Abstract
MicroRNAs are endogenous small RNAs with a high degree of conservation, participating in a variety of vital activities. In present study, to explore the effect of microRNAs on 3T3-L1 adipocyte differentiation and adiponectin expression, the adipo-related microRNAs were screened and identified by micorRNA microarray. The highly expression plasmid of microRNA-21 with obvious expression up-regulation (miR-21) and its anti-sense (miR-21 inhibitor) were constructed and transfected into 3T3-L1 preadipocytes. The effect of miR-21 on 3T3-L1 adipocyte differentiation was observed, and the protein and mRNA expression level of adiponectin and AP-1 were analyzed. Results showed that, the expression profiles of microRNAs significantly changed during 3T3-L1 adipocyte differentiation. The expression of miR-21 was obviously up-regulated. miR-21 could significantly promote adipocyte differentiation, increase adiponectin mRNA and protein expression, while decrease AP-1 protein level. Meanwhil, miR-21 inhibitor blocked the effects of miR-21 mentioned above. The overexpression of AP-1 could absolutely reverse the stimulatory effect of miR-21 on adiponectin. miR-21 plays an important role in regulating adipocyte differentiation and adiponectin expression by inhibiting AP-1 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
microRNAs are endogenous non-coding small RNAs with 18–24 bp. They combine with the 3′ end of targeted mRNA to regulate the gene expression by degrading mRNA or inhibit its translation [1]. microRNAs are involved in the regulation of many biological processes of eukaryotic cells including proliferation and apoptosis [2], lipid metabolism [3] and nerve conduction [4]. Previous researches find that, some microRNAs exhibit tissue specificity and high expression characteristics. The specific microRNAs are involved in regulation of the occurrence and development of major diseases such as diabetes [5], cardiovascular disease [6] and cancer [7].
Adiponectin is a marker protein of adipose tissue expression and secretion in adipocyte differentiation [8]. A large number of studies have confirmed that, adiponectin is involved in the occurrence of insulin resistance, and clinically associated with the obesity, type 2 diabetes and cardiovascular disease [9, 10]. Recent studies find that, microRNAs play an important role in adipocyte differentiation and its function exertion. As reported by Xie et al. [11] the ectopic expression of miR-103 and miR-143 in preadipocytes can accelerate the adipocyte formation. Wang et al. [12] finds that, miR-17-92 can target Rb2/p130 gene and inhibit its protein expression to accelerate adipocyte differentiation and triglyceride accumulation. However, the role of microRNAs on adipocyte differentiation is still unclear.
In this study, the regulating functions of microRNAs in adipocyte differentiation were investigated. Their expression profiles in the inducing differentiation of 3T3-L1 adipocyte were analyzed by micorRNA microarray. The effect of up-regulated miR-21 on adipocyte differentiation and adiponectin expression were discussed.
Materials and methods
Materials
The main materials were as follows: DMEM (Gibco, USA). Fetal bovine serum and fetal calf serum (Hangzhou Sijiqing Biological Engineering Materials Co., Ltd., China), Plasmid kit and PCR kit (Tiangen biochemical technology (Beijing) Co., Ltd., China), Total RNA purification Kit (TRzol) (BioTeke (Beijing) Corporation, China), RT kit (MBI, USA), LipofectamineTM2000 Kit (Invitrogen, USA), Primers and siRNA synthesis kits (Shanghai Sangon Biological Engineering Technology & Services Co., Ltd., China), Rabbit anti-adiponectin polyclonal antibody (Cell Signaling Technology, USA), miRNASelect™ pEGP-miR (Cell Biolabs, USA), Mouse 3T3-L1 preadipocytes (Institute of Basic Medical Science, Chinese Academy of Medical Sciences, China), pCMV6-AP-1 plasmid (MC208799) (Origene, USA), AP-1 monoclonal antibody (sc-166540) (Santa Cruz Biotech, USA) and microRNA chips (Exiqon, Denmark).
Screening and identification of microRNAs associated with 3T3-L1 adipocyte differentiation.
The screening and identification of microRNAs associated with 3T3-L1 adipocyte differentiation was conducted by micorRNA microarray according to the manufacturer’s protocol. The 3T3-L1 preadipocytes and differentiated adipocyte were collected. After extraction of total RNA, the microRNAs were extracted and marked. The hybridization with microRNA chips was conducted on the concentrated marker sample. Then the fluorescence intensity imaging of microRNAs and data analysis was performed, using relative value as expression intensity.
Construction and identification of miR-21 expression plasmid
After screening and identification, miR-21 which had a high expression level was selected as the target microRNA for further study. The nucleotide sequence of miR-21 was 5′-UAGCUUAUCAGACUGAUGUUGA-3′ (http://www.mirbase.org), as well as the anti-sense 5′-UCAACAUCAGUCUGAUAAGCUA-3′(miR-21inhibitor). A same length of sequence, 5′-UCUGACAGUUACCAAUGCUUAA-3′, from luciferase gene was selected as the control. The cDNA was obtained using DNA synthesis techniques, and then the double-stranded DNA was synthesized with DNA connection methods. It was inserted in the miRNASelect™ pEGP-miR carrier for DNA sequence determination.
Culture and transfection of 3T3-L1 preadipocytes
The culture of 3T3-L1 preadipocytes was conducted in DMEM (10 % fetal bovine serum, 100 U/ml penicillin and 100 U/ml streptomycin) with 37 °C temperature, 5 % CO2 and saturated humidity. LipofectamineTM 2000 Kit was used for cell transfection, according to the manufacturer’s protocol. The positive clone with puromycin selection was performed 48 h after transfection.
Inducing differentiation of 3T3-L1 preadipocytes and identification of differentiated adipocytes.
After 2 days of fusion of 3T3-L1 preadipocytes, the classic cocktail method [13] was used to induce their differentiation. 3T3-L1 preadipocytes were cultured in DMEM (0.5 mmol/l IBMX, 1 μmol/l dexamethasone, 10 μg/ml insulin and 10 % FBS) for 48 h, then in DMEM (10 μg/ml insulin and 10 % FBS) for 48 h, followed by continued culture in DEME containing 10 % FBS. After 10–12 days of inducing differentiation, the 3T3-L1 adipocytes were represented as multiple phenotypes. The identification of differentiated adipocytes was conducted using Oil-red-O staining method.
Determination of adiponectin and AP-1 protein expressions after transfection
The expressions of adiponectin and AP-1 protein after transfection were determined using Western blotting method. The differentiated 3T3-L1 adipocytes were treated with protein cleavage enzyme. After centrifugation, the supernatant protein was obtained. The SDS-PAGE (8 % SDS) was conducted on 30 μl of supernatant protein. Then it was transferred to the PVDF membrane and was sealed with skim milk powder for 1 h. After being covered with primary antibody overnight, the membrane was washed with TBST for three times, 10 min for each time. Then the horseradish peroxidase conjugated secondary antibody was added. After incubation for 1 h, the membrane was washed, then colored with aqueous solution of A and B. After X-ray film exposure, developing and fixation, it was observed.
Determination of adiponectin and AP-1 mRNA expressions after transfection
The expressions of adiponectin and AP-1 mRNA after transfection were determined using RT-PCR method. Total RNA was extracted with TRIzol reagent according to the manufacturer’s protocol. The primers of adiponectin, AP-1 and β-actin were designed using Beacon Designer 2.0 software. The sequence of the upstream primer of adiponectin was 5′-GTCCTAAGGGAGACATCGGT-3′, with 5′-CAGTGGAATTTACCAGTGG-3′ for downstream primer. For AP-1, the upstream and downstream primer sequences were 5′-CACGTTAACAGTGGGTGCCA-3′ and 5′-CCCCGACGGTCTCTCTTCA-3′, respectively. For β-actin, the upstream and downstream primer sequences were 5′-GCTATCCAGGCTGTGCTATC-3′ and 5′-CAGCACTGTTTGGCGTACA-3′, respectively. The cDNA was synthesized using RevertAidTM first strand cDNA synthesis kit. Then the RT-PCR amplification was performed using cDNA as template according to the standard protocol, followed by the agarose gel electrophoresis.
Statistical analysis
Data were expressed as mean ± SD. Statistical analysis was performed using SPSS 12.0 statistical software, and P < 0.05 was considered as statistically significant.
Results
Changes of microRNAs expression profiles during adipocyte differentiation
As shown in Tables 1 and 2, there were 30 down-regulated microRNAs (miR-24, miR-296, etc.) and 16 up-regulated microRNAs (miR-27a, miR-27b and miR-21, etc.) in differentiated adipocytes. The up-regulation of miR-21 was the most obvious. These indicated that, the expression profiles of microRNAs were obviously changed during adipocyte differentiation, and the adipocyte-related microRNAs existed.
Effect of miR-21 on adipocyte differentiation
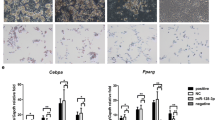
After transfection, inducing differentiation and Oil-red-O staining, the morphological changes of 3T3-L1 adipocytes were observed. Results were shown in Fig. 1. On the 7th day after inducing differentiation, the number and volume of miR-21 transfected adipocytes was singnificantly larger than those of normal preadipocytes in control (Fig 1c). Treatment of miR-21 inhibitor could restore the number and volume of the adipocytes, compared with the miR-21 transfection (Fig 1d). This indicated that, the miR-21 transfected 3T3-L1 adipocytes had a higher degree of differentiation.
Effect of miR-21 on adiponectin mRNA and protein expression
After extraction of total protein, the adiponectin mRNA and protein level was determined. Results showed that, after transfection with miR-21 and inducing differentiation, the expression level of adiponectin mRNA in 3T3-L1 adipocytes was obviously up-regulated (Fig. 2, 0.83 ± 0.07 vs. 0.42 ± 0.05, P < 0.01), and the amount of adiponectin protein significantly increased (Fig. 3, 0.78 ± 0.06 vs. 0.39 ± 0.05, P < 0.01), compared with control. Meanwhile, miR-21 inhibitor co-transfection blocked miR-21’s effects on adiponetcin mRNA (Fig. 2, 0.37 ± 0.06 vs. 0.83 ± 0.07, P < 0.01) and protein expression (Fig. 3, 0.45 ± 0.04 vs. 0.78 ± 0.06, P < 0.01), compared with miR-21 transfection alone. The expression of adiponectin mRNA and protein was not found in 3T3-L1 preadipocytes.
Reversal effect of AP-1 overexpression on miR-21 stimulatory function
The effect of miR-21 overexpression on miR-21 regulated AP-1 mRNA and protein expression was determined. As shown in Figs. 4 and 5, the AP-1 mRNA and protein levels in 3T3-L1 adipocytes with miR-21 overexpression were obviously lower than that in preadipocytes (mRNA: 0.20 ± 0.04 vs. 0.71 ± 0.09, and protein: 0.09 ± 0.02 vs. 0.91 ± 0.07, P < 0.01) and control adipocytes (mRNA: 0.20 ± 0.04 vs. 0.45 ± 0.05, and protein: 0.09 ± 0.02 vs. 0.33 ± 0.03, P < 0.01), respectively. Meanwhile, miR-21 inhibitor co-transfection abolished miR-21’s effects on adiponetcin mRNA and protein expression, compared with miR-21 transfection alone (mRNA: 0.36 ± 0.06 vs. 0.20 ± 0.04, and protein: 0.31 ± 0.03 vs. 0.09 ± 0.02, P < 0.01). This indicated that, the miR-21 overexpression could significantly inhibit the expression of AP-1 mRNA and protein.
Based on above results, the effect of AP-1 oveexpression on miR-21 stimulatory function for adiponectin expression was observed. It was found that, when AP-1 was overexpressed in 3T3-L1 adipocytes, there was no significant difference of adiponectin mRNA and protein level between adipocytes transfected with miR-21 and control (Figs. 6 and 7), indicated that AP-1 overexpression inhibited the miR-21’s effect on adiponectin expression (Figs 2 and 3). As shown in Figs. 2 and 3, miR-21 could significantly increase the adiponectin mRNA and protein expression level. Therefore, it was indicated that, under the condition of AP-1 overexpression, miR-21 could not stimulate the adiponectin expression. In other word, AP-1 overexpression could reverse the stimulatory effect of miR-21 on adiponection expression.
Discussion
The main purpose of our study is to explore the potential regulation function of microRNAs in adipocyte differentiation. The microRNAs which are closely related to adipocyte differentiation have been screened and identified. For miR-21 which has significant regulating activity, the high expression plasmid is constructed and transfected into 3T3-L1 preadipocytes. The regulating effect of miR-21 on adipocyte differentiation and expression of adipocyte-specific gene adiponectin is discussed. Results show that, the expression profiles of microRNAs have significantly changed during 3T3-L1 adipocyte differentiation, and the up-regulated miR-21 overexpression can obviously promote the preadipocyte differentiation, and increase the adiponectin expression, while decrease AP-1 expression.
More and more studies find that, microRNAs play an important role in adipocyte differentiation. For example, in adipocyte differentiation, the expression of miR-27 is down-regulated, and its overexpression can specifically inhibit the differentiation [14]. The expression of miR-143 is up-regulated in adipocyte differentiation, and the inhibition of its expression can effectively reduce the adipocyte differentiation [15, 16]. In addition, the expression of Let-7 is up-regulated in 3T3-L1 preadipocyte differentiation, and its overexpression can inhibit 3T3-L1 cell clonal amplification by targeting HMGA2 [17]. These data suggest that, the microRNAs expression profiles may change in adipocyte differentiation. This has been proved in our study, namely, the microRNAs which are associated with adipocyte differentiation exist. The overexpression of some microRNAs (e.g. miR-21) can significantly promote the adipocyte differentiation, and increase the expression of marker genes such as adiponectin.
There are a variety of adipocyte differentiation marker genes, such as PPAR γ, C/EBP α, LP1 and A-FABP [18]. Existing researches indicate that, the adiponectin is closely related to obesity, insulin resistance, type 2 diabetes and other metabolic syndromes. The adiponectin concentration in serum of patients with obesity decreases significantly. Simpson et al. [19] finds that, the reduced plasma level of adiponectin, especially adiponectin with high molecular weight, is the main cause of type 2 diabetes and cardiovascular diseases leading to obesity. As reported by Spranger et al. [20] the adiponectin plasma level is low in patients with type 2 diabetes. The high concentration of adiponectin can reduce the relative risk in patients with type 2 diabetes, and can greatly reduce the incidence of type 2 diabetes in healthy people. Kubota et al. [21] finds that, in adiponectin knockout mice, the blood sugar and insulin concentration increases significantly. The exogenous supplementation of adiponectin can significantly increase insulin sensitivity and reduce insulin resistance [21]. The research of Bloomgarden et al. [22] shows that, in patients with type 2 diabetes, the adiponectin can promote the burning and consumption of fatty acid in muscle cells reduce insulin resistance. In our study, when miR-21 is over expressed in 3T3-L1 preadipocytes, the expression levels of adiponectin mRNA and protein in differentiated adipocytes is obviously up-regulated. This suggests that, miR-21 may accelerate the adipocyte differentiation by promoting adiponectin expression.
Update, it is unknown about the molecular mechanisms underlying microRNA regulating adipocyte differentiation and adipogenic gene expression. Several reports have indicated the it may be complicated, such as, oxidative stress and inflammation could be involeved in the microRNA’s role in dipogenesis [23]. Of particular interest, miR-21 regulates adipocyte differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue [24]. Recently, it was recognized that some nuclear transcription factors might be responsible for mediating the microRNAs role in adipocyte differentiation such as Osterix [25], KLF5 [26], and Prdm16 [27]. In the present study, it was found that miR-21 had inhibitory effect on AP-1 expression during adipogenesis. The AP-1 is a dimeric complex of Jun and Fos proteins. As a transcription factor, it is involved in regulation of physiological and pathophysiological processe of many diseases. At present, the role of AP-1 in adipocyte differentiation is not clear. It is found that, the activation of JNK-Jun can prevent the lipogenesis by PPAR-γ phosphorylation [28, 29], and inhibit the adipocyte differentiation from mesenchymal stem cells by down-regulating CREB [30]. This study also finds that, after the 3T3-L1 preadipocytes differentiate into adipocytes, the AP-1 expression level decreases. Interestingly, when the miR-21 overexpression promotes adiponectin expression, the AP-1 expression is inhibited. In addition, the AP-1 overexpression can absolutely reverse the stimulating effect of miR-21 on adiponectin, which may be due to the collaborative regulation of miR-21 and AP-1 on adipocyte-specific genes. To our knowledge, this is firstly reported. Whether there are other transcription factors involved in miR-21 promoted adipocyte differentiation and adiponectin expression remains to be further studied.
In conclusion, the key microRNAs which are associated with adipocyte differentiation have been screened and identified in this study. miR-21 is the microRNA with the most obvious expression up-regulation. The high expression system of miR-21 in 3T3-L1 preadipocytes is constructed. Ap-1 mgiht play an important role in miR-21-mediated effects on adipocyte differentiation and adiponectin expression. This study has revealed the effect of microRNAs on adipocyte differentiation and regulating gene expression, and has provided a theory basis for further clarify the regulating effect of microRNAs on adipocyte differentiation and its pathological significance.
References
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM (2003) Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113:25–36
Ambros V (2003) MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell 113:673–676
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E et al (2007) A microRNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224
Pandey AK, Agarwal P, Kaur K, Datta M (2009) MicroRNAs in diabetes: tiny players in big disease. Cell Physiol Biochem 23:221–232
Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M et al (2008) MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456:980–984
Tavazoie SF, Alarcón C, Oskarsson T, Padua D, Wang Q, Bos PD et al (2008) Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451:147–152
Trujillo ME, Scherer PE (2005) Adiponectin: journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med 257:167–175
Couzin J (2008) MicroRNAs make big impression in disease after disease. Science 319:1782–1784
Yamauchi T, Kadowaki T (2008) Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond) 7:S13–S18
Xie H, Lim B, Lodish HF (2009) MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 58:1050–1057
Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ et al (2008) miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci USA 105:2889–2894
Cowherd RM, Lyle RE, McGehee RE Jr (1999) Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol 10:3–10
Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z (2009) A role of miR-27 in the regulation of adipogenesis. FEBS J 276:2348–2358
Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV et al (2004) MicroRNA-143 regulates adipocyte differentiation. J Biol Chem 279:52361–52365
Taksnabe R, Ono K, Abe Y, Takaya T, Horie T, Wada H et al (2008) Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochem Biophys Res Commun 376:728–732
Sun T, Fu M, Bookout AL, Kliewer SA, Kliewer SA, Mangelsdorf DJ (2009) MicroRNA let-7 regulates 3T3-L1 adipogenesis. Mol Endocrinol 23:925–931
Ntambi JM, Young-Cheul K (2000) Adipocyte differentiation and gene expression. J Nutr 130:3122S–3126S
Simpson F, Whitehead JP (2010) Adiponectin-it’s all about the modifications. Int J Biochem Cell Biol 42:785–788
Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H et al (2003) Adiponectin and protection against type 2 diabetes mellitus. Lancet 361:226–228
Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J et al (2002) Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277:25863–25866
Bloomgarden ZT (2002) Adiposity and diabetes. Diabetes Care 25:2342–2349
Hulsmans M, De Keyzer D, Holvoet P (2011) MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J25:2515–2527
Kim YJ, Hwang SJ, Bae YC, Jung JS (2009) MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 27:3093–3102
Zhang JF, Fu WM, He ML, Wang H, Wang WM, Yu SC et al (2011) MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol Biol Cell 22:3955–3961
Kinoshita M, Ono K, Horie T, Nagao K, Nishi H, Kuwabara Y et al (2010) Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5. Mol Endocrinol 24:1978–1987
Trajkovski M, Ahmed K, Esau CC, Stoffel M (2012) MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol 14:1330–1335
Hu E, Kim JB, Sarraf P, Kubota T, Moroi M, Matsui J et al (1996) Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 274:100–2103
Adelmant G, Gilbert JD, Freytag SO (1998) Human translocation liposarcoma-CCAAT/enhancer binding protein (C/EBP) homologous protein (TLS–CHOP) oncoprotein prevents adipocyte differentiation by directly interfering with C/EBPbeta function. J Biol Chem 273:15574–15581
Tominaga S, Yamaguchi T, Takahashi S, Hirose F, Osumi T (2005) Negative regulation of adipogenesis from human mesenchymal stem cells by Jun N-terminal kinase. Biochem Biophys Res Commun 326:499–504
Acknowledgments
The work is supported by the National Natural Science Foundation of China (No. 30871186), and the Research Foundation of the Education Department of Guangxi Province, China (No. 2013YB049).
Author information
Authors and Affiliations
Corresponding authors
Additional information
First author equivalent: Min Kang and Li-Mei Yan.
Rights and permissions
About this article
Cite this article
Kang, M., Yan, LM., Zhang, WY. et al. Role of microRNA-21 in regulating 3T3-L1 adipocyte differentiation and adiponectin expression. Mol Biol Rep 40, 5027–5034 (2013). https://doi.org/10.1007/s11033-013-2603-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2603-6