Abstract
Insulin-like growth factor peptides, play an important role in regulating cell growth, differentiation, and apoptosis, which has been demonstrated to promote the development of cancer. The purpose of our study is to assess the association between circulation insulin-like growth factor peptides and colorectal cancer (CRC) risk. We searched Medline, EMBASE, OVID and Web of Science and picked up epidemiological studies that satisfied our inclusion criteria. A meta-analysis of 19 epidemiological studies containing 5,155 cases and 9,420 controls related with the association of circulation insulin-like growth factor peptides and CRC risk was carried out. Meta-analysis showed that high level IGF-I and IGF-II significantly increased CRC risk, (OR = 1.25, 95 % CI: 1.08–1.45 for IGF-I; OR = 1.52, 95 % CI: 1.16–2.01 for IGF-II; OR = 0.85, 95 % CI: 0.70–1.03 for IGFBP-1; OR = 0.77, 95 % CI: 0.41–1.43 for IGFBP-2 and OR = 0.88, 95 % CI: 0.71–1.10 for IGFBP-3). Subgroup analysis showed that the increased cancer risk by IGF-I was more distinguished in colon cancer (OR = 1.35, 95 % CI: 1.04–1.75) and Caucasian (OR = 1.32, 95 % CI: 1.12–1.56). Our meta-analysis provides comprehensive support for a role of circulation IGF-I and IGF-II in the etiology of CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer, with over 1 million new cases occurring every year worldwide [1]. CRC has been long prevalence in development countries, and even reached up to the second of cancer-related death these years [2]. In United States, there were an estimated 142,570 new cases and 51,370 cancer deaths in 2010 [3]. The incidence and mortality of CRC have also grown rapidly in developing countries, which has become one of the most serious threats to some developing countries [4]. Although the pathogenesis of CRC still remains unclear, a link between CRC and insulin resistance has drawn a lot of interest and has been hypothesized mediated by increasing exposure to the promitotic and antiapoptotic effects of insulin and insulin-like growth factor peptides [5].
Circulation insulin-like growth factor peptides, include insulin-like growth factors (IGF-I and IGF-II) and their binding proteins (IGFBP-1–IGFBP-6). Unlike most other growth factors, IGFs are produced by endocrine, autocrine, paracrine and occur in high concentrations in the blood. Free IGF-I in circulation plays an important role in cell behavior regulation by binding to its receptor. Nevertheless, IGFBPs can inhibit IGF-I action by binding competitively to it and thereby reducing its bioavailability [6]. Circulation insulin-like growth factor peptides are multifunctional peptides in regulating cell proliferation, differentiation, and apoptosis and necessary for the normal development of the colon and rectum [7, 8]. Besides, the cell behaviors mentioned above are all involved in cell malignant growth and transformation, therefore components of IGFs and their binding proteins may be also involved in CRC initiation and progress [9, 10]. Some epidemiological studies have shown that high concentration of IGF-I increased cancer risk, including CRC [11, 12], but others did not [13–15]. In addition, the association between high concentration of IGFBP-3 and CRC risk was more controversial [11, 15–18]. Moreover, there were a limited number of publications exploring the association between other components of IGF peptides, such as IGF-II, IGFBP-1–3, and CRC risk, which required comfirmation by studies with bigger sample size.
To understand the carcinogenesis role of the insulin-like growth factor peptides, an updated and comprehensive meta-analysis focused on more publication and peptides was conducted.
Materials and methods
Data collection
We identified eligible epidemiological studies by searching MEDLINE, EMBASE, OVID and Web of Science from January 1990 to May 2012 with the combination of the search phrases: “colorectal or colon or rectum” (title) and “cancer or carcinoma or neoplasia or tumor or neoplasm” (title) and “IGF* or IGFBP*” (title/abstract). Additional studies were identified by searching references list of key studies and reviews. The search was repeated after the paper writing, and no additional published data was found.
Criteria for inclusion and exclusion
Inclusion criteria: (1) primary research (not reanalysis, note or review); (2) cohort studies or case–control study design; (3) exploring the association between circulation IGF* and/or IGFBP* concentration and colorectal carcinogenesis; (4) presenting odds ratio (OR) and 95 % confidence interval (CI) or sufficient data to calculate OR and 95 % CI; (5) healthy people as control.
Exclusion criteria: (1) unpublished studies; (2) abstract, case report, comment, review and editorial; (3) Whenever reports pertained to overlapping patients, we retained only the largest study to avoid duplication of information.
Data extraction
Two investigators extracted data independently using a standardized data form, including data of author, year of publication, location, study design, sample size, demographic characteristics, CRC ascertainment method, adjusted factor, matching situation, test method and maximally adjusted OR. Then the two sets of extracted data were entered into database and checked for consistency by an automatic procedure.
Statistical analysis
We used maximally adjusted OR comparing the highest with lowest categories as the principle effect measure in this meta-analysis. We obtained adjusted ORs and 95 % CIs from the publications directly. Studies included in our meta-analysis differed in the variables of interest, so any kind of variability among studies may result in heterogeneity. Heterogeneity across studies was checked using the Cochran’s Q-test, which was considered significant at P < 0.05 [19]. The quantity I 2 that presents the percentage of total variation across studies as a result of heterogeneity was also calculated [20]. After that, appropriate method (fixed effect model or random effect model) was applied to calculate summary OR estimates. A fixed effect model was used as all the studies were homogeneous; otherwise, a random effect model was applied [21]. We calculated the combined effect of maximally adjusted ORs of IGFs and IGFBPs. Additional analysis of IGF-I and IGFBP-3 by subsite (colon and rectum) and ethnicity (Caucasian and Asian) was also conducted. The Egger and Begg tests were used to estimate publication bias [22, 23]. All statistic analyses were done with Stata Statistical Package (version 10.0).
Results
Literature search and meta-analysis databases
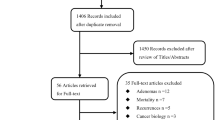
Our research yielded 279 potentially related publications. We finally selected 19 publications into our analysis, which included 16 prospective nested case–control studies and three case–control studies with 5,155 cases and 9,420 controls after screening according to the inclusion and exclusion criteria. Table 1 lists the characteristics of studies included. There were 16, 6, 7, 3 and 15 publications that involved results of IGF-I, IGF-II, IGFBP-1, IGFBP-2 and IGFBP-3 associated with CRC risk, respectively (Fig. 1). There were two publications [16, 24] from the Nurses’ Health Study cohort (NHS), two [25, 26] from the New York University Women’s Health Study (NYUWHS) and two [27, 28] from the Northern Sweden Health and Disease Cohort, but they explored the relationship between CRC risk with different components of IGF system. Four publications were carried out in male and six were done in female exclusively. Fourteen of nineteen studies were conducted in Caucasians, four in Asian, and one in mixed population. The types of sample and assay varied among these studies. One publication [17] displayed its outcomes by gender which did not provide enough information for us to combine the outcomes from the two categories, so in the following analysis, we treated the publication as two independent ones.
Test of heterogeneity
Figure 2 shows the association between the IGF system and CRC risk. The Q values from the heterogeneity tests on IGF peptides showed that all publications about IGF-I, IGF-II and IGFBP-1were homogenous, and that of IGFBP-2 and IGFBP-3 was heterogeneous (IGF-I: P heterogeneity = 0.212, I 2 = 20.7 %; IGF-II: P heterogeneity = 0.318, I 2 = 14.9 %; IGFBP-1: P heterogeneity = 0.397, I 2 = 3.8 %; IGFBP-2: P heterogeneity = 0.043, I 2 = 68.3 %; IGFBP-3: P heterogeneity = 0.024, I 2 = 45.6 %). Therefore, fixed effect model was used for meta-analysis of IGF-I, IGF-II and IGFBP-1, and random effect model was applied for IGFBP-2 and IGFBP-3. Besides publications on IGFBP-3 in Caucasian existed heterogeneity, other subgroup analyses by cancer site and ethnicity on IGF-I and IGFBP-3 found no heterogeneity in related publications (Figs. 3, 4).
Forest plots of the association between IGF system and colorectal cancer risk showing the effect comparing highest versus lowest category. The dashed vertical line and diamonds (95 % CI) are the pooled estimated of IGF-I, IGF-II and IGFBP-1 based on fixed effect models and that of IGFBP-2 and IGFBP-3 based on random effect models. Otani 2007a and Otani 2007b represented data on men and women of Otani’s publication, respectively
Meta-analysis
Overall, the pooled maximally adjusted ORs and 95 % CIs for comparison of highest and lowest categories of all components in IGF system of CRC were: IGF-I, OR = 1.25, 95 % CI = 1.08–1.45, P = 0.003; IGF-II, OR = 1.52, 95 % CI = 1.16–2.01, P = 0.003; IGFBP-1, OR = 0.85, 95 % CI = 0.70–1.03, P = 0.101; IGFBP-2, OR = 0.77, 95 % CI = 0.41–1.43, P = 0.399; IGFBP-3, OR = 0.88, 95 % CI = 0.71–1.10, P = 0.269 (Fig. 2). Subanalysis was conducted to explore the association between IGF-I, IGFBP-3 and CRC risk by cancer site. High level IGF-1 increased colon cancer risk with an OR of 1.35, (95 % CI = 1.04–1.75), but not in rectal cancer (OR = 0.88, 95 % CI = 0.58–1.36). IGFBP-3 presented influence in neither cancer site (Fig. 3). Further stratified analysis by ethnicity showed that IGF-I increased CRC risk more obviously in Caucasian than in Asian, with ORs of 1.35 (95 % CI = 1.12–1.61) and 1.03 (95 % CI = 0.74–1.42), respectively. IGFBP-3 did not display any difference in cancer risk between these two groups (Fig. 4).
Bias diagnosis
Finally, funnel plots and the Egger’s test were used to assess publication bias. None Begg’s funnel plot of IGF system on CRC showed obvious asymmetry (Supplemental Fig. 1). The consistent results of Egger’s test were as follow: IGF-I t = 1.42, P = 0.176; IGF-II t = 0.23, P = 0.832; IGFBP-1, t = −1.13, P = 0.310; IGFBP-2 t = −7.75, P = 0.082; IGFBP-3 t = −0.84, P = 0.415. Therefore, there was no significant publication bias in the studies included in current analyses.
Sensitivity analysis
In the sensitivity analysis, we repeated all above tests with different models to see how different the result would be because the quality of all publications was good in total. The pooled ORs for random models of CRC were as follow: IGF-I, 1.28 (95 % CI: 1.07–1.53); IGF-II, 1.52 (95 % CI: 1.09–2.11); IGFBP-1, 0.85 (95 % CI: 0.69–1.04), and pooled ORs for fixed effect models: IGFBP-2, 0.98 (95 % CI: 0.74–1.29); IGFBP-3, 0.91 (95 % CI: 0.80–1.06). Subgroup and influence trend analysis also showed stable statistical results in different models. All the results were similar to those we mentioned in the above paragraphs.
Discussion
This meta-analysis systematically assessed the relationship between CRC risk and IGF system concentration and revealed that the highest blood IGF-I and IGF-II concentration increased CRC risk by 25.2 and 52.2 %, respectively. However, there was no significant difference of the concentration of other IGF peptides in CRC risk. Moreover, in the stratified analyses, we found that higher concentrations of IGF-I increased colon cancer risk and CRC risk in Caucasian.
IGF family, which involves two ligands (IGF-I and IGF-II), two receptors (IGF-I R and IGF-II R) and six high-affinity binding proteins (IGFBP-1–6), is supposed to play an important role in regulating cell proliferation, apoptosis and transformation [29]. IGFs are mainly produced from liver with mitotic and anti-apoptotic roles and the bioactivities are regulated by their levels as well as the expression of proteases and IGFBPs. The IGF signaling pathways involving multiple interaction components are extremely complex and form networks. The pattern of the interactions within these networks, their kinetics and their subcellular compartmentalization result in alterations to cellular behaviors that increase the risk of deregulated cell growth and, ultimately, permit the growth of neoplastic cells that are more likely to develop into carcinomas in the presence of high IGFs level [30]. As the main ligands, the potential carcinogenesis role of IGF-I and IGF-II has been addressed in various cancers. In vitro models, IGFs have presented potent antiapoptotic and mitogenic properties in both normal and neoplastic cells. IGF-I and IGF-II were found commonly expressed by tumor cells and may act as an autocrine growth factor to promote the initiation and development of tumor [31]. Conversely, blockade of IGF-IR, the principle receptor of IGF-I and IGF-II, inhibits growth and angiogenesis of IGFs in colon cancer. Many tumor cells respond to IGF by increasing growth and many cancer cells have been shown to secrete high levels of IGF-I and IGF-II to sustain autocrine growth [32]. Epidemiological evidences also suggested the association between serum IGFs and the onset or progression of carcinoma [33–38]. For example, increasing IGF-I concentration was associated with a significant increase in breast cancer risk in women who developed breast cancer after 50 years of age [37]. Harman et al. [39] found that IGF-I was a significant risk factor for prostate cancer, and Muti et al. [40] presented the same effect of IGF-I on pre-menopausal breast cancer. London supported this association between plasma IGF-I and lung cancer [41]. The relationship between IGF-I and colorectal has also been summarized by previous meta-analyses, which found a 58 % increased OR (95 % CI 1.11–2.27) [42], a 56 % increased OR (95 % CI 1.14−2.13) [43] and a 31 % increased OR (95 % CI 1.03−1.67) [44] of CRC risk by comparing the highest with the lowest category of IGF-I. Since our meta-analysis was based on more publications, the pooled result tended to more modest and reasonable for complex disease. Besides, IGF-II was also presented association with CRC. Loss of imprinting (LOI), an epigenetic alteration affecting IGF-II gene, was found in normal colonic mucosa of about 30 % of CRC patients, but it was found in only 10 % of healthy individuals [45]. Additionally, IGF-II expression was up-regulated in many cancers including colon cancer [46]. Previous meta-analysis based on thre studies indicated a 95 % increased CRC risk by high level IGF-II [44], which might be overestimated but also indicated the carcinogenesis role of IGF-II.
There are some strengths in our meta-analysis. This is the first meta-analysis on five components of IGF family (IGF-I, IGF-II, IGFBP-1, IGFBP-2 and IGFBP-3) and CRC risk. Compared to previous ones [42, 43], this analysis not only included more IGF components but also attained more epidemiological studies, which makes the pooled results more stable, reasonable and comprehensive. In addition, our meta-analysis showed not only results on CRC risk but also results on colon and rectal cancer risk separately, which is helpful to identify the exact organ where IGF system may play their role and specificity species of cancer risk. Besides, to our knowledge, as a meta-analysis, our study is the first to explore the association between IGFBP-1 and IGFBP-2 and CRC risk.
Our study has several limitations. The number of publications about the association between IGFBP-2 and risk CRC was limited, so we need more epidemiological studies to validate our results. In this meta-analysis, we did not have sufficient data to classify CRC patients into different stages, which may influence the role of hormone levels in cancer risk. Tissue IGF bioactivity is determined by blood circulating and local IGF level, the IGF-IR and IGFBPs, but all researches we attained reported circulating IGFs and IGFBPs level, which may underestimate the real effects of IGFs/IGFBPs.
In summary, our findings suggest that the higher the circulating level of IGF-I, the greater the subsequent risk for CRC especially for colon cancer risk. High level of IGF-II may play a slight role in colorectal carcinogenesis.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917
de la Chapelle A (2004) Genetic predisposition to colorectal cancer. Nat Rev Cancer 4(10):769–780
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics. CA Cancer J Clin 60(5):277–300
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Sandhu MS, Dunger DB, Giovannucci EL (2002) Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst 94(13):972–980
Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE (2000) The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev 21(3):215–244
Jerome L, Shiry L, Leyland-Jones B (2003) Deregulation of the IGF axis in cancer: epidemiological evidence and potential therapeutic interventions. Endocr Relat Cancer 10(4):561–578
Pollak MN, Schernhammer ES, Hankinson SE (2004) Insulin-like growth factors and neoplasia. Nat Rev Cancer 4(7):505–518
LeRoith D, Baserga R, Helman L, Roberts CT Jr (1995) Insulin-like growth factors and cancer. Ann Intern Med 122(1):54–59
Baserga R (1995) The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res 55(2):249–252
Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ (1999) Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst 91(7):620–625
Manousos O, Souglakos J, Bosetti C, Tzonou A, Chatzidakis V, Trichopoulos D, Adami HO, Mantzoros C (1999) IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer 83(1):15–17
Probst-Hensch NM, Yuan JM, Stanczyk FZ, Gao YT, Ross RK, Yu MC (2001) IGF-1, IGF-2 and IGFBP-3 in prediagnostic serum: association with colorectal cancer in a cohort of Chinese men in Shanghai. Br J Cancer 85(11):1695–1699
Nomura AM, Stemmermann GN, Lee J, Pollak MN (2003) Serum insulin-like growth factor I and subsequent risk of colorectal cancer among Japanese-American men. Am J Epidemiol 158(5):424–431
Ollberding NJ, Cheng I, Wilkens LR, Henderson BE, Pollak MN, Kolonel LN, Le Marchand L (2012) Genetic variants, prediagnostic circulating levels of insulin-like growth factors, insulin, and glucose and the risk of colorectal cancer: the Multiethnic Cohort study. Cancer Epidemiol Biomarkers Prev 21(5):810–820
Giovannucci E, Pollak MN, Platz EA, Willett WC, Stampfer MJ, Majeed N, Colditz GA, Speizer FE, Hankinson SE (2000) A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev 9(4):345–349
Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S (2007) Plasma C-peptide, insulin-like growth factor-I, insulin-like growth factor binding proteins and risk of colorectal cancer in a nested case–control study: the Japan public health center-based prospective study. Int J Cancer 120(9):2007–2012
Rinaldi S, Cleveland R, Norat T, Biessy C, Rohrmann S, Linseisen J, Boeing H, Pischon T, Panico S, Agnoli C, Palli D, Tumino R, Vineis P, Peeters PH, van Gils CH, Bueno-de-Mesquita BH, Vrieling A, Allen NE, Roddam A, Bingham S, Khaw KT, Manjer J, Borgquist S, Dumeaux V, Gram IT, Lund E, Trichopoulou A, Makrygiannis G, Benetou V, Molina E, Suarez ID, Gurrea AB, Gonzalez CA, Tormo MJ, Altzibar JM, Olsen A, Tjonneland A, Gronbaek H, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Morois S, Slimani N, Boffetta P, Jenab M, Riboli E, Kaaks R (2010) Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer 126(7):1702–1715
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Andrel JA, Keith SW, Leiby BE (2009) Meta-analysis: a brief introduction. Clin Transl Sci 2(5):374–378
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, Giovannucci E (2005) A prospective study of C-peptide, insulin-like growth factor-1, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 14(4):850–855
Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E (2000) Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst 92(19):1592–1600
Hunt KJ, Toniolo P, Akhmedkhanov A, Lukanova A, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E, Kaaks R (2002) Insulin-like growth factor II and colorectal cancer risk in women. Cancer Epidemiol Biomarkers Prev 11(9):901–905
Palmqvist R, Hallmans G, Rinaldi S, Biessy C, Stenling R, Riboli E, Kaaks R (2002) Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut 50(5):642–646
Palmqvist R, Stattin P, Rinaldi S, Biessy C, Stenling R, Riboli E, Hallmans G, Kaaks R (2003) Plasma insulin, IGF-binding proteins-1 and -2 and risk of colorectal cancer: a prospective study in northern Sweden. Int J Cancer 107(1):89–93
Pollak MN (1998) Endocrine effects of IGF-I on normal and transformed breast epithelial cells: potential relevance to strategies for breast cancer treatment and prevention. Breast Cancer Res Treat 47(3):209–217
Jenkins PJ, Bustin SA (2004) Evidence for a link between IGF-I and cancer. Eur J Endocrinol 151(Suppl 1):S17–S22
LeRoith D, Roberts CT Jr (2003) The insulin-like growth factor system and cancer. Cancer Lett 195(2):127–137
Reinmuth N, Liu W, Fan F, Jung YD, Ahmad SA, Stoeltzing O, Bucana CD, Radinsky R, Ellis LM (2002) Blockade of insulin-like growth factor I receptor function inhibits growth and angiogenesis of colon cancer. Clin Cancer Res 8(10):3259–3269
Wolpin BM, Michaud DS, Giovannucci EL, Schernhammer ES, Stampfer MJ, Manson JE, Cochrane BB, Rohan TE, Ma J, Pollak MN, Fuchs CS (2007) Circulating insulin-like growth factor axis and the risk of pancreatic cancer in four prospective cohorts. Br J Cancer 97(1):98–104
Pham TM, Fujino Y, Kikuchi S, Tamakoshi A, Yatsuya H, Matsuda S, Yoshimura T (2007) A nested case–control study of stomach cancer and serum insulin-like growth factor (IGF)-1, IGF-2 and IGF-binding protein (IGFBP)-3. Eur J Cancer 43(10):1611–1616
Peeters PH, Lukanova A, Allen N, Berrino F, Key T, Dossus L, Rinaldi S, van Gils CH, Bueno-de-Mesquita HB, Boeing H, Schulz M, Chang-Claude J, Linseisen J, Panico S, Sacerdote C, Palli D, Tumino R, Trichopoulou A, Trichopolos D, Bamia C, Larranaga N, Ardanaz E, Pera G, Quiros JR, Martinez-Garcia C, Navarro C, Bingham SA, Khaw KT, Clavel F, Tjonneland A, Olsen A, Overvad K, Tetsche MS, Lund E, Lundin E, Berglund G, Riboli E, Kaaks R (2007) Serum IGF-I, its major binding protein (IGFBP-3) and epithelial ovarian cancer risk: the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer 14(1):81–90
Hernandez W, Grenade C, Santos ER, Bonilla C, Ahaghotu C, Kittles RA (2007) IGF-1 and IGFBP-3 gene variants influence on serum levels and prostate cancer risk in African-Americans. Carcinogenesis 28(10):2154–2159
Rinaldi S, Peeters PH, Berrino F, Dossus L, Biessy C, Olsen A, Tjonneland A, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Tehard B, Nagel G, Linseisen J, Boeing H, Lahmann PH, Trichopoulou A, Trichopoulos D, Koliva M, Palli D, Panico S, Tumino R, Sacerdote C, van Gils CH, van Noord P, Grobbee DE, Bueno-de-Mesquita HB, Gonzalez CA, Agudo A, Chirlaque MD, Barricarte A, Larranaga N, Quiros JR, Bingham S, Khaw KT, Key T, Allen NE, Lukanova A, Slimani N, Saracci R, Riboli E, Kaaks R (2006) IGF-I, IGFBP-3 and breast cancer risk in women: The European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer 13(2):593–605
Aksoy Y, Aksoy H, Bakan E, Atmaca AF, Akcay F (2004) Serum insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in localized, metastasized prostate cancer and benign prostatic hyperplasia. Urol Int 72(1):62–65
Harman SM, Metter EJ, Blackman MR, Landis PK, Carter HB (2000) Serum levels of insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-3, and prostate-specific antigen as predictors of clinical prostate cancer. J Clin Endocrinol Metab 85(11):4258–4265
Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schunemann HJ, Ram M, Freudenheim JL, Sieri S, Trevisan M, Berrino F (2002) Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol Biomarkers Prev 11(11):1361–1368
London SJ, Yuan JM, Travlos GS, Gao YT, Wilson RE, Ross RK, Yu MC (2002) Insulin-like growth factor I, IGF-binding protein 3, and lung cancer risk in a prospective study of men in China. J Natl Cancer Inst 94(10):749–754
Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M (2004) Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 363(9418):1346–1353
Duan QH, Wang ZG, Zhu GB, Lu ZX, Shi LY, Nie SF (2005) Study on the relations between serum insulin-like growth factor-1, insulin-like growth factor binding protein-3 and colorectal cancer: a meta-analysis. Zhonghua Liu Xing Bing Xue Za Zhi 26(2):132–134
Morris JK, George LM, Wu T, Wald NJ (2006) Insulin-like growth factors and cancer: no role in screening. Evidence from the BUPA study and meta-analysis of prospective epidemiological studies. Br J Cancer 95(1):112–117
Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, Wu Y, He X, Powe NR, Feinberg AP (2003) Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science 299(5613):1753–1755
Bertucci F, Salas S, Eysteries S, Nasser V, Finetti P, Ginestier C, Charafe-Jauffret E, Loriod B, Bachelart L, Montfort J, Victorero G, Viret F, Ollendorff V, Fert V, Giovaninni M, Delpero JR, Nguyen C, Viens P, Monges G, Birnbaum D, Houlgatte R (2004) Gene expression profiling of colon cancer by DNA microarrays and correlation with histoclinical parameters. Oncogene 23(7):1377–1391
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chi, F., Wu, R., Zeng, Yc. et al. Circulation insulin-like growth factor peptides and colorectal cancer risk: an updated systematic review and meta-analysis. Mol Biol Rep 40, 3583–3590 (2013). https://doi.org/10.1007/s11033-012-2432-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2432-z