Abstract
Mechanical ventilation can cause direct injury to the lungs, a type of injury known as ventilator-induced lung injury (VILI). VILI is associated with up-regulates angiotensinogen and AT1 receptor expression of in the lung. This work explored effects of losartan on VILI in diabetic mice. Ninty-six C57Bl/6 mice were randomly divided into six groups, control group (C group), diabetes group (D group), diabetes mechanical ventilation group (DV group), losartan control group (L + C group), losartan treatment group in diabetic mice (L + D group) and losartan treatment group in mechanical ventilation diabetic mice (L + DV group). Lung W/D, myeloperoxidase (MPO) activity, microvascular permeability, blood–gas analysis, Ang II concentrations and AT-1R protein expression were measured. Compared with D group, DV group increased Ang II concentrations, AT-1R protein expression, W/D ratio, MPO activity, and microvascular permeability. PaO2 were significantly lower in the DV group than D group or control group. Compared with DV group, L + DV group attenuates ventilator-induced lung injury in diabetic mice and prevented the increase Ang II concentrations, AT-1R protein expression and microvascular permeability caused by ventilation in diabetic mice. This study provides in vivo evidence that losartan attenuates microvascular permeability via down-regulates Ang II and AT-1R expression in mechanical ventilator-induced lung injury in diabetic mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been shown that some mechanical ventilation strategies can cause ventilator-induced lung injury (VILI) [1, 2], which is currently known to present as nonspecific physiological and morphological changes in the lung parenchyma. Changes in the balance of fluids in the lungs, diffuse alveolar injury, increased endothelial permeability and increased epithelial permeability have been described in animals submitted to mechanical ventilation [3]. However, the detailed mechanism behind “endothelial dysfunction” in VILI in diabetic mice is not well understood yet.
Diabetes is associated with increased risk of morbidity and early mortality as a result of its chronic microvascular complications [4]. Recently, Renin-angiotensin system (RAS) blockade induces diabetes protective effects is further supported by a meta-analysis of randomized clinical studies [5]. Circulating and tissue RAS outlines the physiological functions of the RAS major active substance, angiotensin II (ANG II). Circulating ANG II is generated from angiotensin I (ANG I) by carboxypeptidaze angiotensin-converting enzyme (ACE) expressed in the pulmonary endothelial cells [6]. Local RASs play a multitude of autocrine, paracrine and/or intracrine roles in the regulation of specific tissue and organ functions compared with circulating RASs. The existence of a pulmonary RAS and high ANG II concentrations have been demonstrated in normal rat lung [7], In addition, angiotensinogen and ANG II type 1 receptors (AT1) are also expressed in the lung tissue [8]. Furthermore, it has been demonstrated that losartan, a selective inhibitor of subtype AT1 receptors for angiotensin II, can relieve acute lung injury caused by high volume ventilation [9].
Local Ang II synthesis increases vascular permeability by promoting the expression and secretion of vascular endothelial growth factor (VEGF) [10]. Therefore, we hypothesized that VILI could be promoted by local ANG II action in diabetic mice. We tested this hypothesis by investigating the pulmonary expression of the angiotensinogen and AT-1R protein expression in a model of VILI in diabetic mice. Meanwhile, we assessed the effect of losartan, a selective inhibitor of AT1 receptor, on the pulmonary microvascular permeability induced by VILI in diabetic mice.
Materials and methods
We obtained institutional review board approval to perform this study. All animal experiments were performed in accordance with Wuhan University Guide regarding the care and use of animals for experimental procedures.
Animals
Normal female C57Bl/6 wild-type (Wt) mice with weights ranging from 18 to 22 g, 6–8 weeks of age, were obtained from the experimental animal center of Wuhan University School of Medicine. The animals were housed in individual cages in a temperature, humidity, and light-controlled room (12 h light and 12 h dark cycle), and acclimated for at least 7 day prior to use in experiments.
Experimental diabetes was induced by a single intraperitoneal injection of streptozotocin (Sigma Chemical Co., St. Louis, Mo.) at a dose of 150 μg/g body wt in 50 μl of sterile 0.05 mol/l sodium citrate (pH 4.5). The control group received an equal volume of vehicle. At 48 h and 10 days after streptozotocin injection, the blood glucose level was measured with a Glucometer (Abbott Diabetes Care Inc., Alameda, CA). All STZ-induced diabetic mice developed stable hyperglycemia (STZ 22.8 ± 3.1 vs. controls 8.5 ± 0.6 mmol/l, P < 0.001). The experimental protocol was approved by the Animal Experimentation of Wuhan University School of Medicine.
Groups and experimental protocols
Ninty-six C57Bl/6 mice were randomly divided into the following experimental groups: control group (C group), diabetes group (D group), diabetes mechanical ventilation group (DV group), losartan control group (L + C group), losartan treatment group in diabetic mice (L + D group) and losartan treatment group in mechanical ventilation diabetic mice (L + DV group). Animals from each group were divided equally into two subgroups for specific studies: one half of the animals were used for pulmonary microvascular leakage determination, and the other half of animals were used for biochemical analysis. All mice were placed in a supine position and connected to a ventilator with a TOPO small animal ventilator (kent sciantific, Torrington, USA). A Y-tube connector was surgically inserted into the trachea under general anesthesia with 100 mg/kg ketamine, 0.2 mg/kg medetomidine, and 1.0 mg/kg atropine (i.p.). Maintenance anesthesia consisted of 36 mg/kg ketamine (Hengrui medicine co., LTD, Jiangsu, china), 0.04 mg/kg medetomidine(Hongfangde medicine co., LTD, jinan, china), and 0.075 mg/kg atropine (tianjing pharmaceutical group xinzheng Co.,tianjing, china). Maintenance mix was administered via an intraperitoneal catheter (PE 10 tubing; BD, Breda, the Netherlands) every hour. To correct for hypovolemia, 0.9 % NaCl solution was administered via the intraperitoneal catheter every 30 min. DV group mice were ventilated with an inspiratory pressure of 18 cm H2O for 4 h (resulting in injurious VT ~ 15 mL/kg). Respiratory rate was set at 70 breaths/min. Positive end-expiratory pressure was set at 2 cmH2O. FIO2 was kept at 0.5. The inspiration-to-expiration ratio was kept at 1:1 throughout the experiment. Control and D group mice, receiving equate the dose of induction anesthesia, were instrumented, but not ventilated. L + C group, L + D group and L + DV group mice received intraperitoneal injections of Losartan (Merck Sharp & Dohme Corp, USA) 30 mg/kg 30 min before mechanical ventilation. Animals were killed at the end of the experiment. The unventilated control group was killed immediately after tracheotomy in an identical way.
Sample preparation
Plasma samples were collected and stored at −20 °C. Tissue samples were frozen and stored at −70 °C for index assessment.
Analysis of lung water
Lung tissues were taken from the right upper lobes, rinsed in saline, and weighed immediately then dried at 80 °C for 24 h and re-weighed until the weight became constant. Lung W/D was used as an index of pulmonary edema formation.
Myeloperoxidase assay
One of the left lung lobes was frozen at −70 °C until assay. Frozen tissue samples were later thawed, homogenized in 0.2 mmol/L potassium phosphate buffer (PPB, pH 7.4) with homogenizer (~3,400 rpm on ice) and centrifuged at 4,000×g for 30 min at 4 °C. The procedure of MPO content followed the instruction for the MPO assay kit from Jiancheng Co.
Evaluation of microvascular permeability
Pulmonary microvascular permeability was measured according to the method described by Chen [11]. Fluorescein isothiocyanate (FITC)-labeled bovine albumin 10 mg/kg (Sigma-Aldrich, St. Louis, MO) was intravenously administrated to animals 4 h before the end of mechanical ventilation. Eight mice from each group were sacrificed and the pulmonary vascular permeability was determined. At the end of the experiments, mice were exsanguinated by cardiac puncture and serum was collected. The lungs were lavaged in situ three times with phosphate-buffered saline. The bronchoalveolar lavage (BAL) fluid was centrifuged and the supernatant was collected for FITC fluorescence measurement. FITC fluorescence in the serum and BAL fluid was measured using a fluorescence spectrometer (BX-400; Ker Machinery & Engineering Co. Ltd, Wuhan, china) with excitation at 494 nm and emission at 520 nm. The BAL-to-serum FITC fluorescence ratio was calculated and taken as a measure of pulmonary microvascular permeability.
Blood–gas analysis
Arterial blood was assayed immediately after being taken from the carotid artery when the animal was killed by exsanguinations. Arterial blood samples were analysed with a clinical blood gas analyser (I-STAT Portable Clinical Analyser, I-STAT, USA) for partial pressures of oxygen (PaO2).
Microscopy observation
One of the left lung lobes were cut into 1-mm-thick slices, immersion-fixed in 2.5 % buffered glutaraldehyde at 0 to 4 °C for 60 min, buffered in phosphate-buffered saline, and fixed with 1 % osmic acid, then washed with distilled water, and dehydrated by dimethylketone. After being embedded in Epon-812, they were cut into ultrathin sections (70 nm) by an ultramicrotome (Dupont, Newtown, Conn) and stained with uranyl acetate and plumbum citrate. Sections were examined under a Hitachi H-600 electron microscope (Hitachi, Tokyo, Japan).
Enzyme Immunoassay for Ang II
Extraction of peptides from lung tissues or plasma was performed on the basis of instruction provided by the company in the kit (Phoenix Pharmaceuticals, Burlingame, CA). Briefly, homogenize the tissues in ice bath. The homogenate was centrifuged at 1,300×g for 30 min at 4 °C. The supernatant was collected, and the total protein concentration was determined using Bio-Rad protein assay (Bio-Rad, Hercules, CA). The supernatant or plasma was mixed with buffer A at the ratio of 1:3 and centrifuged at 3,500 rpm for 15 min at 4 °C. Sep-column containing 200 mg of C18 was equilibrated by washing once with 1 ml of buffer B and three times with 3 ml of buffer A for each time. The supernatants were loaded onto the pretreated C18 sep-column. The peptides were slowly eluted with buffer B (3 ml, once). The extracts from lung tissue or plasma were reconstituted with 1× assay buffer and assayed with the Ang II Enzyme Immunoassay Kit (SPI Bio, Massy, France). The concentrations of Ang II are expressed as Ang II picograms per milligram protein for lung tissue or milliliters for plasma.
Western blotting for AT-1R protein expression
Western blotting for AT-1R was performed. All samples were kept on ice in lysis buffer (1 % NP-40, 0.5 % sodium deoxycholate, and 0.1 % sodium dodecyl sulfate) and homogenised until the solution turned clear. The total protein concentrations of the samples were measured by the bicinchoninic acid (BCA) method. Equal amounts of protein extract (30 μg) were separated by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (Bio-Rad, CA, USA) and transferred electrophoretically to nitrocellulose membranes. The membranes were then blocked with 5 % non-fat dried milk or 5 % bovine serum albumin in Tris buffered saline Tween (20 mM Tris (pH 7.6), 137 mM NaCl, and 0.1 % Tween 20). The membrane was incubated overnight at 4 °C with primary anti AT-1R antibody (Santa Cruz Biotechnology, CA, USA), and the bound antibody was visualized using the respective horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology Inc.). The immune complex was visualized by using enhance chemiluminescence. The relative densities of AT-1R, and β-actin bands were quantified by an alpha imager 2000 analyzer.
Statistical analysis
All data were presented as mean ± standard deviation. Analysis of variance (ANOVA) and the Student–Newman–Keuls q test were used for statistical analysis to compare values among all groups. A significant difference was presumed for a probability value of <0.05.
Results
Lung wet-to-dry weight ratios and myeloperoxidase activity
We evaluated neutrophil accumulation in lung tissue by MPO assay, and evaluated organ edema by W/D. As shown in Table 1, The DV group had a significantly higher lung W/D and MPO than the D group. However, W/D and MPO significantly decreased in L + DV group compared with DV group.
Blood–gas analysis and histological examination
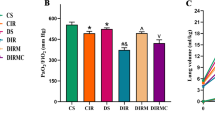
Ventilator-induced lung injury in diabetic mice was confirmed by arterial blood gas analysis (Table 1) and histological examination (Fig. 1 ). As shown in Table 1, DV group mice had about ~20 % lower PaO2 than C group and ~15 % lower PaO2 than D group. L + DV group might protect against this decrease in PaO2 of DV group. Under transmission electron microscope, we could observe the basement membranes of alveolar epithelium and capillary endothelium became swollen, widened; osmiophilic lamellar bodies emptied and the basement membrane were broke to varying degrees in DV group (Fig. 1, slide C). In comparison, L + DV group showed less lung damages and the changes of pathology (Fig. 1, slide D). C group has normal structure of mitochondria and lamellar bodies in alveolar epithelial cell (slide A).
Microvascular permeability
The group D and DV had significantly higher microvascular permeability than the control group. Pulmonary microvascular permeability was increased in DV group compared with D group (Table 2). After treatment of with losartan, this increase was dramatically attenuated.
Lung Ang II and AT-1R protein expression
The expression levels of AT1 receptor protein expression and concentrations of Ang II were significantly different between the Control, D, DV and L/P group. Diabetes increased plasma and lung Ang II levels (Table 2). AT1 receptor expression levels (Fig. 2) and concentrations of Ang II in the DV group significantly increased when compared with the Control and D groups. Mechanical ventilation pretreated with losartan significantly decreased the level of Ang II and AT1 receptor protein expression in lung tissues.
Western blotting for AT-1R protein expression. AT1 receptor expression levels in the DV group significantly increased when compared with the Control and D groups. Mechanical ventilation pretreated with losartan significantly decreased AT1 Receptor protein expression in lung tissues. (Compared with control group, *P < 0.05; Compared with D group, † P < 0.05; Compared with DV group, ∆ P < 0.05)
Discussion
Although mechanical ventilation is an important therapy that is widely used in intensive care medicine, it can result in complications. One major complication is ventilator-induced lung injury.
Damages to the alveolar-capillary barrier, alveolar edema, and inflammation due to excessive mechanical stretch have been recognized as major pathophysiologic events of VILI [12]. This type of lung injury is manifested by increased endothelial and epithelial barrier permeability in the lung [13]. The mechanism of such an increase in permeability is not well understood. It was also reported that mechanical stretch promoted IL-8 synthesis with activation of p38 in HPMVECs. Moreover, cyclic stretch enhanced IL-6 and MCP-1 production and induced endothelial reorientation accompanied by actin polymerization [14]. The induction of a proinflammatory response in the lung epithelium is associated with an alteration in permeability in the local endothelium [15]. VEGF is involved in the regulation of vascular permeability and endothelial cell survival. A hypothetical protective effect of hydroxyethyl starch (HES) against VILI has been associated with the regulation of VEGF [16]. Rearrangement of the cytoskeleton regulates vascular endothelial barrier function and appears to be regulated by nonmuscle myosin light-chain kinase isoform (nmMLCK), which also has a regulatory role in angiogenesis, endothelial cell apoptosis, and leukocytic diapedesis [17, 18].
The present study reveals that, endothelial function of the microcirculation, which is usually evaluated through the vasodilator response to endothelium-dependent vasodilators or physiological stimuli, is characteristically impaired in patients with Type 1 diabetes [19]. Hyperglycaemia also increases endothelial nitric oxide synthase activity, resulting in increased nitric oxide release [20]. This, in turn, leads to microvascular endothelial dysfunction. However, although the endothelial dysfunction plays a fundamental role in the pathogenesis of ventilator-induced lung injury, there have been few in vivo studies during this pathological process. VILI cannot be distinguished from endothelial dysfunction that lead to hyperglycaemia, and VILI can worsen the initial profile when patients with diabetes are subjected to mechanical ventilation.
Blockade of the renin-angiotensin system (RAS) plays an important role in preventing organ injury associated with diabetes. Increasing evidence indicates that Ang II can directly enhance the expression of P-, E-, and L-selectins, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 on vascular endothelial and smooth muscle cells [21]. In addition, it was also found that ANG II induced oxidative stress by increasing the activity of NADPH oxidase in vascular endothelial cells. However, although the RAS plays a fundamental role in the ventilator-induced lung injury and diabetes, there have been few in vivo studies of alterations in the permeability in mechanical ventilator-induced lung injury in diabetic animal model. Therefore, we hypothesized that VILI might be promoted by local ANG II action during diabetes and be alleviated by RAS blockade.
In the current study, we showed that mechanical ventilation with an inspiratory pressure of 18 cmH2O in diabetic mice produced acute lung injury, as manifested by neutrophilia, and significantly decreased PaO2. We ruled out the interference of anesthetic drugs on PaO2 because our present data show that DV group mice had about ~20 % lower PaO2 than control group and ~15 % lower PaO2 than D group. Up-regulation of ANG II and AT1 receptor may contribute to VILI because of the role of ANG II and AT1 receptor in inflammatory response, oxidative stress, and lung cell epithelial apoptosis. We show that losartan was effective in preventing the development of vascular endothelium permeability to VILI in diabetic mice. Up-regulation of ANG II and AT1 receptor probably stimulates the release of inflammatory cytokines and chemokines from lung vascular endothelial cells during diabetes. However, the mechanism of up-regulation of angiotensinogen and AT1 receptor act on VILI remains to be further clarified. In the meantime, our experiments show that losartan can attenuate lung edema, neutrophil accumulation, increased microvascular permeability and alveolar septum fracture caused by VILI in diabetic mice. We suggest that the protection of losartan probably relates to blocking the action of ANG II and AT1 receptor.
In conclusion, our experiments demonstrate that losartan attenuates microvascular permeability via down-regulation of Ang II and AT-1R expression in mechanical ventilator-induced lung injury in diabetic mice.
References
Rocco PR, Dos Santos C, Pelosi P (2012) Pathophysiology of ventilator-associated lung injury. Curr Opin Anaesthesiol 25:123–130
Melsen WG, Rovers MM, Bonten MJ (2009) Ventilator-associated pneumonia and mortality: a systematic review of observational studies. Crit Care Med 37:2709–2718
Dreyfuss D, Saumon G (1998) Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157:294–323
Girgis CM, Scalley BD, Park KE (2012) Utility of the estimated glucose disposal rate as a marker of microvascular complications in young adults with type 1 diabetes. Diabetes Res Clin Pract 96:70–72
Leung PS (2010) Current research of the RAS in diabetes mellitus. Adv Exp Med Biol 690:131–153
Herichova I, Szantoova K (2013) Renin-angiotensin system: upgrade of recent knowledge and perspectives. Endocr Regul 47:39–52
Campbell DJ, Kladis A, Valentijn AJ (1995) Effects of losartan on angiotensin and bradykinin peptides and angiotensin converting enzyme. J Cardiovasc Pharmacol 26:233–240
Campbell DJ, Habener JF (1989) Hybridization in situ studies of angiotensinogen gene expression in rat adrenal and lung. Endocrinology 124:218–221
Yao S, Feng D, Wu Q, Li K, Wang L (2008) Losartan attenuates ventilator-induced lung injury. J Surg Res 145:25–32
Kitayama H, Maeshima Y, Takazawa Y, Yamamoto Y, Wu Y, Ichinose K, Hirokoshi K, Sugiyama H, Yamasaki Y, Makino H (2006) Regulation of angiogenic factors in angiotensin II infusion model in association with tubulointerstitial injuries. Am J Hypertens 19:718–727
Chen XL, Xia ZF, Ben DF, Wang GQ, Wei D (2003) Role of p38 mitogen-activated protein kinase in lung injury after burn trauma. Shock 19:475–479
dos Santos CC, Slutsky AS (2006) The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol 68:585–618
Taniguchi LU, Caldini EG, Velasco IT, Negri EM (2010) Cytoskeleton and mechanotransduction in the pathophysiology of ventilator-induced lung injury. J Bras Pneumol 36:363–371
Iwaki M, Ito S, Morioka M, Iwata S, Numaguchi Y, Ishii M, Kondo M, Kume H, Naruse K, Sokabe M, Hasegawa Y (2009) Mechanical stretch enhances IL-8 production in pulmonary microvascular endothelial cells. Biochem Biophys Res Commun 389:531–536
Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG (2003) Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285:785–797
Li LF, Huang CC, Liu YY, Lin HC, Kao KC, Yang CT, Liao SK (2011) Hydroxyethyl starch reduces high stretch ventilation-augmented lung injury via vascular endothelial growth factor. Transl Res 157:293–305
Dudek SM, Garcia JG (2001) Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91:1487–1500
Ngiam N, Kavanagh BP (2012) Ventilator-induced lung injury: the role of gene activation. Curr Opin Crit Care 18(1):16–22
Gomes MB, Matheus ASM, Tibiric¸a′ E (2008) Evaluation of microvascular endothelial function in patients with type 1 diabetes using laser-Doppler perfusion monitoring: which method to choose? Microvasc Res 76:132–133
Browne D, Meeking D, Shaw K, Cummings M (2003) Endothelial dysfunction and pre-symptomatic atherosclerois in type 1 diabetes pathogenesis and identification. Br J Diabetes Vasc Dis 3:27–29
Alvarez A, Cerdá-Nicolás M, Naim Abu Nabah Y, Mata M, Issekutz AC, Panés J, Lobb RR, Sanz MJ (2004) Direct evidence of leukocyte adhesion in arterioles by angiotensin II. Blood 104:402–408
Acknowledgments
This work was supported by National Natural Science Foundation of China(81000318),the Youth Science Plan for Light of the Morning Sun of Wuhan City (201271031425), and by Hubei Provincial Health Office of the subject of scientific research project (JX5B16).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, C., Zhang, Z., Li, Z. et al. Losartan attenuates microvascular permeability in mechanical ventilator-induced lung injury in diabetic mice. Mol Biol Rep 41, 809–814 (2014). https://doi.org/10.1007/s11033-013-2920-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2920-9