Abstract
PHA665752 (PHA), a selective small molecule c-Met Inhibitor, potently inhibited HGF-stimulated and constitutive c-Met phosphorylation, as well as HGF and c-Met-driven phenotypes of a variety of tumor cells including hepatocellular carcinoma cells. However, these effects were impaired in c-Met-deficient cancer cells. In the present study, we investigated the potential anti-human c-Met-deficient hepatocellular carcinoma effects of Celastrol, a novel triterpene, and its combination with PHA. Human hepatocellular carcinoma cells BEL-7402 (c-Met-positive) and Huh7 (c-Met-deficient) were treated with different dose of PHA with or without equal dose of Celastrol, and cell growth, cell cycle and apoptosis were evaluated, respectively, by MTT assay, flow cytometry and Caspase3/7 activity. Nude mice bearing Huh7 xenografts were used to assess the in vivo anti-tumor activity. Our results showed that Celastrol at high concentration (>1.0 μM) induced G2/M arrest and apoptosis with the activation of Caspase3/7 in Huh7 cells whereas at low concentration (<1.0 μM) had no obvious effects. Low concentration Celastrol presented significant combined effects with PHA on Huh7 cells and Huh7 xenografts in terms of growth inhibition, migration inhibition and apoptosis induction. These results suggest that Celastrol and its combination with PHA present the therapeutic potential on c-Met-deficient hepatocellular carcinoma, and deserve further preclinical and clinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third most common malignancy related mortality rate in worldwide in the last decade [1]. Its occurrence rate has gradually increased, approximately 600,000–700,000 deaths worldwide and it is a great health problem. Especially in china, the percentage of Hepatocellular carcinoma (HCC) have mounted because of the highest carrier prevalences of hepatitis B virus (HBV) and hepatitis C virus (HCV), nearly 10 % of the general Population occurred Hepatocellular carcinoma [2]. Although surgical resection and transplantation are the major curative treatments modality, a minority of patients is amendable because of poor liver function reserve or limited liver donors. In addition, recurrent and metastatic diseases remain the most important factors for survival in HCC transplantation patients.
Hepatocyte growth factor (HGF) is an autocrine and paracrine factor that is produced by stromal cells [3]. HGF acts on c-Met, a high-affinity tyrosine kinase receptor. After c-Met phosphorylation and activation, multiple downstream signaling pathways are involved, including the phospho- inositide 3-kinase (PI3 K)/Akt and mitogen-activated protein kinase (MAPK)/Erk pathways [4]. Thus HGF/c-Met signaling triggers a variety of cellular responses, including proliferation, survival, cytoskeleton rearrangements, cell–cell dissociation, and motility through those downstream events. Within cancer, HGF/c-Met mediates a proliferative advantage and promotes tumor invasion and metastasis [5]. So c-Met has been considered a therapeutic target against tumor growth and metastasis in multiple types of cancers including HCC [6, 7].
A small molecule ATP-competitive inhibitor of c-Met tyrosine kinase, PHA-665752 (PHA), has been identified [8]. PHA-665752 is selective for c-Met with respect to other tyrosine and serine-threonine kinases. IC 50 of PHA-665752 against each of these kinases was more than 300-fold higher than IC 50 against c-Met [9]. Moreover, c-Met inhibition by PHA-665752 may be an effective therapy for HCC in selected patients with strong c-Met expression but may not be of benefit to HCC patients with c-Met–negative disease [10].
In the present study, we investigated the potential anti-human c-Met-deficient hepatocellular carcinoma effects of Celastrol, a novel triterpene, and its combination with PHA. We found that that Celastrol and its combination with PHA could significantly inhibit HCC development both in vitro and in vivo through multiple means. Thus we firstly show that present the therapeutic potential on c-Met-deficient hepatocellular carcinoma by Celastrol and its combination with PHA.
Materials and methods
Reagents and antibodies
Celastrol (C0869, sigma) was prepared for 20 mM stock and frozen at 20 °C. PHA-665752 (PZ0147, sigma). Phospho-MET (#3077, cell signaling Technology), MET (#3127, Cell signaling Technology), Sorafenib (LC Laboratories, S-8599), VEGF (V4512, sigma)
Cell lines and cell culture
Human liver cancer cell lines Bel-7402 and Huh7 were maintained in RPMI-1640 medium (GIBCO) supplemented with 10 % heat-inactivated fetal bovine serum (GIBCO, 10099141), penicillin and streptomycin (GIBCO, 10000U/ml).
Cell proliferation
Cells were seeded in a 96-well plate and cultured overnight. Cells were treated with celastrol or PHA in Triplicate. After72 h of incubation, Cells were then incubated with MTT (5 mg/ml, 20 μl/well). After 4 h, MTT in the cells was dissolved in DMSO and was measured at 570 nm using Flax station3. The inhibition rate on cell proliferation was calculated for each well as [IR(%) = (RLU control-RLU compound)/(RLU control-RLU blank) × 100 %].
Apoptosis assay
Cells were seeded in a 96-well plate, 5 × 103 per well and cultured overnight. Cells were then treated with indicated drugs. After incubated for the indicated time points, cells were then incubated with Caspase3/7 reagent (promega) for 0.5–1 h. Then the luminescence was measured on the Flax station3.
Cell cycle analysis
Cells were seeded into the 6-well plates at 2 × 105 cells/well and culture overnight. Cells were then treated with indicated drugs. After incubated for the indicated time points, cells were harvested and fix with 70 % ice cold ethanol overnight. Spin at 2,000 rpm for 5 min, cells were washed once with PBS, then cells were suspended in 200 ul PBS and treated with 10 ng/ml RNase A to wipe off RNA for 15 min at 37 °C. Cells were then re-suspended in 1 ml of DNA staining solution (2.5 μg/ml propidium iodide) at 4 °C for 30 min and the cell cycle stages were detect by FACS assay.
Transwell migration assay
Celastrol and/or PHA treated Cells (2 × 106) were suspended in serum free media with or without 20 ng/ml recombinant human VEGF. Cells were allowed to migrate for 6 h. After a 6 h incubation period at 37 °C, the non-migrating cells were removed from the upper surface by cotton swabs and the filters were collected with 0.5 mM EDTA. Then cells were placed in a new 96 well plate and MTT were added, the luminescence were then measured on the Flax station3.
Xenograft mouse model of human liver tumor
Male nude mice (4–6 weeks) were inoculated subcutaneously (S.C.) with human liver cancer cell lines Huh7. When tumor grew to about 100 mm3, the mice were treated with celastrol (1 mg/kg) five days per week for three weeks, Intraperitoneal Injection (i.p.) and vehicle was received 1 % DMSO, 7 % Cremophor/ethol (3:1), and 92 % PBS. PHA-665752, (10 mg/kg), solution in L-lactate(pH4.8) and 10 % polyethylene glycol, per day, i.p. Tumor size was measured twice per week. Tumor volume was calculated as [(V = length/width2)/2].
Statistical analysis
All data are presented as mean ± SEM. Statistical differences were determined by a two-tailed t test. *P < 0.05 was considered significant.
Results
Celastrol induces growth inhibition of human hepatocellular carcinoma cell lines
The protein expression levels of Met and p-Met are fluctuated in diverse hepatocellular carcinoma cell lines. To select hepatocellular carcinoma cell lines with relevant low Met/p-Met protein expression levels, we screened several hepatocellular carcinoma cell lines including Huh7, Bel-7402, SMMC-7721, Bel-7404, QGY-7701 and QGY-7703. We found that Met/p-Met protein expression levels were extremely low in Huh7 cell when compared with Bel-7402 cell (Fig. 1a and data not shown). Thus, Huh7 and Bel-7402 cells were ideal models for further biological test. As previously reported, the anti-tumor effects of PHA was highly dependent on the activity of Met/p-Met, we firstly treated Huh7 and Bel-7402 cells with PHA. As expected, PHA significantly inhibited the proliferation of Bel-7402 cell with IC50 = 0.0833 μM. However, PHA failed to inhibited the proliferation of Huh7 cell at low dose and the IC50 was more than 10 times (IC50 = 1.2276 μM) higher than Bel-7402 cell (Fig. 1b). These data was further confirmed the observations that the anti-tumor activity of PHA was indeed dependent on Met/p-Met protein expression levels. Celastrol have also been reported to show anti-tumor efficacy in diverse tumors including hepatocellular carcinoma [11]. To test the efficiency of Celastrol on hepatocellular carcinoma cells, we further treated Huh7 and Bel-7402 cells with Celastrol and found that Celastrol could inhibit the proliferation of these cells, although the IC-50 of Huh7 cell was about 5 times higher than Bel-7402 cell. Taken together, these data suggested that both PHA and Celastrol were able to inhibit the proliferation of hepatocellular carcinoma cells and the differences of the IC-50 may be dependent on Met/p-Met protein expression levels.
Celastrol induces growth inhibition of human hepatocellular carcinoma cell lines. a Lysate from Huh7 and Bel-7402 cells were analyzed by Western blot using indicated antibodies. β-actin was used as loading control. b, c Cells (2,500 cells/well) were incubated with Celastrol (b) or PHA-665752 (c) alone for 72 h. MTT assay was used to determine the antitumor proliferation efficacy and the IC50 of Celastrol and PHA-665752 were calculated
Celastrol enhances the growth inhibition effect of PHA to both Huh7 and Bel-7402 cells
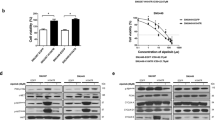
Because the therapeutic efficacy of PHA is limited by Met/p-Met protein expression levels in hepatocellular carcinoma cells, hence, we tested whether Celastrol enhances the efficacy of PHA on Met/p-Met-deficient hepatocellular carcinoma cells. For this purpose, Huh7 cells were treated with different concentrations of Celastrol (from 1 nM to 10 μM) and/or different concentrations (from 1 nM to 10 μM) of PHA for 48 h. As consistent with the data described above (Fig. 1b), PHA has limited inhibitory effect on Huh7 cells, while Celastrol could inhibit Huh7 growth in a dose–dependent manner (Fig. 2a). More intriguingly, the combined treatment of Celastrol with lower concentrations (from 1 nM to 0.1 μM) of PHA made growth of Huh7 cells be greatly inhibited compared with their single treatment (Fig. 2a). In the presence of 0.05 μM of Celastrol, IC50 of PHA against Huh7 cells is 0.05 μM, which was almost 4 % of its alone treatment (1.2276 μM). The similar synergistic effect was also observed in Bel-7402 cells (Fig. 2b). As consistent with this, their combined treatment also more significantly affected the viability of both Huh7 and Bel-7402 cells compared with their alone treatment (Fig. 2a, d).
Celastrol enhances the growth inhibition effect of PHA to both Huh7 and Bel-7402 cells. a, b Huh7 (a) or Bel-7402 (b) cells were incubated with Celastrol and/or PHA-665752 for 72 h, MTT assay was used to determine the antitumor proliferation efficacy and the IC50 of each treatment was calculated. c, d Huh7 (c) or Bel-7402 (d) cells were incubated with indicated drugs for 24 h, then cell viability was determined by the MTT assay
Celastrol enhances the anti-tumor activity of PHA in vivo in Huh7 xenograft model
In order to determine whether Celastrol can affect the growth of preexisting Met/p-Met-deficient hepatocellular carcinoma, male nude mice (4–6 weeks) were inoculated subcutaneously (S.C.) with Huh7 cells on one side, When tumor grew to about 100 mm3, the mice were treated with Celastrol and or PHA. 1 % DMSO was used as vehicle and Sorafenib was used as positive control. After 4 weeks, animals were euthanized and the tumor size was determined. There was no obvious difference in tumor size among Celastrol, PHA and Sorafenib single treatment. However, there was a very significant difference in tumor size between Celastrol and PHA combination and other single drug-treated mice. Celastrol and PHA combination treatment significantly reduced tumor volume (Fig. 3a) without affected the body weight of these mice (Fig. 3b). These data suggested that Celastrol could significantly enhance the anti-tumor activity of PHA in vivo.
Celastrol enhances the anti-tumor activity of PHA in vivo in Huh7 xenograft model. a Huh7 cells were injected into 6-wk-old, nude mice (3 × 106 per mouse). When tumor grew to about 100 mm3, the mice were treated with indicated drugs. Tumor size was measured twice per week. Tumor volume was calculated as [(V = length/width2)/2]. b Huh7 cells were injected into 6-wk-old, nude mice (3 × 106 per mouse). When tumor grew to about 100 mm3, the mice were treated with indicated drugs. Mice body was measured twice per week
Celastrol enhances PHA-induced migration inhibition
To investigate the underline mechanisms of in vivo anti-tumor activity of Celastrol and PHA combination, we first test whether Celastrol and PHA affect hepatocellular carcinoma cells migration. As shown in Fig. 4a, b, the migration of both Huh7 and Bel-7402 cells were enhanced by 10 ng/ml VEGF. Indeed, both Celastrol and PHA could slightly decrease these cells migration. However, when combined with Celastrol and PHA, VEGF-induced migration was almost inhibited.
Celastrol enhances PHA-induced cell cycle arrest
We next test whether Celastrol and PHA affect hepatocellular carcinoma cells cell cycle progress. As depicted in Fig. 5a, b, Celastrol or PHA alone caused the slightly increase in G2/M population of Huh7 or Bel-7402 cells. However, Celastrol and PHA combination significantly arrested cells at G2/M stage.
Celastrol enhances PHA-induced apoptosis in hepatocellular carcinoma cells
We further test whether Celastrol and PHA affect hepatocellular carcinoma cells apoptosis. As shown in Fig. 6a, b in vitro caspase activity test suggested that Celastrol and PHA combination could dramatically increase the activity of Caspase3/7, indicating that apoptosis induction will be contribute to the in vivo anti-tumor activity of Celastrol and PHA combination.
Discussion
The recent clinical success of combining targeted interventions with chemotherapies has extensively stimulated the relevant studies. Consequently, the molecular basis for the improved efficacy of combined treatment remains a topic of active investigation.
PHA is of considerable interest as an anti-tumor agent, in part, because of the specificity of its action in targeting regulating c-Met tyrosine kinase and its downstream signaling pathway[12, 13]. The key mechanism has been implicated in the resistance of cancer cells to the cytotoxicity of PHA—the expression level of c-Met. Indeed, PHA almost has no obvious effect on c-Met deficient tumors.
Celastrol, a natural compound extracted from Tripterygium wilfordii, exerts prominent anti-tumor activities against a variety of tumors through modulating proteasome activity, heat shock response, and NF-kB signaling pathways [14–16]. However, few reports describe the effects of combining celastrol with other anti-cancer agents. Here, we investigated the potential anti-human c-Met-deficient hepatocellular carcinoma effects of Celastrol and its combination with PHA. By using MTT, flow cytometry and Caspase3/7 activity detection assays, we found that Celastrol enhances PHA-induced migration, cell cycle arrest and apoptosis in hepatocellular carcinoma cells.
In conclusion, our results show that the combination of Celastrol and PHA could effectively inhibit c-Met-deficient hepatocellular carcinoma cells growth, migration and apoptosis both in vitro and in vivo. We found that even at low concentration, this combination also exerted significant anti-c-Met-deficient hepatocellular carcinoma activity which could overcome the disadvantages of both Celastrol and PHA alone. These results suggest that Celastrol and its combination with PHA present the therapeutic potential on c-Met-deficient hepatocellular carcinoma, and deserve further preclinical and clinical studies.
References
Baatarkhuu O, Kim do Y, Bat-Ireedui P, Han KH (2011) Current situation of hepatocellular carcinoma in Mongolia. Oncology 81(Suppl 1):148–151
Tanaka M, Katayama F, Kato H, Tanaka H, Wang J, Qiao YL, Inoue M (2011) Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol 21:401–416
Nakamura T, Matsumoto K, Kiritoshi A, Tano Y, Nakamura T (1997) Induction of hepatocyte growth factor in fibroblasts by tumor-derived factors affects invasive growth of tumor cells: in vitro analysis of tumor-stromal interactions. Cancer Res 57:3305–3313
Yap TA, de Bono JS (2010) Targeting the HGF/c-Met axis: state of play. Mol Cancer Ther 9:1077–1079
Grzelakowska-Sztabert B, Dudkowska M (2011) Paradoxical action of growth factors: antiproliferative and proapoptotic signaling by HGF/c-MET. Growth Factors 29:105–118
Yap TA, Sandhu SK, Alam SM, de Bono JS (2011) HGF/c-MET targeted therapeutics: novel strategies for cancer medicine. Curr Drug Targets 12:2045–2058
Sierra JR, Tsao MS (2011) c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol 3:S21–S35
Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, Chen J, Wang X, Ruslim L, Blake R, Lipson KE, Ramphal J, Do S, Cui JJ, Cherrington JM, Mendel DB (2003) A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res 63:7345–7355
Hov H, Holt RU, Ro TB, Fagerli UM, Hjorth-Hansen H, Baykov V, Christensen JG, Waage A, Sundan A, Borset M (2004) A selective c-met inhibitor blocks an autocrine hepatocyte growth factor growth loop in ANBL-6 cells and prevents migration and adhesion of myeloma cells. Clin Cancer Res 10:6686–6694
You H, Ding W, Dang H, Jiang Y, Rountree CB (2011) c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma. Hepatology 54:879–889
Rajendran P, Li F, Shanmugam MK, Kannaiyan R, Goh JN, Wong KF, Wang W, Khin E, Tergaonkar V, Kumar AP, Luk JM, Sethi G (2012) Celastrol suppresses growth and induces apoptosis of human hepatocellular carcinoma through the modulation of STAT3/JAK2 signaling cascade in vitro and in vivo. Cancer Prev Res (Phila) 5:631–643
Lau PC, Wong EY (2012) Targeting MET by tyrosine kinase inhibitor suppresses growth and invasion of nasopharyngeal carcinoma cell lines. Pathol Oncol Res 18:357–363
Kim S, Lee SH, Kang S, Lee L, Park JD, Ryu DY (2011) Involvement of c-Met- and phosphatidylinositol 3-kinase dependent pathways in arsenite-induced downregulation of catalase in hepatoma cells. Biol Pharm Bull 34:1748–1752
Yang H, Chen D, Cui QC, Yuan X, Dou QP (2006) Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res 66:4758–4765
Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G, Kim S, Gu W, Devlin JP, Silverman RB, Morimoto RI (2004) Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem 279:56053–56060
Kim Y, Kang H, Jang SW, Ko J (2011) Celastrol inhibits breast cancer cell invasion via suppression of NF-kB-mediated matrix metalloproteinase-9 expression. Cell Physiol Biochem 28:175–184
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, HL., Jin, JZ., Wu, D. et al. Celastrol exerts synergistic effects with PHA-665752 and inhibits tumor growth of c-Met-deficient hepatocellular carcinoma in vivo. Mol Biol Rep 40, 4203–4209 (2013). https://doi.org/10.1007/s11033-013-2501-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2501-y