Abstract
The amiloride-sensitive epithelial sodium channel (ENaC) is a major contributor to intracellular sodium homeostasis. In addition to epithelial cells, osteoblasts (Obs) express functional ENaCs. Moreover, a correlation between bone Na content and bone disease has been reported, suggesting that ENaC-mediated Na+ regulation may influence osteogenesis. Obs were isolated and cultured by enzyme digestion. Cell proliferation and differentiation were evaluated by WST-8 assay kit and AKP assay kit respectively. PKGII expression was silenced by siRNA. The mRNA expression was investigated by semi-quantitative PCR and the protein expression was determined by Western-blot. The cell-permeable cGMP analog 8-(4-chlorophenylthio)-cGMP (8-pCPT-cGMP) increased α-ENaC channel expression in primary rat Obs as indicated by RT-PCR. In addition, 8-pCPT-cGMP stimulation enhanced expression of the mRNA encoding cGMP-dependent protein kinases II (PKGII). The cGMP analog also promoted osteoblast proliferation, differentiation and induced the expression of several osteogenic genes, including core binding factor al, osteocalcin, alkaline phosphatase, collagen type I, and osteopontin. Furthermore, the expression of α-ENaC, the main functional subunit of ENaC, was reduced when a small interfering RNA specific for PKGII was introduced into Obs. Treatment with 8-pCPT-cGMP in cells transfected with the siRNA for PKGII partially reversed downregulated α-ENaC mRNA expression. Our results suggest that 8-pCPT-cGMP stimulates proliferation, differentiation, and osteogenic gene expression in Obs through cGMP/PKGII-dependent regulation of ENaC channel expression. The cGMP/PKGII signaling pathway is a potential target for pharmaceutical interventions to treat metabolic bone diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal cells such as osteoblasts (Obs) and chondrocytes express a number of distinct ion channel types that may contribute to osteogenesis [7, 18]. The epithelial sodium channel (ENaC), first described in 1994, is a unique amiloride-sensitive non-voltage dependent sodium ion channel that belongs to the ENaC/DEG family of ion channels. The ENaC is a large glycosylated molecule found not only in the apical membranes of epithelial tissues like urinary bladder, renal collecting duct, distal colon, sweat and salivary glands, lung, and taste buds [14], but also in bone cells such as Obs and chondrocytes [1, 11]. The ENaC is responsible for transepithelial Na+ transport and plays a crucial role in sensing acidosis, maintaining intracellular sodium homeostasis, and in transducing mechanical stimuli [2, 8, 15]. The adult human body contains 90–130 g of sodium and roughly half is contained within bone. Although the exact role of bone sodium is unknown, extracellular sodium bathes the bone forming site during mineralization [26].
Osteoblasts are intimately linked to bone formation, repair, and remodeling. Indeed, the proliferation and differentiation of Obs help lay down new bone, while Obs also indirectly mediate osteoclastic bone resorption during remodeling [16]. Osteoblast proliferation and differentiation are regulated by many growth factors and cytokines [12] that activate numerous intracellular signaling pathways, including cAMP- and cGMP-dependent kinase cascades [17]. cGMP is the principle activator of PKG signaling in muscle and nerve cells [23], moreover, it can act as a direct external ENaC activator in oocytes [20, 21]. cGMP has three major intracellular receptors: cGMP-dependent protein kinases (PKGII, PKGIα, PKGIβ), PKG-dependent phosphodiesterases, and cyclic nucleotide-gated ion channels. Both PKGI and PKGII are expressed in Obs and chondrocytes, but they may have different functions in bone development [22, 27].
Little is known about the effects of ENaC activity on osteogenesis, however, and the molecular mechanisms through which cGMP regulates ENaCs are still unclear. Therefore, the aim of the present study was to examine the role cGMP/PKG signaling in ENaC expression and to investigate whether ENaC activity influences osteogenesis. To this end, we investigated the effect of exogenous 8-pCPT-cGMP, a cell-permeable cGMP analog, on osteoblast proliferation, differentiation, and induction of osteogenic genes [core binding factor al (Cbfa1), alkaline phosphatase (ALP), osteopontin (ONP), osteocalcin (OC) and collagen lα (Coll 1α)]. In addition, cGMP-induced expression of ENaC was examined in Obs transfected with an siRNA to PKGII to investigate whether cGMP/PKG signaling is necessary for ENaC activity.
Results
8-pCPT-cGMP promoted the proliferation and differentiation of Obs
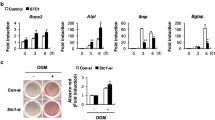
Primary cultured Obs were stimulated with osteogenic medium in the presence of 0, 0.05, 0.1, 0.2, 0.5, or 0.8 mM 8-pCPT-cGMP for 24 h (Figs. 1, 2). Both cell number and ALP activity (a marker for Ob differentiation) increased significantly in cells treated with 8-pCPT-cGMP compared to Obs cultured in the absence of 8-pCPT-cGMP (P < 0.05). While there were no differences among concentration groups, the proliferation rate reached a maximum at 0.2 mM 8-pCPT-cGMP (P < 0.01 vs. control).
Effects of 8-pCPT-cGMP on proliferation of Obs. To determine the effects of 8-pCPT-cGMP on proliferation of Obs, the cell viability of cells treated with 8-pCPT-cGMP at different concentrations (0.05, 0.1, 0.2, 0.5, and 0.8 mM) for 24 h were detected using the WST-8 assay kit. Results are pooled from three independent experiments and expressed as mean ± SD. * P < 0.05 compared to untreated cells. ** P < 0.01 compared to untreated cells
Effects of 8-pCPT-cGMP on the differentiation of Obs. To determine the effects of 8-pCPT-cGMP on the differentiation of Obs, the AKP activity of cells treated with 8-pCPT-cGMP at different concentrations (0.05, 0.1, 0.2, 0.5, and 0.8 mM) for 48 h were detected using the AKP assay kit. Results pooled from three independent experiments are expressed as mean ± SD. * P < 0.05 compared to untreated cells. ** P < 0.01 compared to untreated cells
8-pCPT-cGMP enhanced the expression of ENaC subunits
In addition to promoting Ob proliferation and osteogenic differentiation, 8-pCPT-cGMP treatment for 24 h also significantly increased α-ENaC mRNA expression (P < 0.05) (Fig. 3). For α-ENaC, there was a significant effect of concentration (P < 0.05) and the stimulatory effect was greatest at 0.2 mM 8-pCPT-cGMP, and the concentration dependence was correlated with expression of the OC gene.
The effect of 8-pCPT-cGMP on the expression of α-ENaC genes. a mRNA of α-ENaC was examined in Obs. b Relative mRNA levels of α-ENaC in Obs were compared. α-ENaC mRNA levels were normalized to β-actin mRNA expression in the same sample using the RT-PCR system (y axis, relative mRNA level). The images represent three independent experiments with observation of multiple microscopic fields and the results are expressed as mean ± SD. * P < 0.05 compared to untreated cells
8-pCPT-cGMP enhanced the expression of PKGII
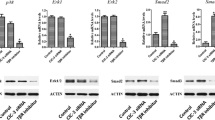
The cGMP analog (0.2 or 0.5 mM for 24 h) also increased the expression of PKGII subunits (Fig. 4). In contrast, 8-pCPT-cGMP had no influence on PKGIα or PKGIβ at any concentration tested (Fig. 4).
The effect of 8-pCPT-cGMP on the gene expression of PKGI (α and β) and PKGII. a mRNA of PKG was examined in Obs. b Relative mRNA levels of α PKGI in Obs were compared. α-PKGI mRNA levels were normalized to β-actin mRNA expression in the same sample using the RT-PCR system (y axis, relative mRNA level). c Relative mRNA levels of beta PKGI in Obs were compared. β-PKGI mRNA levels were normalized to β-actin mRNA expression in the same sample using the RT-PCR system (y axis, relative mRNA level). d Relative mRNA levels of PKGII in Obs were compared. PKGII mRNA levels were normalized to β-actin mRNA expression in the same sample using the RT-PCR system (y axis, relative mRNA level). The images represent three experiments with observation of multiple microscopic fields and the results are expressed as mean ± SD. * P < 0.05 compared to untreated cells
8-pCPT-cGMP promoted the expression of osteogenic genes
We used semi-quantitative RT-PCR to assess the effect of 8-pCPT-cGMP on the expression of mRNA levels encoding Coll 1α, OC, ALP, OPN, and Cbfa1 (Fig. 5). Stimulation of Obs with 0.1 mM or 0.2 mM 8-pCPT-cGMP significantly enhanced Coll 1α gene expression (normalized to β-actin expression as an internal control) relative to the control group (P < 0.05), but a high concentration (0.5 mM) had no significant effect on Coll 1α mRNA levels. Expression of OC was strongly induced by 8-pCPT-cGMP at all concentration, but significantly higher at 0.2 mM (P < 0.05). All concentrations of 8-pCPT-cGMP upregulated ON, ALP, and Cbfa1 gene expression (P < 0.05), with no dose-dependent effect, indicating that low cGMP concentrations induced maximal expression of these genes in cultured Obs.
The effect of 8-pCPT-cGMP on the gene expression of bone formation. a Expression of genes associated with bone formation was examined in Obs. b–f Relative mRNA levels of Coll 1α, OC, ALP, ON and Cbfa1 in Obs were compared, Coll 1α, OC, ALP, ON and Cbfa1 were normalized by mRNA of β-actin in the same sample using the RT-PCR system (y axis, relative mRNA level). The images represent three experiments with observation of multiple microscopic fields and the result is expressed as a mean of three different experiments. * P < 0.05 compared to untreated cells
Effects of PKGII siRNA and 8-pCPT-cGMP on α-ENaC expression in Obs
To determine the signaling mechanisms that mediate cGMP-dependent ENaC expression, we silenced PKGII expression using a specific small interfering RNA (siRNA). Transfection of Obs with siRNA directed against PKGII for 60 h significantly reduced the expression of both PKGII mRNA (Fig. 6) and α-ENaC, the main functional subunit of ENaC (P < 0.05 vs. control). The combination of siRNA PKGII and 8-pCPT-cGMP partially reversed siRNA-mediated downregulation of α-ENaC mRNA expression (P < 0.01), although expression was still lower than after treatment with 8-pCPT-cGMP alone (P < 0.05). Similarly, Obs treated with 8-pCPT-cGMP alone exhibited a significant increase in α-ENaC expression compared to both the untreated control group (P < 0.05) and the 8-pCPT-cGMP plus siRNA group (P < 0.05) (Fig. 7).
Effect of si-RNA transfection on the gene expression of PKGII. To determine the effects of si-RNA (si-RNA-1, -2, -3, and -4) transfection on the gene expression of PKGII, the mRNA of PKGII was examined in Obs. mRNA expression of PKGII decreased following si-RNA transfection compared to parental cells, and the si-RNA3 showed the strongest suppression
The effect of PKGII si-RNA and 8-pCPT-cGMP on the gene expression of α-ENaC. a mRNA of α-ENaC was examined in Obs. b Relative mRNA levels of α-ENaC in Obs were compared, α-ENaC were normalized by mRNA of β-actin in the same sample using the RT-PCR system (y axis, relative mRNA level). The images represent three experiments with observation of multiple microscopic fields and the result is expressed as a mean of three different experiments. * P < 0.05 compared with group NC, Mock and Blank respectively. △ P < 0.05 compared with group siRNA3. □ P < 0.05 compared with group siRNA3 + 8-pCPT-cGMP
Expression of α-ENaC protein was inhibited in pKGII-null mice
Western blot analysis was performed in bone tissue harvested from mice carrying a null mutation in the pKGII gene (Fig. 8). Expression of α-ENaC protein was significantly lower in pKGII-null mice (normalized to GADPH expression) compared to wild type mice (P < 0.01).
Analysis of α-ENaC protein expression in normal and pKGII KO mice. a Protein levels of pKGII were examined in wild type and pKGII knockout mice. b Relative protein expression of each sample was quantified by ImageJ software (National Institute of Health) and then normalized with GADPH levels. Results show the mean value from two (or three or more here) independent experiments. * P < 0.01 compared to wild type mice
Discussion
Osteoblast differentiation and bone formation are regulated by endocrine, paracrine, and autocrine factors that activate complex intracellular signaling pathways [3]. As one of the most important osteogenic intracellular second messengers, cGMP and cGMP signaling pathway play a positive role in bone formation [5, 9]. In this study, we found that exogenous 8-pCPT-cGMP, a cell-permeable cGMP analog, could stimulate the proliferation and differentiation of Obs. In addition, semi-quantitative RT-PCR revealed a significant upregulation of several osteogenesis-related marker mRNAs (products of osteogenic genes). These osteogenic gene products included mRNAs encoding ALP, OPN, and OC, which are specific osteoblast markers during matrix deposition, and Coll lα, the major component of the extracellular bone matrix. In addition, Cbfal, an essential transcription factor in osteoblast differentiation and bone development, was also upregulated at the mRNA level by 8-pCPT-cGMP. Thus, a cGMP analogue increased Ob proliferation and the expression of genes associated with Ob differentiation (ALP) and osteogenesis.
The cGMP-dependent protein kinase family (PKGIα, PKGIβ, and PKGII) is one of the three major intracellular downstream targets for cGMP [10]. Both PKGI and II are expressed in Obs and chondrocytes, but they may make different contributions to osteogenesis [22, 27], with PKGII now considered a seminal regulator of endochondral ossification and bone growth [17, 24]. Our results demonstrated that 8-pCPT-cGMP could increase the expression of PKGII mRNA in Obs, but had no significant effect on PKGIα and PKGIβ mRNA expression. This result is in agreement with earlier studies by [22] and [17], who found that PKGII-deficient mice developed dwarfism as a result of a severe defect in endochondral ossification at the growth plates, and showed increased numbers of postmitotic, non-hypertrophic cells, confirming an important role for PKGII in endochondral ossification [17, 22]. It was also reported that the hypertrophic differentiation of chondrocytes by SOX9 was inhibited in PKGII-deficient mice [6]. In addition, PKGII phosphorylates GSK3β and inhibits its activity, and reduced GSK3β is necessary for enhanced hypertrophic differentiation of growth plate chondrocytes [13]. Moreover, Rangaswami et al. [24] reported that mechanotransduction in Obs was mediated by cGMP/PKGII signaling, while PKGI had no effect. Both these previous studies and our current findings suggested that PKGII is the major functional downstream molecule mediating cGMP signaling in bone.
Several recent studies have established an association between dysfunctional sodium metabolism and osteoporosis. High NaCl intake was linked to increased calcium excretion in urine. Although the exact role of bone sodium is still unknown, sodium in extracellular fluid (ECF) bathes the bone forming site during mineralization [26]. Similar to previous experiments [15, 19, 28], our study confirmed the existence of ENaC in osteoblast. As the interaction of cGMP-ENaC channel previously validated in Xenopus oocytes, we then demonstrated that the mRNA levels of α-ENaC were enhanced by 8-pCPT-cGMP treatment in primary cultured Obs (derived from BMSCs), suggesting that cGMP or a cGMP-activated pathway induces expression of α-ENaC mRNA in Obs. Our previous study showed that 17β-estradiol stimulated osteogenesis-related genes and ENaC mRNA expression in rat Obs (derived from BMSCs) [4, 29]. These results demonstrated that ENaC may participate in Ob bone formation and that cGMP signaling may regulate bone formation through ENaC expression.
To confirm such a role for the cGMP/ENaC pathway in osteogenesis, we measured α-ENaC mRNA expression in Obs transfected with PKGII siRNA. Knockdown of PKGII led to reduced expression of α-ENaC and this reduced expression was partially reversed by co-application of the cGMP analog. Moreover, we found the PKGII-null mice showed significantly downregulated α-ENaC protein expression, indicating that that 8-pCPT-cGMP regulated ENaC expression by activating the cGMP-PKGII signaling pathway. In addition, 8-pCPT-cGMP or cGMP could directly impact the expression of α-ENaC, in accordance with a previous report indicating that without a direct demonstration that cGMP can activate Ob ENaCs in isolated membrane patches of in the presence of PKG inhibitors [21].
Taken together, our results indicate that 8-pCPT-cGMP can enhance proliferation and differentiation of Obs, in addition to promoting the expression of osteogenic genes. These osteogenic effects may be mediated by the expression and function of ENaC channels that are under the regulation of cGMP and/or the cGMP-PKGII signal pathway. Furthermore, these experiments indicated that ENaC may be a potential target for pharmaceutical interventions to treat metabolic bone diseases.
Materials and methods
Isolation and culture of Obs
The newborn Sprague–Dawley rats were purchased from Experimental Animal Center of Guangzhou University of Traditional Chinese Medicine (SPF animals SCXK 2008-0020), and sacrificed by cervical dislocation. Obs obtained from newborn Sprague–Dawley rat calvariae were isolated and cultured according to the protocols reported previously [25]. Briefly, calvariae were isolated and cleaned with 0.1 M phosphate-buffered saline (1× PBS), then minced and digested in 0.2 % collagenase I (Sigma, USA) twice for 10 min and three times for 15 min at 37 °C in the shaking waterbath. The first and second digestion supernatant were not be used for culture. Thereafter, each supernatant containing cells was collected by centrifuge for 6 min at 1500 rpm, pooled and resuspended in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL, USA) with antibiotics and 10 % fetal bovine serum (Hyclone, USA). Cells were then cultured in complete DMEM (supplemented with 10 % fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin) at 37 °C in 5 % CO2. When 90 % confluence was reached, cells were released from the culture substratum using trypsin/EDTA (0.25 % w/v trypsin, 0.02 % EDTA), and were moved to dishes (100 mm in diameter) at 1.0 × 105 cell/ml in 10 ml. Culture medium was further supplemented with 50 μg/ml ascorbic acid, 10 mmol/l β-glycerophosphate, and 10−8 mol/l dexamethasone after 2 passages. Cells at passage 3–5 were used for the following studies.
Determination of cell proliferation
Cells (2 × 103) on 96-well plates were evaluated for proliferation by WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] assay kit (Beyotime Inst Biotech, China) according to manufacture’s instructions. Briefly, WST-8 solution was added to each dish at a concentration of 1/10 volume 24 h after administration (8-pCPT-cGMP) and the plates were incubated at 37 °C for an additional 1 h. Absorbance was then measured at 450 nm with a Microplate Reader 680 (Bio-Rad Laboratories, Hercules, CA, USA).
Determination of cell differentiation
Cells (2 × 103) on 96-well plates were evaluated for differentiation by AKP assay kit (Nanjing jiancheng Inst Biotech, China) according to manufacture’s instructions. Briefly, the supernatant was collected to assay the activity of AKP 48 h after administration (8-pCPT-cGMP), the AKP assay kit solutions (buffer and media) were added to each sample, and 15 min after incubated at 37 °C the chromogenic reagent was added. Absorbance was measured at 490 nm with a Microplate Reader 680 (Bio-Rad Laboratories, Hercules, CA, USA).
RNA purification and reverse transcription-PCR
Total RNA was extracted from cultured cells using Trizol reagent (Life Technologies Inc., Gaithersburg, MD, USA) according to the manufacturer’s protocol. Primers (synthesized by Invitrogen, China) for rat ENaC-α, PKGIα, PKGIβ, PKGII, Coll Iα, OC, ALP, ON, Cbfa 1 and β-actin are shown in Table 1. RT-PCR using Access RT-PCR Reagent (One Step PrimeScript™ RT-PCR Kit, TaKaRa, China) was performed as follows: an initial denaturation step at 94 °C for 15 min, then 30 cycles of 94 °C for 30 s, 50–60 °C for 30 s and 72 °C for 1 min. Samples (5 μl) of each PCR product were size-fractionated by 2.0 % agarose gelelectrophoresis and the bands were visualized with Tanon 4100 (Tanon Science & technology Co., China).
PKGII small interfering RNA (siRNA)
PKGII expression was silenced in Obs by transient transfection of cells with pre-annealed siRNA oligonucleotides, corresponding to specific regions of the murine PKGII genes (pre-validated siRNAs, GenePharma, Shanghai, China). Cells were plated at 50–60 % density (about 1 × 105/ml in a 60 mm dish) and allowed to grow over-night. The next morning, cells were transfected with 50 nmol of siRNA and 10 μl Lipofectamine 2000 (Invitrogen, CA, USA) in 5 ml of serum-free media for 5 h. Then, cells were placed in DMEM with FBS and incubated for 48 h before 8-pCPT-cGMP stimulation, RNA was extracted 60 h later, and RT-PCR was performed as described above.
Western blot assay for ENaC isoforms
pKGII-null mice were used for Western blotting, the bone sample (skull of mice) was skinned, the brain was removed, and bones were homogenized in Sigma CelLyticTM MT Mammalian Tissue Lysis/Extraction Reagent (Catalog number: C3228-500 ml). α-ENaC was detected by Western blotting using immunopurified goat polyclonal IgG α-ENaC antibodys (SantaCruz,1:2,000). Protein expression of each sample was quantified by Image J software (National Institute of Health) and then normalized with GADPH levels.
Statistical analysis
All results are expressed as mean ± SEM (standard error of the mean) of at least three independent experiments performed in duplicate. Statistical analysis was performed by paired t test or One-way ANOVA computation combined with the Bonferroni test. Values were considered statistically significant at a probability level of 0.05.
References
Alvarez de la Rosa D, Canessa CM, Fyfe GK, Zhang P (2000) Structure and regulation of amiloride-sensitive sodium channels. Ann Rev Physiol 62:573–594
Butterworth M, Edinger R, Frizzell R, Johnson J (2009) Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol 296:F10–F24
Centrella M, McCarthy TL, Canalis E (1988) Skeletal tissue and transforming growth factor beta. FASEB J 2:3066–3073
Chen J, Wu XR, Lu L, Yang GZ, Lu XY, Gao JL, Ji HL, Li QN (2011) Effects of estrogen on osteoblast via epithelial sodium channel. Chin Pharm J 3:180–184
Cheng G, Zhai Y, Chen K, Zhou J, Han G, Zhu R, Ming L, Song P, Wang J (2011) Sinusoidal electromagnetic field stimulates rat osteoblast differentiation and maturation via activation of NO-cGMP-PKG pathway. Nitric Oxide 25:316–325
Chikuda H, Kugimiya F, Hoshi K, Ikeda T, Ogasawara T, Shimoaka T, Kawano H, Kamekura S, Tsuchida A, Yokoi N, Nakamura K, Komeda K, Chung UI, Kawaguchi H (2004) Cyclic GMP-dependent protein kinase II is a molecular switch from proliferation to hypertrophic differentiation of chondrocytes. Genes Dev 18:2418–2429
Duncan RL, Akanbi KA, Farach-Carson MC (1998) Calcium signals and calcium channels in osteoblastic cells. Semin Nephrol 18:178–190
Fyfe GK, Quinn A, Canessa CM (1998) Structure and function of the Mec-ENaC family of ion channels. Semin Nephrol 18:138–151
Haqiwara H, Inoue A, Yamaquchi A, Yokose S, Furuya M, Tanaka S, Hirose S (1996) cGMP produced in response to ANP and CNP regulates proliferation and differentiation of osteoblastic cells. Am J Physiol 270:C1311–C1318
Hofmann F, Ammendola A, Schlossmann J (2000) Rising behind NO: cGMP-dependent protein kinases. J Cell Sci 113:1671–1676
Horisberger JD, Chraïbi A (2004) Epithelial sodium channel: a ligand-gated channel? Nephron Physiol 96(2):37–41
Hughes FJ, Turner W, Belibasakis G, Martuscelli G (2006) Effects of growth factors and cytokines on osteoblast differentiation. Periodontol 2000 41:48–72
Kawasaki Y, Kugimiya F, Chikuda H, Kamekura S, Ikeda T, Kawamura N, Saito T, Shinoda Y, Higashikawa A, Yano F, Ogasawara T, Ogata N, Hoshi K, Hofmann F, Woodgett JR, Nakamura K, Chung U, Kawaguchi H (2008) Phosphorylation of GSK-3beta by cGMPdependent protein kinase II promotes hypertrophic differentiation of murine chondrocytes. J Clin Invest 118:2506–2515
Kellenberger S, Schild L (2002) Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82(3):735–767
Kizer N, Guo XL, Hruska K (1997) Reconstitution of stretch-activated cation channels by expression of the alpha-subunit of the epithelial sodium channel cloned from osteoblasts. Proc Natl Acad Sci USA 94:1013–1018
Manolagas S, Jilka R (1995) Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 332:305–311
Miyazawa T, Ogawa Y, Chusho H, Yasoda A, Tamura N, Komatsu Y, Pfeifer A, Hofmann F, Nakao K (2002) Cyclic GMP-dependent protein kinase II plays a critical role in C-type natriuretic peptide-mediated endochondral ossification. Endocrinology 143(9):3604–3610
Mobasheri A, Golding S, Pagakis SN, Corkey K, Pocock AE, Fermor B, O’brien MJ, Wilkins RJ, Ellory JC, Francis MJ (1998) Expression of cation exchanger NHE and anion exchanger AE isoforms in primary human bone-derived osteoblasts. Cell Biol Int 22:551–562
Mobasheri A, Pocock AE, Trujillo E, Ferraz I, Vasallo PM, Francis MJO (2001) Detection of mRNA and protein of the α subunit of the epithelial sodium channel (ENaC) in human osteoblasts and human ACL cells. J Physiol 535P:S046
Nie HG, Chen L, Han DY, Li J, Song WF, Wei SP, Fang XH, Gu X, Matalon S, Ji HL (2009) Regulation of epithelial sodium channels by cGMP/PKGII. J Physiol 587(Pt 11):2663–2676
Nie HG, Zhang W, Han DY, Li QN, Li J, Zhao RZ, Su XF, Peng JB, Ji HL (2010) 8-pCPT-cGMP stimulates alphabetagamma-ENaC activity in oocytes as an external ligand requiring specific nucleotide moieties. Am J Physiol Renal Physiol 298:F323–F334
Pfeifer A, Aszódi A, Seidler U, Ruth P, Hofmann F, Fässler R (1996) Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinaseII. Science 274:2082–2086
Pilz RB, Casteel DE (2003) Regulation of gene expression by cyclic GMP. Circ Res 93:1034–1046
Rangaswami H, Marathe N, Zhuang S, Chen Y, Yeh JC, Franqos JA, Boss GR, Pilz RB (2009) Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J Biol Chem 284:14796–14808
Rath B, Nam J, Knobloch TJ, Lannutti JJ, Agarwal S (2008) Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J Biomech 41:1095–1103
Robert P, Heaney MD (2006) Role of dietary sodium in osteoporosis. J Am Coll Nutr 25:271S–276S
Schlossmann J, Feil R, Hofmann F (2005) Insights into cGMP signalling derived from cGMP kinase knockout mice. Front Biosci 10:1279–1289
Trujillo E, Alvarez RD, Mobasheri A, González T, Canessa CM, Martín-Vasallo P (1999) Sodium transport systems in human chondrocytes. II. Expression of ENaC, Na+/K+/2Cl-cotransporter and Na+/H+ exchangers in healthy and arthritic chondrocytes. Histol Histopathol 14:1023–1031
Yang GZ, Nie HG, Lu L, Chen J, Lu XY, Ji HL, Li QN (2011) Regulation of the epithelial sodium channel subunits by estrogen in primary osteoblasts. Cell Mol Biol 57(Suppl):OL1480-OL1486
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 30971172), the Planned Science and Technology Project of Guangdong Province, China (Grant No. 2010B050700022) and the Planned Science and Technology Project of Dongguan, Guangdong Province, China (Grant No. 201010815207).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, J., Zhang, H., Zhang, X. et al. Epithelial sodium channel enhanced osteogenesis via cGMP/PKGII/ENaC signaling in rat osteoblast. Mol Biol Rep 41, 2161–2169 (2014). https://doi.org/10.1007/s11033-014-3065-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3065-1