Abstract
Calnexin (CNX) is an integral membrane protein of endoplasmic reticulum (ER) and is a critical component of ER quality control machinery. It acts as a chaperone and ensures proper folding of newly synthesised glycoproteins. CNX shares a considerable homology with its luminal counterpart calreticulin (CRT). Together, they constitute CNX/CRT cycle which is imperative for proper folding of nascent proteins. CNX deficient organisms develop severe complications because of improper folding of proteins and consequently ER stress. CNX maintains calcium homeostasis by binding to the Ca2+ which is a central node in various signaling pathways. Phosphorylation of cytoplasmic tail of CNX controls the sarco endoplasmic reticulum calcium ATPase and thus the movement of Ca2+ in and out of its store-house, i.e. ER. Our studies on Oryza sativa CNX (OsCNX) reveal constitutive expression at various developmental stages and various tissues, thereby proving its requirement throughout the plant development. Further, its expression under various stress conditions gives an insight of the crosstalk existing between ER stress and abiotic stress signaling. This was confirmed by heterologous expression of OsCNX (OsCNX-HE) in tobacco and the OsCNX-HE lines were observed to exhibit better germination under mannitol stress and survival under dehydration stress conditions. The dehydration tolerance conferred by OsCNX appears to be ABA-dependent pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcium ion (Ca2+) plays the role of secondary messenger, regulating diverse activities like cytoplasmic streaming, cell division, cell elongation, cell differentiation, cell polarity, photomorphogenesis, thigmotropism, gravitropism, plant defense and most importantly biotic and abiotic stress responses [24, 43, 45]. Ca2+ is central to various signaling pathways. These signals are perceived by the receptors located on plasma membrane and is further transduced by sensors and transducers in the cytoplasm, where Ca2+ buffering takes place. Change in cytoplasmic Ca2+ concentration is reflected in the form of signatures which are specific to the external stimuli [30, 45, 50]. Considerable evidences hints towards the involvement of organeller Ca2+ oscillations downstream to these pathways. Calcineurin, a well documented Ca2+ signaling transducer is proposed to play its role via CRT [31] and/or CNX [25].
As ER is the major storehouse of Ca2+ in the cell, the Ca2+ oscillations in the ER are gaining considerable importance in stress signaling. Various Ca2+ binding proteins in the ER play significant role in the buffering of ER Ca2+, and are termed “Ca2+-buffers”. Calnexin is one such ER resident Ca2+ binding protein with non-EF-hand motif which plays role in Ca2+ homeostasis within the cell. It is a 90 kDa integral membrane protein, conserved from worm to human. The main activity of CNX is in ER quality control by functioning in protein folding, assembly, and retention/retrieval of newly synthesized protein [43, 44]. It possesses large (~50 kDa) N-terminal calcium binding luminal domain, a single transmembrane helix, and a short acidic cytoplasmic tail consisting of only 90 aa residues. Phosphorylation of this cytoplasmic tail plays an important role in controlling the Ca2+ homeostasis within the cell [43].
The role of CNX in protein folding is very critical as protein misfolding and aggregation can lead to a variety of neurodegenerative diseases like Alzheimer’s, Parkinson’s and Huntington’s disease [43]. Misfolded proteins accumulate inside cell thus causing activation of unfolded protein response (UPR) or ER stress response [54]. During UPR, the chaperone proteins like CNX, CRT, Grp78 (BiP), Grp94 are upregulated and eventually the general protein synthesis is declined. They all lead to ER-associated degradation (ERAD) of proteins [6, 27, 32, 54]. Arabidopsis plants subjected to drug induced ER stress showed upregulation of ER chaperones, folding enzymes and translocons [23].
Studies on plant UPR has revealed upregulation of certain proteins which are involved in signal transduction. These include protein kinases and variety of transcription factors. This suggests a possible integration of UPR with other signaling pathways, like oxidative and light stress. One such candidate, Zat 12, was demonstrated to be involved in ROS and abiotic stress signaling and exhibit induced expression during plant UPR [11].
First report on plant CNX came from Arabidopsis thaliana, when Huang et al. [19] isolated cDNA clone encoding a CNX homologue CNX1p. CNX has been isolated from maize [26], soybean [18] and Pisum sativum [15] as well. Studies on tissue expression studies on pea and rice do not show tissue-specific isoforms of CNX [42, 44]. Expression studies on soybean calnexin show decreased level of protein when treated with 10 % polyethylene glycol for 2 days. Other abiotic stresses (salinity, cold and abscisic acid treatment) also reduced accumulation of calnexin protein [38].
In this study, we have isolated and cloned the CNX gene from rice and investigated its expression under various types of stresses and at various developmental stages, showing its role in embryonic development and further. We have done heterologous expression of rice calnexin gene in tobacco to further analyze its role in stress tolerance. This study also reveals the role of CNX in ER stress and abiotic stress cross-talk.
Materials and methods
Plant material and growth conditions
Seeds of Oryza sativa were surface sterilized by solution of Clorox plus 0.05 % Triton X-100 for 10 min, washed with sterilized water and imbibed in sterilized water for 4 h. These pre-soaked seeds were germinated in trays containing sterilized, moist vermiculite and grown at 20 ± 2 °C for 7 days in light/dark conditions in the greenhouse.
Stress treatment and RNA isolation
Abiotic stress treatments were given to old rice plants at various time points up to 7 days. For treatments, water containing the desired solute was used. Sodium chloride was added at a final concentration of 200 mM, abscisic acid (ABA; Sigma) was dissolved in water to make a stock of 200 μM. For cold and heat stress, the seedlings were maintained at 4 and 42 °C, respectively. Dehydration was simulated by drying the plants on tissue paper and keeping them wrapped in dry tissue paper for the desired time. For drought condition plants were treated with 20 % PEG. As calnexin is a protein of ER, plants were subjected to ER stress by treatment with azetidine decarboxylic acid (AZC) at a concentration of 50 mM. Plants were also treated with cycloheximide (apoptosis inducer) and zeatin (senescence inhibitor) at a concentration of 5 μg/ml and 10 μM, respectively. In addition to this 50 mM CaCl2 and 10 μM H2O2 are also given as a part of stress treatment. Total RNA was isolated from these stress treated plants using the GITC method of Chomcznsky and Sacchi (1987). RNA was also isolated from different tissues of rice representing different stages of the plant development; and from the T1 transgenic lines.
Isolation and cloning of OsCNX
The rice cDNA was used as template for the isolation of rice calnexin gene (OsCNX). The PCR amplified product from the above step was then cloned in pGEMT Easy vector (Promega). Colony PCR was done for confirmation. The construct was also restricted with single cutters as well as double cutters for further confirmation, followed by sequencing. The sequence is submitted to NCBI under accession GQ121138. The amino acid sequence alignment of OsCNX protein from various plants was done by CLUSTALW and based on that the phylogenetic tree is prepared showing relation to various calnexin proteins of plants, Principle signature motifs and prominent sites were studied.
To express OsCNX in tobacco (Nicotiana tabacum var. Xanthi), the cDNA was cloned in pRT100 at Sac I and Xho I site. The resulting vector, pRT100-OsCNX, has the CaMV35S promoter and poly-A tail on either sides of the gene. This whole cassette was taken out by Hind III enzyme and cloned at the Hind III site of the MCS of plant transformation vector pCAMBIA 1301, so as to make the construct pCAMBIA-OsCNX (Fig. 4a).
Agrobacterium-mediated transformation of tobacco
To express OsCNX in tobacco (N. tabacum var. Xanthi), the construct pCAMBIA 1301-OsCNX was mobilized into Agrobacterium tumefaciens strain LBA4404 by chemical transformation. Agrobacterium-mediated transformation of tobacco was carried out as per standard protocol: The strain taken is A. tumefaciens strain LBA4404. YEM medium (50 mg/l kanamycin, 50 mg/l streptomycin, and 10 mg/l rifamycin) was used with an OD600 of ca. 0.2. to infect leaf disks (1 cm in diameter). Co-cultivation was done in dark, at 26 °C for 2 days. After co-cultivation, explants were cultured in ROMP medium (MS + 1 mg/l BA + 0.1 mg/l NAA + 3 % sucrose + 250 mg/l cefotaxime + 30 mg/l hygromycin) 16 h (in light): 8 h (in dark) at 26 °C for selection/regeneration. After 5–6 weeks, shoots were transferred to MS medium + 3 % sucrose + 30 mg/l hygromycin + 250 mg/l cefotaxime for rooting. The whole plants were transferred to soil pots in the green house. Along with the transformation experiments, control experiments were performed where leaf disc were not infected with Agrobacterium but cultured on selection/regeneration medium along with the transformed leaf disks.
Genomic DNA PCR
Genomic DNA was isolated from the T1 transgenics as well as vector control plants by grinding leaf tissue followed by extraction using the CTAB (N-acetyl-N,N,N-trimethylammonium bromide) method [13]. PCR was performed by using the oligonucleotides CaMV35S as the forward primer and OsCNX-R as the reverse primer.
Southern blot analysis
For Southern analysis, 20 μg of genomic DNA from PCR positive tobacco lines was digested with EcoRI (for analyzing the number of copies inserted; Fig. 4e), electrophoresced, and blotted onto Hybond N membranes (GE Healthcare). Radiolabeled 1.6-kb complete ORF of OsCNXcDNA was used as a probe. Hybridization and washing were carried out at 55 °C as described in Pham et al. [40].
RT-PCR
Total RNA was isolated from the PCR positive transgenic lines by using an extraction method described [9]. First strand cDNA was prepared by the cDNA synthesis kit (Invitrogen) and RT-PCR was performed with OsCNX specific primers.
Northern blot analysis
About 30 μg of total RNA samples resolved on a 1.0 % formaldehyde-agarose gel were transferred on Hybond N membranes (GE Healthcare) and probed as described above.
Germination sensitivity test
Seeds of the T0 transgenic tobacco plants were selected by germinating them on Murashige and Skoog [33] medium in the presence of hygromycin. These seedlings representing the T1 generation were tested for their sensitivity to drought stress (0.5 M Mannitol). Their germination percentage was calculated and compared with the plants growing in MS medium (without supplements). The root, shoot and length of leaves were measured.
Short term dehydration stress
One week old soil grown seedlings of T1 generation were taken and allowed to dehydrate on tissue paper for subsequent time points and their fresh weight loss is compared with Wild type and vector control plants.
Bioinformatical analyses of OsCNX
The three dimensional Homology Based Modeling of OsCNX was done by taking the atomic coordinates of the protein towards which the protein of interest exhibited maximum homology. Structural predictions were made using the ExPASy modelling programmer and visualised using Swiss-pdb-viewer software.
Results
Isolation and sequence analysis of OsCNX
The rice calnexin gene sequence was retrieved from the Rice Genome Database by BLAST analysis with A. thaliana Calnexin (AtCNX). This sequence is annotated in RGD as calreticulin. Primers were designed from the 5′ and 3′ regions of the complete coding region (CDS) of the retrieved sequence. These primers were used for amplifying OsCNX gene from the rice cDNA (prepared from pool of mRNAs isolated from NaCl, PEG and heat-stressed rice seedlings). The amplified product was cloned in pGEMT Easy vector by TA cloning method. The sequence was submitted to GenBank with accession number GQ121138. Sequence analysis of OsCNX shows that it encodes a full-length cDNA, 1645 bp in size and encodes a protein of 516 amino acid residues with a predicted molecular mass of 57.9 kDa and pI 8.23.
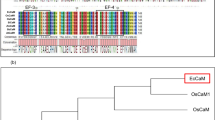
The amino acid sequence alignment of OsCNX with A. thaliana calnexin (AtCNX2), Brassica napus calnexin (BnCNX), Solanum lycopersicon calnexin (SlCNX) and P. sativum calnexin (PsCNX) is shown in Fig. 1b.
a Taxonomic coverage represents sequence diversification of calnexin across various organisms. The figure shows that calnexin protein is expressed across the prokaryotes, lower and upper eukaryotes, plant and animal kingdom. b CLUSTALW amino acid sequence alignment of calnexin proteins from different plant sources. Principle signature motifs and prominent sites are marked in the figure. c Phylogenetic tree depicting the relationship between the amino acid sequences of OsCNX with calnexin proteins from other plants. The names and accession numbers of the sources are mentioned in the figure. Genetic distances are marked in black and bootstrap values at various nodes are marked in red. d Homology modeling of OsCNX. Structural predictions were made using the ExPASy modelling programmer and visualised using Swiss-pdb-viewer software. N N-terminal, C C-terminal of the protein. Protein is divisible into two parts, the globular domain and arm domain. Conserved domain search of the protein revealed three important motifs present in the calreticulin family proteins, marked in figure as CRT repeat motif 1, motif 2 and motif 3 present in the extended arm domain
As previously described, the protein consists of an N-terminal luminal domain, a transmembrane domain and a short cytoplasmic tail like domain. The N-terminal luminal domain comprises majority of the protein. It contains various specific sites like N-myristoylation site, PKC phosphorylation site and CK2 phosphorylation site. Various important signals like. The characteristic feature of this protein is the presence of highly conserved signature motifs (RLQNGLECGGAYLKYI, IMFGPDKCG, IPDPDDKKPEDWD), which shows considerable homology with calreticulin protein. These are denoted in the figure as calreticulin family signature 1, calreticulin family signature 2 and calreticulin family repeated motif signature.
Phylogenetic tree constructed (Fig. 1c) on the basis of sequence alignment indicates that OsCNX (BAF14606) exhibit highest homology with Pea calnexin (PsCNX, CAA76741) followed by SlCNX (BAD99512). On the other hand, both AtCNX (CAA79144) and BnCNX (AAK84429) are clustered together and evolutionary distant to OsCNX.
Homology modelling of calnexin protein from rice (OsCNX) is shown as a ribbon diagram (Fig. 1d). Structural predictions made using the ExPASy modelling programmer shows the protein to be divisible into two parts, the globular domain and an arm domain. The three important characteristic motifs of calreticulin family proteins are marked in figure as CRT repeat motif 1, motif 2 and motif 3 and are present on the extended arm domain.
OsCNX is ubiquitously expressed in different tissues
RNA isolated from various tissues of rice (Fig. 2a) was used for cDNA preparation and consequently RT analysis. The transcript levels of OsCNX in different tissues of rice indicated the ubiquitous presence of CNX in all the tissues examined viz., prepollinated flowers, panicle, seed, young leaf, mature leaf, culm and root (Fig. 2b). Across these tissues, OsCNX expression was highest in leaves and lowest in prepollinated flowers.
OsCNX expression in various rice tissues. (But in the figure “a” is “b”) a Tissue-specific expression of OsCNX transcript in Oryza sativa indica cultivar Swarna. Lower panel shows amplification of rice actin gene used here for normalization. b Rice tissues taken for tissue-level expression of OsCNX, a young and mature leaves, b pollinated flowers, c inflorescence, d flowers, e root, f seeds bearing panicles, g panicle, h seed, i culm
OsCNX expression is modulated by abiotic stresses
The response of OsCNX expression in various abiotic stresses was investigated. It was noticed that induction of calnexin is a general response to several abiotic stresses. The expression levels of calnexin exhibit cyclic pattern when subjected to cold treatment. It increased within 30 min after (Fig. 3a), decreased instantly after that (1 h), subsequently increases after that, until 24 h, and declines at higher incubation. Conversely, heat stress does not affect the OsCNX transcript level at early time points (3 and 6 h) but decreases afterwards (Fig. 3b). In case of salt stress, calnexin transcript levels decreases as the time progresses (Fig. 3c). After subjecting to drought stress (Fig. 3d), although, the transcript level slightly increases till 3 h, a gradual decrease was observed subsequently.
Semi-quantitative RT-PCR showing transcript level of OsCNX after different stress treatments to rice seedlings: cold (a), heat (b), salt (c), drought (d), AZC (e), ABA (f), cycloheximide (g) and zeatin (h), CaCl2 (i) and H2O2 (j) for subsequent time points. c Wild type rice control without exposure to stress. Reverse transcribed RNA from samples collected at different time points was PCR amplified using OsCNX primers. Actin was used as endogenous control
We next explored the role of ABA in the signal transduction pathway mediated by calnexin. For this, we have studied the transcript level of OsCNX over a broad range of time points because ABA has very critical roles in signaling pathways as it can effect both early as well as late gene expression. The OsCNX, transcript levels peaked as early as 30 min and declined after 1 h (Fig. 3f). Then slowly increases and at 6 h, the mRNA level was more than that of the control, after which it decreases. The expression studies show that CNX works in calcium signalling in an ABA-dependent manner and is an early responsive gene to ABA hormone.
Upregulation of OsCNX under ER stress and PCD
Azetidine decarboxylic acid which is a marker of ER stress results in a very high accumulation of OsCNX transcript at 12 h time point (Fig. 3e), which is maintained higher than control even after 24 h, indicating the role of calnexin protein during ER stress. As ER chaperones are involved in ER stress associated cell death, we have included cycloheximide, an apoptotic inducer, in our study. It is evident that CNX plays key role in such case as the transcript remained at elevated levels until 24 h (Fig. 3g). In the case of Zeatin stress, which is reported to protect the cells from ER stress induced injury, different induction kinetics was observed (Fig. 3h). The gene was induced strongly within 3 h, remain induced till 6 h, after which its mRNA levels declined dramatically to a much lower level than control.
Upregulation of OsCNX by calcium
We noticed that OsCNX transcript is strongly upregulated in response to various abiotic stresses. Signaling pathways for these stresses are often mediated by Ca2+ and CNX is itself a Calcium binding protein. Thus, Ca2+ was applied exogenously, and the CNX transcript level was analysed at different time points. As shown in Fig. 3i, the transcript level of OsCNX was upregulated in response to Ca2+, in the first 3 h, reaching a maximum at 6 h and declined at higher time points. At 12 h, CNX expression was similar to the untreated controls and afterwards it shows a surprisingly sharp increase at 24 h (Fig. 3i).
Involvement of H2O2 in OsCNX mediated signaling
To analyse the possible involvement of H2O2 in OsCNX mediated stress responses, the effect of H2O2 was also tested. Plants treated with 10 μM H2O2 shows progressively increase in the transcript level till 12 h and then a sharp decreases at 24 h (Fig. 3j). This suggests that the protein participates early in the oxidative stress.
Molecular confirmation of OsCNX-expressing tobacco transgenics
For functional validation of OsCNX gene, it was expressed in tobacco by using pCAMBIA-OsCNX construct. The successful transgenic expressing OsCNX were confirmed by employing various techniques. The genomic DNA isolated from the OsCNX-OE lines and the vector control plants was used for PCR analysis using CaMV35S as the forward primer and OsCNX-R as the reverse primer. Figure 4b shows single band of 1.6 Kb in transgenic lines L1, L2, L3 and L5. It is absent in L4 and the vector control plants. These four PCR positive transgenic lines were then carried for subsequent analyses.
Analysis of transgenic plants (T1) to confirm the integration, copy number, and expression of the gene. a Schematic diagram of the pCAMBIA-OsCNX construct used to express OsCNX in tobacco plants. b PCR with CaMV 35S and OsCNX-R primers of DNA isolated from plants, c RT-PCR analysis with OsCNX-F and OsCNX-R primers and d Northern analysis to confirm OsCNX transcript in the PCR positive plants, and e Southern blot for copy number analysis of the integrated OsCNX gene. L1–L5 OsCNX-OE lines, VC vector control, M molecular weight marker
Reverse-transcription PCR analysis of the RNA prepared from OsCNX-OE lines by OsCNX specific primers amplified product of desired size in all the PCR positive transgenic lines (Fig. 4c), which is absent from the vector controls as expected. The northern analysis further confirms the results (Fig. 4d) showing presence of a ~1.6 kb transcript in all of the transgenic plants (lanes 2–6), the corresponding band was missing in the vector control plants (lane 1), as the heterologous probe did not bind to CNX of N. tabacum. In order to determine the copy number of OsCNX genes in transgenic plants, Southern analysis was performed. Genomic DNA isolated from the OsCNX-OE PCR positive lines were digested by EcoRI restriction enzyme. A 1.6-kb complete ORF of OsCNX cDNA was used as radio-labelled probe. Figure 4e shows single site insertion in all the lines. This also confirms independent transformation events as the band sizes are different in all lines.
Improved drought tolerance by T1 transgenics heterogenously expressing OsCNX
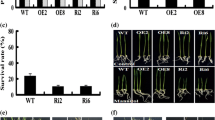
To evaluate the effect of OsCNX heterogenously expressed in tobacco plants for their abiotic stress response, seeds were germinated on supplemented MS medium. Homozygous lines with single copy insertion were identified and used for further analysis. For germination sensitivity test, OsCNX-OE T1 transgenic tobacco plants were obtained by growing the seeds of T0 lines on MS medium supplemented with selection marker hygromycin. These seedlings, representing the T1 generation, were tested for their sensitivity to drought stress by growing them on MS medium supplemented with 0.4 M mannitol (Fig. 5a, b). Their germination percentage was calculated (Table 1) and compared with the plants grown on MS medium (without supplements). Evaluation of three experiments with 25 seedlings of each (L1–L5) line showed that in the presence of mannitol, germination in control plants reduced to 70–80 %, but in transgenic line L3 more than 90 % of the seeds germinated (p < 0.01).
OsCNX confers drought tolerance to transgenic plants. a, b Germination sensitivity test for drought stress tolerance seeds of the T0 transgenic tobacco plants (n = 25) germinated on MS medium in the presence of hygromycin and with or without Mannitol (0.5 M), represented by germination percentage. c Measurement of organ lengths after drought stress treatment of T1 plants grown on MS with or without supplements. L1, L3 OsCNX-OE* lines, VC vector control, Wt wild type plants. The results shown here are the mean ± SD for three independent experiments. *The p values were calculated by Student's two-tailed t test
Seeds grown on MS medium for seven days were shifted to MS supplemented with 0.4 M mannitol to check for drought stress tolerance. It was observed that the length of roots, shoots and leaves of OsCNX-OE lines were increased significantly under drought stress and were more than double compared with the unstressed seedlings. In case of control plants (Wt and VC) although a slight increase in root length was noticed, but there was significant reduction in shoots and leaves.
Transgenics expressing OsCNX have increased leaf length compared to control plants and which can be attributed to high level of tissue-specific expression of CNX in non-stressed conditions. However, the difference in root length between control and transgenics in drought stress is surprising as the OsCNX transcript level in roots is very low in control conditions. We speculated that OsCNX might be playing some crucial role in roots under drought stress conditions.
The T1 transgenics were also subjected to short term dehydration stress. For this, 1 week old soil grown T1 seedlings were taken and allowed to dehydrate on tissue paper. Photos were taken for subsequent 8, 12 and 24 h interval (Fig. 6a).Their fresh weight loss was compared with wild type and vector control plants at 3, 6, 9 and 12 h interval (Fig. 6b). Both the transgenic lines L3 and L5 performed better than wild type control and vector control plants, even after 12 h of stress they were able to retain 40 % of their fresh weight.
Fresh weight loss in 4 weeks old soil grown T1 transgenic plants subjected to short term dehydration stress, L1, L3 OsCNX-OE lines, VC vector control and Wt wild type plants. Photos were taken for subsequent 8, 12 and 24 h interval (a). Fresh weight loss was compared with wild type and vector control plants at 3, 6, 9 and 12 h interval (b)
One month old soil grown plants were checked for drought stress tolerance and the readings were recorded (Table 2). In the presence of 0.5 M mannitol, the controls (WT and VC) and transgenic seedlings (L3 and L5) both were able to survive. The growth of controls were retarded (Table 2) under drought stress, whereas the T1 transgenics show better performance in terms of plant height, fresh weight of leaves, time required for flowering, and seed weight. No chlorosis was observed as the chlorophyll content remained unaltered both in transgenics and controls under stress conditions. Roots continue to grow in transgenics (L3 and L5) in soil too. The fresh weight of leaves is also recorded to be high in young plants, middle level planta and mature plants. The seed weights are also reported to be high in stressed transgenics than controls in our study. The flowering time in drought-stressed plants was delayed by approximately a fortnight in controls, conversely, the transgenics report early flowering in similar stress circumstances. Interestingly, the OsCNX-OE transgenics performed much better in stress conditions than in H2O (Table 2) in terms of height, seed weight and flowering time.
Discussion
Calnexin is present from prokaryotes to eukaryotes and from plants to humans. Del Bem [12] reported calnexin to be present along all green plant lineages. It is ubiquitous in occurrence (Fig. 1a). Given its universal presence, its importance is imperative in all the organisms. This is an important and integral component of protein folding machinery of the cell and along with other ER proteins ensure proper folding and secretion of nascent glycoproteins. Some organisms like yeast possess only calnexin as a whole and sole member of the machinery to carry out the function [43].
In the present study we have isolated and cloned the full-length CDS of rice calnexin (OsCNX). It contains an ORF of 1,614 bp which encodes for 537 amino acids protein. The deduced amino acid sequence alignment (Fig. 1b) indicates that OsCNX has high sequence identity with other plant CNXs. The phylogenetic analysis of these CNX proteins confirms high similarity between the proteins and closeness of OsCNX with PsCNX. Moreover, the luminal part of OsCNX is highly similar to OsCRT, so much so that OsCNX was wrongly annotated as OsCRT in rice genome database. The points on which the two proteins show highest level of similarity are the two–three principal regions that are termed as signature motifs, depicted in the figure (Fig. 1b). The homology based model of OsCNX (Fig. 1d) also shows these three motifs. With high level of similarity exhibited by the two proteins and common functions in ER quality control system, earlier it was thought that these proteins might have lapping functions [7, 35]. But, later studies made it clear that although being highly similar these proteins play distinct roles in cellular functions and they are not interchangeable [2, 22]. Calreticulin has been reported in various parts of the cell like Golgi compartment, nucleus, nuclear envelope, spindle apparatus of dividing cells, plasmodesmata, cytoplasm and the cell surface [21], but CNX has been localized to ER only. This highlights crucial role of calnexin in cellular physiology.
In Arabidopsis three different calnexins has been reported (AtCNX1, AtCNX2 and AtCNX3) while rice encodes only one (OsCNX, [44], showing its functional divergence in different plant systems. Role of calnexin in plants is not very well documented, however, few reports shows its involvement in protein folding. For instance, increase in CNX expression is recorded from the floury-2(fl2) mutants of maize, where an abnormal zein polypeptide is accumulated, thus showing its potential in folding of zein protein, the major seed storage protein of maize [17].
Calnexin being the calcium binding protein of ER is reported to be involved in Ca2+ buffering in ER lumen by modulating SERCA [41]. Calcineurin plays an important role in the dephosphorylation of CNX and activation of SERCA [1]. Since most of the biotic and abiotic stress signaling pathways converge on Ca2+ as a secondary messenger, one can hypothesize the role of CNX in stress tolerance. Our expression studies on OsCNX under various abiotic stress conditions throw light on this signaling pathway (Fig. 2). The rapid increase in OsCNX transcript level under cold treatment shows its involvement in cold stress adaptation to plants. Conversely, heat has no significant effect on the transcript level of this gene. This suggests its distinct role from the heat shock proteins of the cell. The decline in transcript level of OsCNX due to osmotic stresses (drought and salinity), is in sync with findings of other researchers where down-regulation of ER molecular chaperones and folding catalysts under these stresses was observed. Irsigler et al. [20] have reported down-regulation of ER chaperones due to PEG-induced dehydration stress in soybean while, Valente et al. [48] demonstrated the repression of BiP and CNX transcript level in cases of severe drought, in soybean plants. These results indicated that among the different type of stresses, it is the drought stress that cause serious harm to ER folding machinery by inversely regulating the UPR-specific targets.
Our detailed study on the OsCNX expression under ABA stress and the time course oscillations in the transcript level showed the role of CNX in abiotic stress tolerance is ABA-dependent. Constitutively higher expression of OsCNX under Azetidine de carboxylic acid (AZC, marker of ER stress) confirms the potent role of calnexin protein during ER stress. To further prove the point that continuous ER stress leads to programmed cell death (PCD), we mimicked the situation by providing plants with cycloheximide, an apoptotic inducer. Not surprisingly, CNX transcript remained at elevated levels until 24 h. The intriguing induction kinetics of OsCNX when subjected to zeatin treatment (a protectant of ER stress induced injury), will need further investigation before coming to any conclusion.
As drought promotes rapid and excessive accumulation of reactive oxygen species (ROS) in plant cells, we have also checked the expressions of OsCNX under H2O2 stress which is the major contributor of ROS signaling, and reported considerable elevation of OsCNX transcript level, thus proving further the role of this gene in drought signaling. We have also checked the expression of OsCNX transcript under calcium stress and found differences in the transcript level corresponding to different time points.
To give further strength to our hypothesis, we have done heterologous expression of the rice calnexin gene in tobacco and the OsCNX-OE lines show better performance than wild type and vector control plants in the presence of drought stress both at the level of germination and further development. The T1 seedlings were observed to be stable and functional as the transgenics were able to grow, flower, and set viable seeds with no loss in seed number and seed weight under normal and drought stress conditions (Tables 2, 3).
We have also checked the occurrence of this gene (OsCNX) in various tissues of rice exhibiting different developmental stages of the plant. Interestingly, Calnexin transcript level was present in all the tissues tested. However, the expression level varies across different tissues, with leaves having the highest level of expression and least in pre-pollinated flowers. A surprising observation came from OsCNX expressing lines which exhibit enhanced performance under drought stress conditions, with early flowering and increased length of roots. This clearly indicates specific requirement of OsCNX at the time of flowering and root development under these stress conditions.
We postulate the role of Ca2+ signaling in the OsCNX mediated drought tolerance. As Ca2+ stores of ER are controlled by CNX mediated opening and closing of SERCA pump, it is likely that the heterologous expression of OsCNX in tobacco increased the Ca2+ storage capacity of ER which eventually triggered Ca2+ mediated stress signaling of drought stress tolerance. Studies have revealed that the Ca2+ signaling in cytoplasm is also responsible in controlling the turgor pressure of plant stomata that regulates their opening and closing and controls leaf dehydration. Therefore, the regulation of cellular Ca2+ homeostasis by CNX might have contributed to CNX mediated drought stress tolerance in tobacco seedlings. Nouri and Komatsu [37] reported enhanced accumulation of calnexin in the plasma membrane fraction of soybean after subjecting it to 10 % PEG treatment. What can be the reason of the presence of CNX in the plasma membrane? The authors hypothesize the escape of calnexin from the ER to the plasma membrane when subjected to osmotic stress, where calnexin may associate with membrane proteins to play role in signaling. Studies on animal cells also reveal the localization of low levels of CNX on the plasma membrane under normal cellular conditions; however, more than 80 % of calnexin is localized to the ER. An ER cytosolic sorting protein PACS-2 controls the CNX distribution between the ER and plasma membrane. The protein kinase CK2 phosphorylates CNX (on Ser554/564 sites) and reduces its binding to PACS-2, thus limiting it to ER membrane [34]. PACS-2 might play important role in controlling stress signaling, but its role in plants is still not clear.
Although calnexin phosphorylation is not new to the field, several kinases have been reported to phosphorylate calnexin and CK2 phosphorylation of CNX at Ser534 and Ser544 has been well documented [39, 51]. The role of calnexin phosphorylation in ER quality control was also reported earlier [36]. MEK1-stimulates ERK1 which then enhance the phosphorylation of calnexin at Ser563 during the misfolding of 1-antitrypsin (AAT). An enhanced secretion of AAT was reported following the inhibition of calnexin phosphorylation at the ERK1 site [3, 8]. Nonetheless, a clear role of CNX phosphorylation in mediating abiotic stress signaling is yet to be unfolded. Our data shed some light on involvement of calnexin in stress pathways additional to ER stress. Other ER chaperones have also been reported to be involved in abiotic and biotic stress responses. It thus appears that calnexin and other ER chaperons establish crosstalk across different stress pathways in plants. In mammals, although, such crosstalks are very well documented [53], but not many reports are there in plants and the field is still in infancy [28]. Gao et al. [16] reported AtbZIP28 mediated heat tolerance in plants where an ER chaperone BiP2 is found to be upregulated. It is found that the bZIP domain of AtbZIP28 cause the activation of BiP2. In soybean, it has been demonstrated that the ER stress and osmotic stress pathways together activates another pathway of PCD that causes leaf senescence through activation of plant-specific N-rich proteins [10]. This pathway differs considerably from the molecular chaperone-inducing branch of the UPR.
ER chaperones have also shown to be involved in plant-innate immunity [5], [47], [52]. Crosstalk of G-protein signaling and ER stress signaling has also been reported from Arabidopsis [49]. A heterodimer of Gβ and Gγ proteins shows involvement in UPR-associated cell death. So much so, that the growth of G-null mutant plants is not restricted by the ER stress inducer tunicamycin, while the wild type plants suffers growth retardation. A recent study by Liu et al. [29] reveals that ER associated degradation (ERAD) is necessary for plants to combat salt stress. Salt treatment causes accumulation of unfolded proteins that consequently leads to ER stress responses. Ca2+ released from the ER is crucial in this response and elevation of UPR also involves ROS. Further, studies have shown the involvement of calcium (Ca2+) alongwith nitric oxide (NO) in plant senescence signalling cascade [46]. Severe drought stress cause accumulation of ROS and consequently trigger the cell death process in the root meristematic cells, the apical root dominance is inhibited and the thick and short lateral roots are induced which have increased tolerance to the water stress. According to the studies by Cao and Li [4] and Duan et al. [14] this modulation of root architecture is controlled by BAX inhibitor-1 (AtBI1) and the ER stress response. The increase in root length observed in our study can also be attributed to the inhibition of root apical dominance. However, it needs further investigation before coming to any conclusion.
The novel aspects of this work are highlighted below
This is the first report on the characterization of Calnexin proteins from rice. The downregulation of OsCNX under osmotic stresses (NaCl and PEG) corroborates with the findings of other reports on ER chaperones. Heterogenous expression of OsCNX in tobacco conferred protection against various stresses supporting its role in abiotic stress tolerance and also a possible crosstalk between ER Stress and abiotic stress. The constitutive elevation of OsCNX subjected to AZC, cycloheximide further signifies its role in ER stress signaling. Heat stress is ineffective on OsCNX transcript level, thus showing the characteristic difference between CNX and the heat shock proteins. Our report on involvement of rice CNX in this crosstalk is first of its kind and when probed further will surely lead to better understanding of various unanswered questions in stress biology.
References
Bollo M, Paredes RM, Holstein D, Zheleznova N, Camacho P, Lechleiter JD (2010) Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs. PLoS One 5:e11925
Brodsky JL (2007) The protective and destructive roles played by molecular chaperones during ERAD (endoplasmic-reticulum-associated degradation). Biochem J 404:353–363
Cameron PH, Chevet E, Pluquet O, Thomas DY, Bergeron JJ (2009) Calnexin phosphorylation attenuates the release of partially misfolded alpha 1-antitrypsin to the secretory pathway. J Biol Chem 284:34570–34579
Cao M, Li X (2010) Die for living better: plants modify root system architecture through inducing PCD in root meristem under severe water stress. Plant Signal Behav 5:1645–1646
Caplan JL, Zhu X, Mamillapalli P, Marathe R, Anandalakshmi R, Dinesh-Kumar SP (2009) Induced ER chaperones regulate a receptor-like kinase to mediate antiviral innate immune response in plants. Cell Host Microb 6:457–469
Caramelo JJ, Parodi AJ (2008) Getting in and out from calnexin/calreticulin cycles. J Biol Chem 283:10221–10225
Ceirotti A, Duranti M, Bollini R (1998) Effect of N-glycosylation on the folding and quality control of plant proteins. J Exp Bot 49:1091–1103
Chevet E, Smirle J, Cameron PH, Thomas DY, Bergeron JJ (2010) Calnexin phosphorylation: linking cytoplasmic signaling to endoplasmic reticulum lumenal functions. Sem Cell Dev Biol 21:486–490
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Annal Biochem 162:156–159
Costa MDL, Reis PAB, Valente MAS, Irsigler AST, Carvalho CM, Loureiro ME (2008) A new branch of endoplasmic reticulum stress signaling and the osmotic signal converge on plant-specific asparagine-rich proteins to promote cell death. J Biol Chem 283:20209–20219
Davletova S, Schlauch K, Coutu J, Mittler R (2005) The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol 139:847–856
Del Bem LE (2011) The evolutionary history of calreticulin and calnexin genes in green plants. Genetica 139:255–259
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Duan Y, Zhang W, Li B, Wang Y, Li K, Han C, Zhang Y, Li X (2010) An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol 186:681–695
Ehtesham NZ, Phan TN, Gaikwad A, Sopory SK, Tuteja N (1999) Calnexin from Pisum sativum: cloning of the cDNA and characterization of the encoded protein. DNA Cell Biol 18:853–862
Gao H, Brandizzi F, Benning C, Larkin RM (2008) A membrane-tethered transcription factor defines a branch of the heat stress response in Arabidopsis thaliana. Proc Natl Acad Sci USA 105:16398–16403
Gillikin JW, Zhang F, Coleman CE, Bass HW, Larkins BA, Boston RS (1997) A defective signal peptide tethers the floury-2 zein to the endoplasmic reticulum membrane. Plant Physiol 114:345–352
Goode JH, Settlage SB, Wilson RF, Dewey RE (1995) Isolation of a calnexin homolog from developing soybean seeds. Plant Physiol 108:1341
Huang L, Franklin AE, Hoffman NE (1993) Primary structure and characterization of an Arabidopsis thaliana calnexin-like protein. J Biol Chem 268:6560–6566
Irsigler AST, Costa MDL, Zhang P, Reis PAB, Dewey RE, Boston RS, Fontes EPB (2007) Expression profiling on soybean leaves reveals integration of ER and osmotic-stress pathways. BMC Genomics 8:431
Jia XY, Xu CY, Jing RL, Li RZ, Mao XG, Wang JP, Chang XP (2008) Molecular cloning and characterization of wheat calreticulin (CRT) gene involved in drought-stressed responses. J Exp Bot 59:739–751
Jin H, Hong Z, Su W, Li J (2009) A plant-specific calreticulin is a key retention factor for a defective brassinosteroid receptor in the endoplasmic reticulum. Proc Natl Acad Sci USA 106:13612–13617
Kamauchi S, Nakatani H, Nakano C, Urade R (2005) Gene expression in response to endoplasmic reticulum stress in Arabidopsis thaliana. FEBS J 272:3461–3476
Kim MC, Chung WS, Yun D, Cho MJ (2009) Calcium and calmodulin-mediated regulation of gene expression in plants. Mol Plant 2:13–21
Klee CB, Ren H, Wang X (1998) Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem 273:13367–13370
Kwiatkowski BA, Zielinkska-Kwiatkowska AG, Migdalski A, Kleczkowski LA, Wasilweska LD (1995) Cloning of two cDNAs encoding calnexin-like proteins from maize (Zea mays) leaves: identification of potential calcium-binding domains. Gene 65:219–222
Lederkremer GZ (2009) Glycoprotein folding, quality control and ER-associated degradation. Curr Opin Struct Biol 19:515–523
Liu JX, Howell SH (2010) Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 22:2930–2942
Liu L, Cui F, Li Q, Yin B, Zhang H, Lin B, Wu Y, Xia R, Tang S, Xie Q (2011) The endoplasmic reticulum-associated degradation is necessary for plant salt tolerance. Cell Res 21:957–969
Luan S (2009) The CBL–CIPK network in plant calcium signaling. Trends Plant Sci 14:37–42
Lynch J, Michalak M (2003) Calreticulin is an upstream regulator of calcineurin. Biochem Biophys Res Commun 311:1173–1179
Meusser B, Hirsch C, Jarosch E, Sommer T (2005) ERAD: the long road to destruction. Nat Cell Biol 7:766–772
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–479
Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G, Simmen T (2008) The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell 19:2777–2788
Nash PD, Opas M, Michalak M (1994) Calreticulin: not just another calcium-binding protein. Mol Cell Biochem 135:71–78
Nguyen DT, Kebache S, Fazel A, Wong HN, Jenna S, Emadali A (2004) Nck-dependent activation of extracellular signal-regulated kinase-1 and regulation of cell survival during endoplasmic reticulum stress. Mol Biol Cell 15:4248–4260
Nouri MZ, Komatsu S (2010) Comparative analysis of soybean plasma membrane proteins under osmotic stress using gel-based and LC MS/MS-based proteomics approaches. Proteomics 10:1930–1945
Nouri MZ, Hiraga S, Yanagawa Y, Sunohara Y, Matsumoto H, Komatsu S (2012) Characterization of calnexin in soybean roots and hypocotyls under osmotic stress. Phytochemistry 74:20–29
Ou WJ, Thomas DY, Bell AW, Bergeron JJ (1992) Casein kinase II phosphorylation of signal sequence receptor alpha and the associated membrane chaperone calnexin. J Biol Chem 267:23789–23796
Pham XH, Reddy MK, Ehtesham NZ, Matta B, Tuteja N (2000) A DNA helicase from Pisum sativum is homologous to translation initiation factor and stimulates topoisomerase activity. Plant J 24:219–229
Roderick HL, Lechleiter JD, Camacho P (2000) Cytosolic phosphorylation of calnexin controls intracellular Ca2+ oscillations via an interaction with SERCA2b. J Cell Biol 149:1235–1248
Sarwat M (2011) Calnexin: a candidate for crosstalk of ER stress and abiotic stress in plants. In: Society for experimental biology annual meeting 2011, Glasgow, 1–4 July 2011, p 4.26
Sarwat M, Tuteja N (2007) Calnexin: a versatile calcium binding integral membrane chaperone of endoplasmic reticulum. Calcium Bind Proteins 2:36–43
Sarwat M, Tuteja N (2010) Overexpression of rice calnexin protect transgenic tobacco plants from ER stress. In: Society of experimental biology annual meeting 2010, Prague, 30 June–3 July 2010
Sarwat M, Nabi G, Parvaiz A, Hu X (2013a) Ca2+ signals: the versatile decoders of environmental cues. Crit Rev Biotechnol. 33:97–109
Sarwat M, Naqvi A R, Ahmad P, Ashraf M, Akram NA (2013b) Phytohormones and microRNAs as sensors and regulators of leaf senescence: assigning macro roles to small molecules. Biotech Adv. doi:10.1016/j.biotechadv.2013.02.003
Shirasu K (2009) The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu Rev Plant Biol 60:139–164
Valente MAS, Faria JAQA, Soares-Ramos JRL, Reis PAB, Pinheiro GL, Piovesan ND, Morais AT, Menezes CC, Cano MAO, Fietto LG, Loureiro ME, Aragao FJL, Fontes EPB (2009) The ER luminal binding protein (BiP) mediates an increase in drought tolerance in soybean and delays drought-induced leaf senescence in soybean and tobacco. J Exp Bot 60:533–546
Wang S, Narendra S, Fedoroff N (2007) Heterotrimeric G protein signaling in the Arabidopsis unfolded protein response. Proc Natl Acad Sci USA 104:3817–3822
Weinl S, Kudla J (2009) The CBL–CIPK Ca2+-decoding signaling network: function and perspectives. New Phytol 184:517–528
Wong HN, Ward MA, Bell AW, Chevet E, Bains S, Blackstock WP (1998) Conserved in vivo phosphorylation of calnexin at casein kinase II sites as well as a C/proline-directed kinase site. J Biol Chem 273:17227–17235
Ye C, Dickman MB, Whitham SA, Payton M, Verchot J (2011) The unfolded protein response is triggered by a plant viral movement protein. Plant Physiol 156:741–755
Yoshida H (2007) ER stress and diseases. FEBS J 274:630–658
Zhang K, Kaufman RJ (2004) Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem 279:25935–25938
Acknowledgments
We sincerely thank Department of Biotechnology, Government of India for the postdoctoral fellowship grant to M.S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarwat, M., Naqvi, A.R. Heterologous expression of rice calnexin (OsCNX) confers drought tolerance in Nicotiana tabacum . Mol Biol Rep 40, 5451–5464 (2013). https://doi.org/10.1007/s11033-013-2643-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2643-y