≤ 0.0001). Subjects having CT genotype had lower levels of ox-LDL than CC genotype (p ≤ 0.005). C242T polymorphism of p22phox gene of NADPH oxidase is a novel genetic marker associated with reduced susceptibility to AMI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI) is a complex multifactorial polygenic disorder that is thought to result from an interaction between a person’s genetic makeup and various environmental factors [1]. In general, the incidence of MI increases additively as a function of the number of conventional risk factors, which include hypertension, diabetes mellitus, and hypercholesterolemia. Although each risk factor is partly under genetic control, a family history of MI is also an independent predictor, suggesting the existence of additional susceptibility genes for this condition [2].
Nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) is a major source of the O −2 anion, which may play an important role in the development of atherosclerosis and coronary artery disease (CAD). p22phox, a component of the NADPH oxidase and encoded by the cytochrome b-245 a (CYBA) gene, is essential for the activation of this enzyme, and its over-expression has been reported in human atherosclerotic arteries [3].
Four types of polymorphism have been reported in the p22phox gene, namely C242T, C549T, G508A, and A640G. The C242T and C549T polymorphisms affect amino acid sequence, whereas the G508A and A640G polymorphisms do not, due to the degeneracy of the genetic code in the former case and the location of the latter in the 39 untranslated region [4].The C242T gene variant of p22phox gene causes a structural modification in the protein from histidine-to-tyrosine possibly leading to functional changes in the protein. Therefore, the functional consequences of the C242T polymorphisms in the p22phox gene could modify the risk of CAD by producing varying amounts of the O −2 anion [5, 6]. Analysis of coronary arteries indicated that O −2 is distributed homogeneously throughout normal vessels, whereas intense ROS production is found in the plaque shoulder of atherosclerotic arteries, suggesting that increased oxidative stress contributes to coronary atherosclerosis [7].
A growing body of evidence demonstrates that markers of inflammatory activity are increased in CAD patients [8]. High levels of inflammatory markers are also associated with an increased risk for development of CAD [9]. These associations are not surprising as inflammation of the arterial intima is one of the major characteristics of atherosclerosis [10]. Accumulation, aggregation and oxidative modification of LDL are believed to play an important role in activation of this inflammation [11]. Oxidation of LDL occurs mainly in the extracellular matrix of the arterial intima and ox-LDL is cleared locally by macrophages through the scavenger receptor pathway. The recent development of highly-sensitive ELISAs for ox-LDL using monoclonal antibodies has made it possible to identify ox-LDL in the circulation [10]. Increased levels of plasma ox-LDL have been demonstrated in patients with CAD. Moreover, patients with AMI and unstable angina, have been reported to have higher plasma levels of ox-LDL than controls [12].
The major aims of this study were to investigate the contribution of the C242T polymorphism of the p22phox gene of NADPH oxidase to the predisposition of early onset AMI in Egyptians and to correlate the different genotypes with the levels of plasma ox-LDL, a marker of oxidative stress.
Patients and methods
Study population
Random unrelated 101 healthy controls were recruited for the study from the volunteers attending the blood bank at 57357 Hospital in Cairo, Egypt. They were included if they had no clinical or diagnostic evidence for CHD and having controlled blood pressure below 140/90 mm. Out of the controls, 32 were females, ages between 18 and 62 years, and 69 were males, 19–54 years of age. On the other hand, random unrelated 104 AMI patients, divided into 35 females (age range 34–55 years) and 69 males (age range 35–55 years), were recruited from the intensive care unit of the National Heart Institute, Imbaba, Giza. Patients were included if they had a diagnosis of an acute single or multi-vessel CAD verified by clinical presentation, ECG changes, and/or cardiac markers elevation.
Written informed consent was obtained from each participant in the study that abided by the Helsinki declaration. Information on personal and family medical history and health-relevant behaviors, including exercise and diet was obtained by a routine questionnaire filled in by blood donors at the time of venesection. Exclusion criteria included any concomitant acute or chronic severe diseases such as renal failure, hepatic insufficiency, cardiovascular disease other than MI and diabetes mellitus.
Specimen collection
Fasting blood samples (4 ml) were collected into two sets of tubes; the first set was EDTA-coated vacuum tubes stored at 4 °C for DNA extraction. The second set was non EDTA-coated vacuum tubes, which were centrifuged at 1,000 rpm for 10 min and the resulting serum was separated and stored at −20 °C in 0.25 ml aliquots. The aliquots were used for determination of ox-LDL.
Purification of DNA from human blood by spin protocol
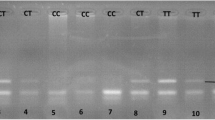
DNA extraction and purification was performed using QIAamp DNA Blood Mini Kits (Qiagen, Hilden, Germany). The purified DNA, free of protein, nucleases, and other contaminants or inhibitors, was used directly in the PCR reaction [13, 14]. DNA fragments containing C242T polymorphic site of p22 phox gene were amplified from genomic DNA by PCR with sense primer 5′-TGCTTGTGGGTAAACCAAGGCCGGTG-3′ and antisense primer 5′-AACACTGAGGTAAGTGGGGGTGGCTCCTGT-3′. The PCR product was then digested with restriction enzyme Rsa I. C → T mutation at nucleotide 242 creates an Rsa I digestion site, which digests the 348-bp fragment into 160- and 188-bp fragments [4]. A representative gel after electrophoresis of Rsa I digested p22 phox gene of the C242T polymorphism is shown in Fig. (1).
Assay of ox-LDL
Serum ox-LDL was determined by quantitative enzyme-linked immunosorbent assay (ELISA) using a commercial kit provided by Immunodiagnostik, Germany [15].
Data processing and statistical analysis
All statistical analyses were performed using the GraphPad Prism statistics software (GraphPad software, Inc.). Data are represented as mean ± standard error of mean (SEM). Comparison of the differences between groups was done using one-way analysis of variance (ANOVA) and Mann–Whitney test. Correlations between different parameters were tested by Pearson coefficient (r). A two-tailed p value ≤ 0.05 was considered statistically significant.
Results
Genotyping of C242T variants of the p22 phox gene of NADPH oxidase
The genotype distribution and allele frequencies of NADPH oxidase gene variants among the control subjects and AMI patients are shown in Figs. 2 and 3, respectively. A significant difference was observed in the genotype distribution pattern (Mann–Whitney test, p = 0.0259) between the patients and controls, while the difference in the allele frequencies was not significant (Mann–Whitney test, p = 0.1665). It was quite noticeable that the TT genotype is rare in both healthy (one subject) and patients (zero subjects).
Serum levels of ox-LDL
A highly significant difference (p ≤ 0.005) was observed between the mean serum levels of ox-LDL in patients (202.3 ± 21.3) (mean ± SEM) as compared to controls (113.5 ± 4.9).
Correlation of NADPH oxidase genotypes with serum ox-LDL levels in control and AMI subjects
There was a significant difference in the serum ox-LDL levels when compare the CC vs. CT genotypes of the NADPH oxidase gene either in the controls (p ≤ 0.05) or in patients (p ≤ 0.005) (Fig. 4). Because of the limited number of TT in both controls (one subject) and in patients (zero subjects), they were excluded from the comparison.
Serum ox-LDL concentrations for the various NADPH oxidase genotypes in control subjects and AMI patients. There was a significant difference in the serum ox-LDL levels between the CC and CT genotypes of the NADPH oxidase gene in the controls (p ≤ 0.05) and patients (p ≤ 0.005). Results are expressed as mean ± SEM. **Significantly different from CT at p ≤ 0.05
Discussion
Genotype distribution and allele frequency of C242T p22phox gene of NADPH oxidase
All cell types within the heart, including cardiomyocytes, endothelial cells; vascular smooth muscle cells (VSMCs), fibroblasts and infiltrating inflammatory cells generate ROS. Potential sources of ROS in these cell types include the mitochondrial electron transport chain, xanthine oxidases, ‘uncoupled’ nitric oxide synthases (NOSs), cytochrome P450 and NADPH oxidase. Among these sources, NADPH oxidase may be considered unique in that they generate ROS in a highly regulated manner whereas ROS are generated as a by-product of enzymatic activity by all the other sources [16–18].
In the last decade, five NADPH oxidase isoforms, each encoded by a separate gene and with distinct tissue distributions, have been identified [16, 17]. These isoforms are distinguished by the presence of distinct catalytic subunits, Nox1-Nox-5, which mediate the electron transfer process. In addition to the core catalytic Nox subunit, the enzymatic activity of the oxidase depends on additional subunits, which vary according to the isoform. These subunits include gp91phox and p22phox, and a cytosolic component composed of subunits p47phox, p40phox, p67phox, and a G protein, Rac [19]. In vessels from patients with CAD, expression of Nox2 and Nox4 is enhanced [3].
To our knowledge, this study represents the first clinical investigation in the Egyptian population on the significance of C242T polymorphism of NADPH oxidase in CAD. We found that the (CT + TT) genotypes were significantly more frequent in control subjects than in CAD patients, indicating that the C242T polymorphism of the potential heme-binding site in the p22 phox gene might have a protective effect on coronary risk. The p22 phox, a heme-binding protein, contains two histidine residues at amino acids 72 and 94 [20] which are the potential heme-binding sites. Because the C242T polymorphism substitutes the histidine-72 to tyrosine residues, this base substitution may have a direct functional role in the association between the C242T polymorphism and coronary risk. It is interesting to speculate that this mutation of the p22 phox gene might modulate the activity and regulation of NADPH oxidase, which leads to a decrease in oxidative stress in the vasculature, which in turn, might reduce susceptibility to CAD [4].
Our results were consistent with several studies done in Asians [4, 21–23] and in a Finnish population [24]. In harmony, Schachinger et al. [25] observed a significant increase in the flow-dependent dilation in patients bearing the T allele of the C242T polymorphism and an impaired coronary arterial dilator response to nitroglycerin in patients carrying the CC genotype. However, our results were in contrast with others done on Japanese [26] and Caucasian Italians [27]. Moreover, many researchers found no correlation between the C242T polymorphism of p22 phox gene and the risk of CAD and MI [28, 29].
Serum levels of ox-LDL
In the present study, the mean serum levels of ox-LDL were significantly elevated in patients compared to controls (p ≤ 0.05). The reason behind the observed increase in ox-LDL levels in AMI patients remains unknown. Previous in vitro studies have documented that macrophages and lymphocytes are capable of oxidizing LDL [12].
The culprit lesions of patients with AMI contain abundant macrophages and T lymphocytes [30]. Under these circumstances, ox-LDL in macrophage-derived foam cells may be enhanced within unstable plaques. One could hypothesize that the ox-LDL present within unstable plaques may be released into the blood stream in patients with severe endothelial injuries, such as in plaque erosion or rupture. Moreover, previous in vitro studies have demonstrated that neutrophils can oxidatively modify LDL into a form that is rapidly incorporated by macrophages [31]. Neutrophils are known to accumulate at sites of plaque rupture or erosion in patients with AMI. Hence, one could also hypothesize that these neutrophils could contribute to an increase in the blood ox-LDL levels, especially at early stages after injuries.
The causal role of ox-LDL is suspected but not established [32, 33]. The observed association between CAD and plasma levels of ox-LDL suggests investigating the causal role of ox-LDL in atherosclerotic cardiovascular disease in a prospective study. Previously, malodialdehyde-modified LDL was isolated from the plasma of AMI patients [34]. It was concluded that it did not originate from extensive metal ion-induced oxidation of LDL but that it may be generated by MDA released by oxidation of arachidonic acid present in LDL. Ischemic injury may induce not only the activation of the cyclooxygenase-dependent pathway of prostaglandin synthesis in endothelial cells, but also increased production of F2-isoprostanes, non-cyclooxygenase-derived prostaglandin F2-like compounds, that are strong inducers of platelet activation. Activated platelets may then produce large amounts of aldehydes, further enhancing the modification of LDL [35]. Experimental studies have identified several mechanisms through which ox-LDL may contribute to the development of atherosclerosis. Ox-LDL may cause intimal inflammation by activating the expression of adhesion molecules on endothelial cells, stimulating leucocyte chemotaxis and inducing release of growth factors from macrophages [11].
Many studies coincide with our results [10, 12, 35, 36] suggesting that ox-LDL plays an important role in the progression of CAD and AMI. Tsimikas et al. [37] reported that circulating levels of ox-LDL are strongly associated with angiographically documented CAD, and with serum lipoprotein (a), which binds oxidized phospholipids in LDL.
Association between C242T p22phox genotypes and ox-LDL in AMI patients
As shown in Fig. 4, serum ox-LDL levels were significantly reduced in the CT genotype compared to the wild genotype, CC, in both patients and controls confirming our finding that the T allele and the C242T polymorphism of the p22phox gene decreases the risk and has a protective role in CAD.
The above finding may be explained by the fact that O2 − production by vascular NADPH oxidase in the CC homozygous subjects may result in the oxidation of small LDL particles, and a reduction in the uptake of these oxidized particles by the hepatic LDL receptor. Thus the fraction of small LDL particles may be increased in the CC homozygotes without advanced atherosclerosis, because ox-LDL appears to induce expression of scavenger receptor mRNA in advanced atherosclerosis [38].
In summary, the salient findings of this work include:
-
(1)
The C242T polymorphism of the p22phox gene of NADPH oxidase may reduce susceptibility to MI and is a novel genetic marker that has a protective effect on coronary risk in the Egyptian Population. In harmony, subjects having CT genotype of the p22 phox gene of NADPH oxidase were found to have significantly lower levels of serum ox-LDL than the CC wild genotypes.
-
(2)
AMI Patients had higher serum ox-LDL levels compared to controls confirming the role of oxidative stress in the incidence of AMI.
References
Tobin MD, Braund PS, Burton PR, Thompson JR, Steeds R, Channer K, Cheng S, Lindpaintner K, Samani NJ (2004) Genotypes and haplotypes predisposing to myocardial infarction: a multilocus case-control study. Eur Heart J 25:459–467
Yamada Y, Izawa H, Ichihara S, Takatsu F, Ishihara H, Hirayama H, Sone T, Tanaka M, Yokota M (2002) Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N Engl J Med 347:1916–1923
Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybyowski P, Wierzbicki K, Korbut R, Harrison DG, Channon KM (2006) Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 26:333–339
Inoue N, Kawashima S, Kanazawa K, Yamada S, Akita H, Yokoyama M (1998) Polymorphism of the NADH/NADPH oxidase p22 phox gene in patients with coronary artery disease. Circulation 97:135–137
Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM (2000) Functional effect of the C242T polymorphism in the NAD(P)H oxidase p22phox gene on vascular superoxide production in atherosclerosis. Circulation 102:1744–1747
Wyche KE, Wang SS, Griendling KK, Dikalov SI, Austin H, Rao S, Fink B, Harrison DG, Zafari AM (2004) C242T CYBA polymorphism of the NADPH oxidase is associated with reduced respiratory burst in human neutrophils. Hypertension 43:1246–1251
Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK (2002) Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105:1429–1435
Ridker P, Libby P (1998) Nontraditional coronary risk factors and vascular biology: the frontiers of preventive cardiology. J Invest Med 46:338–350
Ridker P, Cushman M, Stampfer M, Tracy RP, Hennekens CH (1997) Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 336:973–979
Fredrikson GN, Hedblad B, Berglund G, Nilsson J (2003) Plasma oxidized LDL: a predictor for acute myocardial infarction? J Intern Med 253:425–429
Glass CK, Witztum JL (2001) Atherosclerosis: the road ahead. Cell 104:503–516
Ehara S, Ueda M, Naruko T, Haze K, Itoh A, Otsuka M, Komatsu R, Matsuo T, Itabe H, Takano T, Tsukamoto Y, Yoshiyama M, Takeuchi K, Yoshikawa J, Becker AE (2001) Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 103:1955–1960
Greenspoon SA, Scarpetta MA, Drayton ML (1998) QIAamp spin columns as a method of DNA isolation for forensic casework. J Forensic Sci 43:1024–1030
Fahle GA, Fischer SH (2000) Comparison of six commercial DNA extraction kits for recovery of cytomegalovirus DNA from spiked human specimens. J Clin Microbiol 38:3860–3863
Craig WY (1995) Auto antibodies against oxidized low density lipoprotein: a review of clinical findings and assay methodology. J Clin Lab Anal 9:70–74
Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4:181–189
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313
Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM (2006) NADPH oxidases in cardiovascular heth and disease. Antioxid Redox Signal 8:691–728
Lassegue B, Clempus RE (2003) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol Rev 285:R277–R297
Parkos CA, Dinauer MC, Walker LE, Allen RA, Jesaitis AJ, Orkin SH (1988) Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc Natl Acad Sci USA 85:3319–3323
Fang S, Wang L, Jia C (2010) Association of p22phox gene C242T polymorphism with coronary artery disease: a meta-analysis. Thromb Res 125:e197–e201
Lee WH, Hwang TH, Oh GT, Kwon SU, Choi YH, Park JE (2001) Genetic factors associated with endothelial dysfunction affect the early onset of coronary artery disease in Korean males. Vasc Med 6:103–108
He MA, Cheng LX, Jiang CZ, Zeng HS, Wang J, Wang F, Chen Y, Yang M, Tan H, Zheng HY, Hu FB, Wu TC (2007) Associations of polymorphism of P22(phox) C242T, plasma levels of vitamin E, and smoking with coronary heart disease in China. Am Heart J 153:e1–e6
Fan M, Kahonen M, Rontu R, Lehtinen R, Viik J, Niemi M, Nieminen T, Niemela K, Porsti I, Koobi T, Turjanmaa V (2006) The p22phox C242T gene polymorphism is associated with a reduced risk of angiographically verified coronary artery disease in a high-risk Finnish Caucasian population. The Finnish Cardiovascular Study. Am Heart J 152:538–542
Schachinger V, Britten MB, Dimmeler S, Zeiher AM (2001) NADH/NADPH oxidase p22 phox gene polymorphism is associated with improved coronary endothelial vasodilator function. Eur Heart J 22:96–101
Ito D, Murata M, Watanabe K, Yoshida T, Saito I, Tanahashi N, Fukuuchi Y (2000) C242T polymorphism of NADPH oxidase p22 PHOX gene and ischemic cerebrovascular disease in the Japanese population. Stroke 31:936–939
Nasti S, Spallarossa P, Altieri P, Garibaldi S, Fabbi P, Polito L, Bacino L, Brunelli M, Brunelli C, Barsotti A, Ghigliotti G (2006) C242T polymorphism in CYBA gene (p22phox) and risk of coronary artery disease in a population of Caucasian Italians. Dis Markers 22:167–173
Saha N, Sanghera DK, Kamboh MI (1999) The p22 phox polymorphism C242T is not associated with CHD risk in Asian Indians and Chinese. Eur J Clin Invest 29:999–1002
Mata-Balaguer T, de la Herran R, Ruiz-Rejon C, Ruiz-Rejon M, Garrido-Ramos MA, Ruiz-Rejon F (2004) Angiotensin-converting enzyme and p22(phox) polymorphisms and the risk of coronary heart disease in a low-risk Spanish population. Int J Cardiol 95:145–151
van der Wal AC, Becker AE, van der Loos CM, Das PK (1994) Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 89:36–44
Katsura M, Forster LA, Ferns GAA, Anggarda EE (1994) Oxidative modification of low density lipoprotein by human polymorphonuclear leukocytes to a form recognized by the lipoprotein scavenger pathway. Biochim Biophys Acta 1213:231–237
Chen CH, Nguyen HH, Weilbaecher D, Luo S, Gotto AM, Henry PD (1995) Basic growth factor reverses atherosclerotic impairment of human coronary angiogenesis-like responses in vitro. Atherosclerosis 116:261–268
Holvoet P, Collen D (1997) Thrombosis and atherosclerosis. Curr Opin Lipidol 8:320–328
Holvoet P, Perez G, Zhao Z, Brouwers E, Bernar H, Collen D (1995) Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J Clin Invest 95:2611–2619
Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D (1998) Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation 98:1487–1494
Vasankari T, Ahotupa M, Toikka J, Mikkola J, Irjala K, Pasanen P, Neuvonen K, Raitakari O, Viikari J (2001) Oxidized LDL and thickness of carotid intima-media are associated with coronary atherosclerosis in middle-aged men: lower levels of oxidized LDL with statin therapy. Atherosclerosis 155:403–415
Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB (2005) Oxidized phospholipids, Lp(a) lipoproteins, and coronary artery disease. N Engl J Med 353:46–57
Okana RH, Yamasaki Y, Ohtoshi K, Yasuda T, Katakami N, Hirano T, Yoshino G, Kajimoto Y (2002) NAD(P)H oxidase p22 Phox C242T polymorphism affects LDL particle size and insulin resistance in Japanese subjects. J Atheroscler Thromb 9:200–205
Acknowledgments
This study was supported by the Science and Technology Development Fund (STDF) Grant No. 2951.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hashad, I.M., Abdel Rahman, M.F., Abdel-Maksoud, S.M. et al. C242T polymorphism of NADPH oxidase p22phox gene reduces the risk of coronary artery disease in a random sample of Egyptian population. Mol Biol Rep 41, 2281–2286 (2014). https://doi.org/10.1007/s11033-014-3081-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3081-1