Abstract
In the present investigation a novel series of chalcone analogues were synthesized and evaluated for their anti-proliferative activity in human umbilical vein endothelial cells (HUVECs). Among 14 tested compounds, chalcone analogue (E)-3-(2′-methoxybenzylidene)-4-chromanone (KRP6) exhibited the most potent activity with IC50 19 μM. Moreover, HUVECs exhibited divergent, even opposing concentration-dependent responses to KRP6. This compound was the most potent inhibitor of cell proliferation and extracellular matrix formation (fibronectin and type IV collagen) at higher concentrations (20–50 μM). In contrast, KRP6 stimulated the compensatory increase in proliferative activity including extracellular matrix formation at low concentrations (1, 10 μM). KRP6 concentration-dependently modulated phosphorylation of Akt and mitogen-activated protein kinases such as extracellular signal-regulated kinase-1/-2 and p38 kinase, suggesting that these pathways play a role in the effect mediated by this compound. In addition, we found a selective effect on activated endothelial cells, in particular with resting endothelial cells. In conclusion, KRP6 is a potent modulator of selected steps of the angiogenic process in vitro. Accordingly, further in vivo research should be performed to facilitate its use in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past decades anti-tumor chemotherapy has not significantly reduced the mortality of several cancer-suffering patients, thus the search for new anti-cancer drugs has also been partially refocused on natural compounds. It has been found that they can prevent cancer development by many mechanisms, such as anti-oxidant [1], anti-inflammatory [2], anti-proliferative, and anti-cancer [3] activities. These bioactive compounds are generally safe and efficacious, given that they have been consumed by humans for centuries. However, understanding their mechanisms of action as therapeutic modalities is the major challenge for current medicine [4, 5].
Chalcones are naturally occurring compounds acting as intermediates in the biosynthesis of flavonoids, which are of high interest due to their wide range of biological activities [6]. Chalcones are also well known due to their pharmacological activity as anti-cancer and anti-proliferative agents [7, 8]. Published data suggest that the anti-proliferative activity of chalcone derivatives against various human carcinoma cell lines is tightly linked with cell cycle arrest and initiation of the cellular apoptotic machinery [9–12]. Although their mechanism of action seems to be rather non-specific, chalcones are able to selectively target distinct regulatory proteins that modulate many other downstream signaling pathways [13, 14].
Many lines of scientific evidence indicate the prominent role of plant polyphenols, including members of the chalcone family, in the fight against pathological angiogenesis [15, 16]. The angiogenic cascade is a complex process involving endothelial cell adhesion, proliferation, survival, migration, tube formation, and protease secretion. Several studies have reported that synthetic or semi-synthetic chalcone analogues exhibit anti-angiogenic activities in vitro and in vivo [17, 18]. These compounds inhibited the growth of human vascular endothelial cells, preferentially through multiple signaling pathways including the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, extracellular signal-regulated kinase (ERK)-1/-2, nuclear factor-κB pathway, as well as through inhibition of receptor tyrosine kinases and many other mechanisms [19–21].
Encouraged by these previous findings and our on-going interest in developing more effective anti-cancer therapeutics using several different approaches, we synthesized a series of new cyclic chalcone analogues that underwent preliminary in vitro testing in our laboratory. Among the tested compounds the compound (E)-3-(2′-methoxybenzylidene)-4-chromanone (KRP6) presented itself as the most potent modulator of cell proliferation and has therefore now become the main focus of our interest.
Materials and methods
Reagents
Medium 199 (M199) supplemented with 20 mM HEPES (M199), and newborn calf serum (heat-inactivated prior use) were obtained from Cambrex (Verviers, Belgium). l-glutamine, sodium dodecyl sulfate (SDS), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Human serum (heat-inactivated prior use) was obtained from PAA (Pasching, Austria). Vascular endothelial growth factor (VEGF)-A was purchased from Biosource (Camarillo, CA, USA). The tested cyclic chalcone analogues (synthesized in our laboratory): (E)-3-benzylidene-4-chromanone (KRP1), (E)-3-(4′-methoxybenzylidene)-4-chromanone (KRP2), (E)-3-(4′-methylbenzylidene)-4-chromanone (KRP3), (E)-3-(3′-methylbenzylidene)-4-chromanone (KRP4), (E)-3-(3′-methoxybenzylidene)-4-chromanone (KRP5), KRP6, (E)-3-(4′-bromobenzylidene)-4-chromanone (KRP7), (E)-3-(2′-bromobenzylidene)-4-chromanone (KRP8), (E)-3-benzylidene-4-thiochromanone (KRP9), (E)-3-(4′-fluorobenzylidene)-4-chromanone (KRP10), (E)-3-(4′-methoxybenzylidene)-4-thiochromanone (KRP11), (E)-3-(4′-chlorobenzylidene)-4-chromanone (KRP12), (E)-3-(4′-chlorobenzylidene)-4-thiochromanone (KRP13), (E)-3-(4′-fluorobenzylidene)-4-thiochromanone (KRP14) were dissolved in DMSO. The final concentration of DMSO in the culture medium was <0.2 % and exhibited no cytotoxic effect. Fetal bovine serum and antibiotics penicillin and streptomycin were purchased from Invitrogen (Carlsbad, CA, USA). Other materials used in the methods described below are specified in detail in related references or in the text or were purchased from standard commercial sources.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated, cultured, and characterized as previously described [22, 23]. Cells were cultured on gelatin-coated dishes in cM199 (= M199 medium supplemented with 10 % heat-inactivated human serum, 10 % heat-inactivated new born calf serum, 150 μg/ml crude endothelial cell growth factor (ECGF), 5 U/ml heparin, 100 IU/ml penicillin, and 100 μg/ml streptomycin) at 37 °C under 5 % CO2/95 % air atmosphere. Twenty-four hours prior to the experiments the endothelial cell cultures were refreshed with a medium without crude ECGF and heparin. Cell viability, estimated by trypan blue exclusion, was >95 % before each experiment.
Methyl-thiazole-tetrazolium (MTT) assay
The anti-proliferative effect of the tested compounds was studied using colorimetric microculture assay with the MTT end-point [24]. Briefly, aliquots of 80 μl cell suspension (4,000 cells/well) were added to 96-well microculture plates. Twenty-four hours after seeding, 20 μl aliquots of drug solutions (1, 3, 10, 30, 100 μM) were added to HUVECs in triplicate wells. After 48 h of culturing, 10 μl of the MTT solution was added to each well, incubated for another 4 h. Then the formazan crystals were dissolved with 100 μl of 10 % SDS. The optical density was measured at 540 nm with an EL-312 microplate reader (Biotek Instruments Inc., Winooski, VT, USA), and cell survival was calculated.
Assessment of monolayer integrity and cell viability
Endothelial cells were seeded and grown to confluence in 6-well plates and then treated with serial dilutions of KRP6 (1-100 μM) in cM199 medium. The number of cells was counted in 3 independent visualization fields recorded by a digital camera at time points 24, 48 and 72 h (Leica Microsystems, Wetzlar, Germany). In parallel, cell viability at 24 h was determined by Trypan blue exclusion test.
5-Bromo-2′-deoxyuridine (BrdU) cell proliferation assay
Cell proliferation activity was directly monitored by quantification of BrdU incorporated into the genomic DNA during cell growth. DNA synthesis was assessed using colorimetric cell proliferation ELISA assay (Roche Diagnostics GmbH, Mannheim, Germany) following the vendor’s protocol. Briefly, 4000 cells/well in 80 μl medium were plated in a 96-well tissue culture grade flat bottom plate. The next day, cells were treated with or without the studied compound KRP6 (1–100 μM) in the presence of 25 ng/ml of recombinant VEGF for 48 h. After 24 h of treatment, cells were incubated with BrdU labeling solution (10 μM final concentration) for another 24 h at 37 °C followed by fixation and incubation with anti-BrdU peroxidase conjugate for an additional 1.5 h at room temperature. Finally, after substrate reaction, color intensity was measured with multi-well microplate ELISA reader at 450 nm (reference wavelength: 690 nm).
Immunocytochemistry of HUVECs
HUVECs were seeded on cover glasses (22 × 22 mm) coated with gelatin at a density of 10,000 cells/cm2 in 6-well plates and cultured for 24 h. Tested chalcone with or without VEGF (25 ng/ml) was then added to the medium at final concentrations of 1–100 μM. VEGF was added to another well as a positive control. Cells were cultured for another 48 h. After draining, cells were washed with phosphate buffered saline (PBS) and fixed in 2 % paraformaldehyde (pH = 7.2). Consecutively, the cell membrane was permeabilized with 0.02 % Triton-X solution in PBS (not in the case of CD31). Non-specific binding of the secondary antibody was blocked by pre-incubation with normal Swine Serum (DAKO, Glostrup, Denmark) diluted in PBS (1:30) for 30 min.
To visualize endothelial cells either monoclonal mouse anti-human vimentin or polyclonal rabbit anti-human CD31 primary antibodies were used. To visualize proliferating cells monoclonal mouse anti-human vimentin, Ki67 primary antibody (1:50) (DAKO, Glostrup, Denmark) was used. To demonstrate extracellular matrix production by endothelial cells polyclonal rabbit anti-human fibronectin (1:1,000) (DAKO, Glostrup, Denmark) and monoclonal mouse anti-human collagen-IV (1:50) (DAKO, Glostrup, Denmark) antibodies were employed.
To visualize stained structures either goat anti-mouse immunoglobulin labeled by TRITC (diluted 1:30) (Sigma-Aldrich, St. Louis, MO, USA) or swine anti-rabbit immunoglobulin labeled by FITC (1:30) (Sigma-Aldrich, St. Louis, MO, USA) were used as the second step antibodies. Control of specificity was performed by replacing the first-step antibody with a monoclonal antibody of the same isotype directed against antigens not occurring in the cells. The nuclei of cells were counterstained by SlowFade® Gold Antifade Reagent with DAPI (Invitrogen, Carlsbad, CA, USA), specifically recognizing DNA.
Coverslips containing cultured cells were analyzed by fluorescence microscopy using a Nikon Eclipse 90i apparatus (Nikon, Tokyo, Japan) equipped with filterblocks specific for FITC, TRITC and DAPI, respectively, a high-resolution CCD camera Cool-1300Q (Vosskühler, Osnabrück, Germany) and a LUCIA 5.1 computer-assisted image analysis system (Laboratory Imaging, Prague, Czech Republic). Fluorescence intensity was measured under standardized conditions [25] using the software given above.
By evaluating Ki67 expression all cells were counted in three visualization fields of one coverslip followed by counting the Ki67 positive cells. The proliferation activity was then expressed as the percentage of Ki67 positive cells to the total number of cells.
Western blot analysis
Cell lysates were prepared as follows: equal numbers of HUVECs cultured on gelatin-coated wells were washed with ice-cold PBS and lysed using a lysis buffer containing: 0.02 M Tris/HCl (pH = 8), 0.15 M NaCl, 0.09 M KCl, 2 mM EDTA/NaOH, 5 % Igepal (Sigma-Aldrich, St. Louis, MO, USA), 0.5 % Triton-X-100, 1 mM Na3VO4, 0.05 M NaF, protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) and phosphatase inhibitor cocktail. Lysates were clarified by centrifugation at 14,000 rpm for 15 min at 4 °C. Protein concentration was measured using Bio-Rad Dc Protein Assay (Bio-Rad, Hercules, CA, USA). Equal amounts (20 μg) of protein samples were separated on 12 % SDS-polyacrylamide gel and electrophoretically transferred (100 V, 2 h) onto nitrocellulose membrane (Pall Gelman Laboratory, Ann Arbor, MI, USA). Afterwards the membrane was blocked for 1 h using 5 % non-fat dry milk. The following primary antibodies were used: anti-phospho-ERK1/2 (Cell Signaling Technology, Beverly, MA, USA, 1:2000), anti-phospho-Akt (Cell Signaling Technology, Beverly, MA, USA, 1:2000) and anti-phospho-p38 (Cell Signaling Technology, Beverly, MA USA, 1:1000). The membranes were incubated with the indicated antibodies in 1 % non-fat milk at 4 °C for 18 h. After quadruple washing with 0.2 % PBS-Tween 20, goat-anti-rabbit-HRP (Santa-Cruz Biotechnology, Santa Cruz, CA, USA, sc-2004, 1:2000) or goat-anti-mouse-HRP (Dako, Carpinteria, CA, USA; p0447, 1:2000) antibodies were added and membranes were incubated for 2 h at room temperature. Each membrane was washed in 0.2 % PBS-Tween 20 and protein was visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA) according to the manufacturer’s instructions on X-ray film (Pierce, Rockford, IL, USA). Signal intensity of p-ERK, p-Akt and p-p38 was determined densitometrically (software Quantity One, Bio-Rad) and expressed relative to total ERK, Akt or p38.
Two-dimensional migration (wound healing) assay
The motility of HUVECs was assayed using a wound healing assay [26]. Briefly, endothelial cells were cultured on a 24-well plate in the cM199 medium until confluent. A 2 mm pipette tip was used to wound the monolayer of cells. Afterwards, the medium was replaced with fresh ECGF and heparin-free medium containing the studied compound at different concentrations in the presence of 25 ng/ml of recombinant VEGF. The wounded area was photographed at the start (t = 0 h) and at a specific time point t = 17 h. The migration distance (gap size) was determined using image analysis software. The experiments were performed in duplicate wells and repeated three times with cells from different donors.
In vitro matrigel angiogenesis assay
The effect of KRP6 on the ability of HUVECs to reorganize and differentiate into capillary-like networks was assessed in the in vitro Matrigel morphogenesis assay as described previously [27]. Matrigel (9.8 mg/ml) was thawed at 4 °C, and 50 μl was quickly added to wells of a 96-well plate and allowed to solidify for 30 min at 37 °C. HUVECs were seeded at a cell density of 15,000 cells/well. A medium supplemented with VEGF (25 ng/ml) and different concentrations of studied compound (1–100 μM) was added for 8 h. The formation of tube-like structures was examined microscopically and photographs were taken using a camera (Leica Microsystems, Wetzlar, Germany) and Leica DM IL microscope (Leica Microsystems, Wetzlar, Germany).
Statistical analysis
Results are expressed as mean ± SEM (standard error mean). Statistical analyses of the data were performed using standard procedures, with one-way ANOVA followed by the Bonferroni multiple comparisons test. Differences were considered significant when P values were smaller than 0.05.
Results
Effect of newly-synthesized chalcone analogues on HUVECs
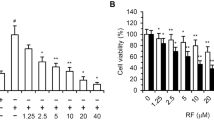
A series of newly-synthesized cyclic chalcone analogues was screened for potential anti-proliferative activity using the MTT assay. Among the tested compounds, compound KRP6 (see Fig. 1) exhibited the most significant modulatory effects on the growth of HUVECs. KRP6 reduced the proliferation capacity of HUVECs with an IC50 value of 19 μM. Other tested cyclic chalcone analogues displayed a very limited effect or no effect on cell proliferation at all (Fig. 2a).
Effect of KRP6 on HUVECs. a Inhibitory effect of chalcones analogues on proliferation of HUVECs. IC50 was the concentration causing 50 % growth inhibition for HUVECs. The results represent the mean values of three independent experiments. b Proliferation assay using quantitative ELISA analysis of BrdU incorporation into HUVECs during exposure to KRP6 (1–50 μM) in the presence of 25 ng/ml of recombinant VEGF for 48 h. Data are presented as mean ± SEM (***P < 0.001). c, d Influence of KRP6 on the monolayer of endothelial cells. HUVECs were cultured in cM199 medium in the presence or absence of various concentrations (10, 20, 30, 40 and 50 μM) of KRP6 for 72 h. At 24, 48 h and after 72 h pictures were taken and the cells were counted. Values are mean ± the standard error of the mean (SEM) from 3 cultures in two independent experiments. The pictures shown are representative of two independent experiments
The lack of cytotoxic activity of KRP6 in the range of concentrations (10–50 μM) was confirmed when the compound was added to confluent HUVECs (Fig. 2c, d) cultured in gelatin-coated wells. In this experiment, no significant decrease in cell number or increase in cell death, as assessed by trypan blue exclusion staining (data not shown), was observed when cells were incubated in the presence of KRP6 up to 72 h. However, higher concentrations of the compound were cytotoxic.
KRP6 as a modulator of cell proliferation
To confirm the potential anti-proliferative effect of this compound, the BrdU proliferation assay was used and the proportion of cells expressing Ki67 was established.
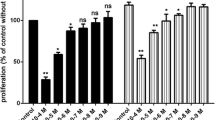
To quantify the inhibition of cellular proliferation we used the BrdU to monitor cellular proliferation at the DNA level in HUVECs at concentrations 1–100 μM. As shown in Fig. 2b, the tested compound showed different effects on DNA synthesis in HUVECs at the concentration used. Unlike in the VEGF control, dose-dependent inhibition of proliferation capacity of HUVECs was observed. At the concentrations of 20, 30, 40 and 50 μM of KRP6, a significant decrease in BrdU incorporation absorbance (A450nm–A690nm) in cells was found from 0.161 to 0.126 (approximately a 22 % decrease compared with the control, P < 0.001), 0.161 to 0.116 (approximately a 28 % decrease compared with the control, P < 0.001), 0.161 to 0.082 (approximately a 49 % decrease compared with the control, P < 0.001) and from 0.161 to 0.065 (approximately a 60 % decrease compared with the control, P < 0.001), respectively. The inhibitory effect of BrdU on proliferation was not observed at the concentrations 1 and 10 μM. Conversely, KRP6 treatment of HUVECs resulted in 121 % (P < 0.001) and 104 % stimulation in BrdU incorporation at these particular concentrations. Cell death was observed at the higher tested concentrations (60–100 μM). These findings suggest that KRP6 leads to reduced cellular expansion at concentrations of 20–50 μM.
In the next step, the human Ki67 nuclear antigen (pKi67) was used to confirm the potential anti-proliferative effect of KRP6. The expression of human Ki67 protein is strictly associated with cell proliferation. HUVECs were incubated with KRP6 at different concentrations. The anti-proliferative action of tested chalcone was confirmed by decreasing the percentage of Ki67 positive human endothelial cells. KRP6 added to cell culture at 30 and 50 μM statistically significantly reduced the percentage of proliferating cells as compared to the control (Fig. 3a). At higher concentrations (60, 70 μM) Ki67-expressing cells were not observed, since these concentrations were already cytotoxic to HUVECs (data not shown). Interestingly, when 80 and 90 μM concentrations were used, Ki67 was rarely expressed in cells with pyknotic nuclei, which may be related either to the DNA repair or to the effort of cells to undergo mitosis as a last resort. Surprisingly, the tested compound significantly stimulated the growth of human endothelial cells at the lowest tested concentration (1, 10 μM).
Immunocytochemistry of HUVECs. a The anti-proliferative action of KRP6 was confirmed by decreasing the percentage of Ki67 positive HUVECs. The pictures shown are representative of three independent experiments. The results show the mean ± SEM of three independent experiments, each performed in triplicate (***P < 0.001). Immunofluorescence staining of HUVECs shows the expression of extracellular matrix proteins such as collagen IV (red) b and fibronectin (green) c in the presence or absence of KRP6 at indicated concentrations. Cell nuclei are stained with DAPI staining (blue). (Color figure online)
Effect of KRP6 on extracellular matrix production by HUVECs
Cells isolated from umbilical veins were positive for both vimentin, which confirms the mesenchymal origin of cells, and CD31, which is a commonly-accepted marker of endothelium. Our study demonstrates that HUVECs seeded on a gelatin surface and cultured in a VEGF-free medium are able to produce a fine-structured extracellular matrix containing fibronectin and no type IV collagen. In contrast, cells in VEGF-stimulated culture synthesized rather rough bundles of fibronectin in a lower amount with poor expression of type IV collagen. Interestingly, the lowest tested concentration of KRP6 induced the most prominent fibronectin network formation, but the type IV collagen expression remained weak. Both fibronectin and collagen production decreased with the increasing concentration of the tested compound. The non-toxic concentration of 50 μM provided complete inhibition of both fibronectin and type IV collagen production (Fig. 3b, c).
Effect of KRP6 on endothelial intracellular signaling
The question whether signaling pathways (important in stimulation of cell proliferation, differentiation, survival, and growth) are involved in chalcone-mediated cell growth inhibition in HUVECs was also investigated. We attempted to elucidate the anti-proliferative activity and machinery targets of KRP6 in the VEGF-induced signaling pathways. These results provide evidence that KRP6 dose dependently inhibited the VEGF-induced activation of ERK, p38 MAPK and Akt in HUVECs. Western blot analysis showed that the levels of p-ERK (20–50 μM), p-Akt (20–50 μM) and p-p38 (30–50 μM) were reduced in KRP6 treated cells compared to control cells, although the total level remained the same. These results indicate that KRP6 could inhibit the activation of these pathways. Conversely, lower concentrations of this chalcone (1, 10 μM) promoted the phosphorylation of ERK and p38 MAPK, but not Akt kinase (Fig. 4). The results presented here corroborate our previous results discussed above.
Western blot analysis after KRP6 treatment. HUVECs were pre-treated with the indicated concentrations of KRP6 for 1 h and then stimulated with 25 ng/ml of VEGF for 30 min before collection. Phosphorylated and total ERK1/2 (a), p38 (b) or Akt (c) were detected by specific antibodies. The pictures shown are representative of three independent experiments. Western blots were quantified by densitometry and the ratio of phosphorylated ERK, phosphorylated p38 or phosphorylated Akt to their total counterpart was expressed as mean ± SD of three experiments (***P < 0.001 vs VEGF alone)
Effect of KRP6 on migration and tube formation of endothelial cells
Sprouting angiogenesis includes successive phases of microvessel formation, neovessel growth, and neovessel stabilization [28]. These steps require the migration of endothelial cells from the parent vessel toward angiogenic growth factors, proliferation of endothelial cells behind the migration front, and the organization of endothelial cells into capillary-like structures. Since we observed the potent capacity of KRP6 to inhibit proliferation of endothelial cells, it prompted us to evaluate the potential effect of this chalcone to inhibit other steps in the angiogenic process. First, the ability of the examined compound to inhibit endothelial cell migration observed by the overgrowth of a cellular line in the wounded areas of the confluent monolayer was studied. However, KRP6 did not influence the migration of endothelial cells in the range of concentrations (1–50 μM, data not shown). The studied compound displayed cytotoxic effect on endothelial cells at higher concentrations. Similarly, KRP6 treatment did not prevent the ability of HUVECs to form cord-like structures when seeded on matrigel (data not shown). These results indicate the specificity of the effect of KRP6 on cell proliferation.
Discussion
In the present study, we show for the first time that HUVECs exhibit divergent, even opposing—concentration dependent—responses to newly-synthesized chalcone analogue KRP6. Intriguingly, cells exposed to low concentrations of KRP6 showed markedly stimulated cell proliferation, while higher concentrations significantly reversed the proliferation activity. Similarly, our results also suggest that KRP6 initiated modulation of DNA synthesis in HUVECs in a concentration-dependent manner. Monitored Ki67 expression confirmed that KRP6 is a potent inhibitor of cell proliferation at concentrations of 20–50 μM (poor/no Ki67 expression) and a strong stimulator at concentrations of 1 and 10 μM (marked Ki67 expression). The important observation was that the efficient inhibitory concentrations of KRP6 (20–50 μM) had no cytotoxic effects on endothelial monolayers, since prolonged incubation of HUVECs with these concentrations did not change the morphology of the cells and did not induce cell death. Accordingly, based on the findings presented here it may be concluded that this compound is a potent modulator of cell proliferation.
The anti-proliferative activity could be explained by interference with VEGF-induced signal transduction cascades. Significantly, the MAPKs family including ERK and p38 MAPK and PI3K/Akt are thought to play an important role in cell proliferation [29]. Previous studies have shown that several chalcones could inhibit the phosphorylation of signaling molecules in cancer cell lines and in HUVECs [19, 30]. In the case of KRP6, tested chalcone was capable of suppressing VEGF-induced phosphorylation of ERK, Akt and p38 at non-toxic concentrations. In contrast, KRP6 stimulated phosphorylation of ERK and p38 at low concentrations, whereas no effect was observed on the phosphorylation of Akt. Maximum levels of ERK and p38 activation were observed as a result of treatment with 1 and 10 μM of KRP6. These results are apparently consistent with our experiments mentioned above and confirmed the pleiotropic effect of KRP6. Only a limited number of studies have been published that demonstrate similar pleiotropic effects of natural compounds or their chemical analogues. In breast cancer cells, apigenin was reported to possess a biphasic effect on cell proliferation. At lower concentrations, apigenin stimulated MCF-7 cell growth. However, at high concentrations, the drug inhibited cell growth [31]. Also quercetin exhibited a biphasic effect on cancer cells, stimulation at 1 and 10 μM, and inhibition at 100 μM of cell growth [32]. Furthermore, Baron-Menguy et al. [33] reported similar dual effect of red wine polyphenols. Recently, Choi and Kim [34] found that the isoflavones daidzein and genistein exhibited biphasic effects (stimulatory or inhibitory) on proliferation in breast cancer cells. Both flavonoids significantly stimulated cell growth at low concentrations and inhibited cell proliferation at high concentrations.
Although the concentration-dependent pleiotropic effect is not a typical feature of chalcones, KRP6 is the first published compound from this group with this effect in HUVECs. On the base of our results, dose-dependent effect on proliferation may be mediated in part by modulation of activities of protein kinases p38 and ERK. However, the precise explanation of why KRP6 exerts both proliferative and anti-proliferative effects at different concentrations remains unclear.
The majority of chalcones are highly multifunctional and have been investigated not only for their anti-proliferative effects [6, 35, 36], but also for their potential to inhibit tumor angiogenesis [15, 17, 37], which is an important consideration because the growth and metastatic behavior of tumors also depends on the formation of new blood vessels from pre-existing ones. The compound KRP6 did not affect the subsequent steps of angiogenesis such as migration and formation of tubular structures, stimulated by VEGF. However, KRP6 at non-toxic concentrations of 40–50 μM reduced fibronectin and collagen IV (basement membrane component) production in vitro. This observation indicates some specificity of this chalcone in the initial stage of the angiogenic cascade.
In conclusion, we have demonstrated that chalcone may be a promising modulator of cellular proliferation in HUVECs. A unique dual effect of KRP6 offers therapeutic perspectives for the treatment of angiogenesis-related diseases such as cancer, rheumatic disorders, myocardial infarction. However, it is necessary to perform many other studies both in vitro and in vivo to determine its true potential for the development of medicine.
References
Senevirathne M, Kim SK (2012) Utilization of seafood processing by-products: medicinal applications. Adv Food Nutr Res 65:495–512
Costa G, Francisco V, Lopes MC, Cruz MT, Batista MT (2012) Intracellular signaling pathways modulated by phenolic compounds: application for new anti-inflammatory drugs discovery. Curr Med Chem 19:2876–2900
Tsubura A, Lai YC, Kuwata M, Uehara N, Yoshizawa K (2011) Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anticancer Agents Med Chem 11:249–253
Aggarwal BB, Van Kuiken M, Iye LH, Harikumar KB, Sung B (2009) Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp Biol Med 234:825–849
Kim YH, Shin EK, Kim DH, Lee HH, Park JHY, Kim JK (2010) Antiangiogenic effect of licochalcone. A Biochem Pharmacol 80:1152–1159
Dyrager C, Wickström M, Fridén-Saxin M, Friberg A, Dahlén K, Wallén EA, Gullbo J, Grøtli M, Luthman K (2011) Inhibitors and promoters of tubulin polymerization: synthesis and biological evaluation of chalcones and related dienones as potential anticancer agent. Bioorg Med Chem 19:2659–2665
Batovska DI, Todorova IT (2010) Trends in utilization of the pharmacological potential of chalcones. Curr Clin Pharmacol 5:1–29
Yadav VR, Prasad S, Sung B, Aggarwal BB (2011) The role of chalcones in suppression of NF-κB-mediated inflammation and cancer. Int Immunopharmacol 11:295–309
Hseu YC, Lee MS, Wu CR, Cho HJ, Lin KY, Lai GH, Wang SY, Kuo YH, Kumar KJ, Yang HL (2012) The chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ROS generation and downregulation of the Akt/p38 MAPK signaling pathway. J Agric Food Chem 60:2385–2397
Kamal A, Mallareddy A, Suresh P, Shaik TB, Lakshma Nayak V, Kishor C, Shetti RV, Sankara Rao N, Tamboli JR, Ramakrishna S, Addlagatta A (2012) Synthesis of chalcone-amidobenzothiazole conjugates as antimitotic and apoptotic inducing agents. Bioorg Med Chem 20:3480–3492
Pilatova M, Varinska L, Perjesi P, Sarissky M, Mirossay L, Solar P, Ostro A, Mojzis J (2010) In vitro antiproliferative and antiangiogenic effects of synthetic chalcone analogues. Toxicol In Vitro 24:1347–1355
Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni SJ, Wang LS, Du X (2011) Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett 302:69–75
Jing H, Zhou X, Dong X, Cao J, Zhu H, Lou J, Hu Y, He Q, Yang B (2010) Abrogation of Akt signaling by isobavachalcone contributes to its anti-proliferative effects towards human cancer cells. Cancer Lett 294:167–177
Rajendran P, Ong TH, Chen L, Li F, Shanmugam MK, Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP, Hui KM, Sethi G (2011) Suppression of signal transducer and activator of transcription 3 activation by butein inhibits growth of human hepatocellular carcinoma in vivo. Clin Cancer Res 17:1425–1439
Mojzis J, Varinska L, Mojzisova G, Kostova I, Mirossay L (2008) Antiangiogenic effect of flavonoids and chalcones. Pharmacol Res 57:259–265
Orlikova B, Tasdemir D, Golais F, Dicato M, Diederich M (2011) Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr 6:125–147
Kong Y, Wang K, Edler MC, Hamel E, Mooberry SL, Paige MA, Brown ML (2010) A boronic acid chalcone analog of combretastatin A-4 as a potent anti-proliferation agent. Bioorg Med Chem 18:971–977
Robinson TP, Hubbard RB, Ehlers TJ, Arbiser JL, Goldsmith DJ, Bowen JP (2005) Synthesis and biological evaluation of aromatic enones related to curcumin. Bioorg Med Chem 13:4007–4013
Lee JS, Kang Y, Kim JT, Thapa D, Lee ES, Kim JA (2012) The anti-angiogenic and anti-tumor activity of synthetic phenyl propenone derivatives is mediated through the inhibition of receptor tyrosine kinases. Eur J Pharmacol 677:22–30
Noonan DM, Benelli R, Albini A (2007) Angiogenesis and cancer prevention: a vision. Recent Results Cancer Res 174:219–224
Park SY, Ku SK, Lee ES, Kim JA (2012) 1,3-Diphenylpropenone ameliorates TNBS-induced rat colitis through suppression of NF-κB activation and IL-8 induction. Chem Biol Interact 196:39–49
van Hinsbergh VW, Sprengers ED, Kooistra EA (1987) Effect of thrombin on the production of plasminogen activators and PA inhibitor-1 by human foreskin microvascular endothelial cells. Thromb Haemost 57:148–153
Defilippi P, van Hinsbergh VWM, Bertolotto A, Rossino P, Silengo L, Tarone G (1991) Differential distribution and modulation of expression of alpha1/beta1 integrin on human endothelial cells. J Cell Biol 114:855–863
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application for proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Klima J, Lacina L, Dvorankova B, Herrmann D, Carnwath JW, Niemann H, Kaltner H, Andre S, Motlik J, Gabius HJ, Smetana K Jr (2009) Differential regulation of galectin expression/reactivity during wound healing in porcine skin and in cultures of epidermal cells with functional impact on migration. Physiol Res 58:873–884
Martínez-Poveda B, Quesada AR, Medina MA (2005) Hypericin in the dark inhibits key steps of angiogenesis in vitro. Eur J Pharmacol 516:97–103
Grant DS, Tashiro KI, Segui-Real B, Yamada Y, Martin GR, Kleinman HK (1989) Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro Cell 58:933–943
Vailhé B, Vittet D, Feige JJ (2011) In vitro models of vasculogenesis and angiogenesis. Lab Invest 81:439–452
Santarpia L, Lippman SM, El-Naggar AK (2012) Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets 16:103–119
Zhang X, Yeung ED, Wang J, Panzhinskiy EE, Tong C, Li W, Li J (2010) Isoliquiritigenin, a natural anti-oxidant, selectively inhibits the proliferation of prostate cancer cells. Clin Exp Pharmacol Physiol 37:841–847
Long X, Fan M, Bigsby RM, Nephew KP (2008) Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-alpha-dependent and estrogen receptor-alpha-independent mechanisms. Mol Cancer Ther 7:2096–2108
ElAttar TM, Virji AS (1999) Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anticancer Drugs 10:187–193
Baron-Menguy C, Bocquet A, Guihot AL, Chappard D, Amiot MJ, Andriantsitohaina R, Loufrani L, Henrion D (2007) Effects of red wine polyphenols on postischemic neovascularization model in rats: low doses are proangiogenic, high doses anti-angiogenic. FASEB J 21:3511–3521
Choi EJ, Kim GH (2013) Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Mol Med Rep. doi:10.3892/mmr.2013.1283
Panchapakesan G, Dhayalan V, Dhatchana Moorthy N, Saranya N, Mohanakrishnan AK (2011) Synthesis of 2-substituted 17β-hydroxy/17-methylene estratrienes and their in vitro cytotoxicity in human cancer cell cultures. Steroids 76:1491–1504
Solomon VR, Lee H (2011) Anti-breast cancer activity of heteroaryl chalcone derivatives. Biomed Pharmacother 66:213–220
Varinska L, van Wijhe M, Belleri M, Mitola S, Perjesi P, Presta M, Koolwijk P, Ivanova L, Mojzis J (2012) Anti-angiogenic activity of the flavonoid precursor 4-hydroxychalcone. Eur J Pharmacol 691:125–133
Acknowledgments
This work was supported by the Slovak Research and Development Agency under contract No. APVV-0325-07, by SEPO-II (ITMS code: 26220120039) and by Charles University (Grant No. PRVOUK 27-1). We would like to thank Tom Billingham for his careful proof-reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Ivanova and L. Varinska contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ivanova, L., Varinska, L., Pilatova, M. et al. Cyclic chalcone analogue KRP6 as a potent modulator of cell proliferation: an in vitro study in HUVECs. Mol Biol Rep 40, 4571–4580 (2013). https://doi.org/10.1007/s11033-013-2547-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2547-x