Abstract
SGT1 (suppressor of G2 allele of Skp1) plays a role in various cellular processes including kinetochore assembly and protein ubiquitination by interacting with Skp1, a component of SCF E3 ligase complex. However, the function of SGT1 in cancer is largely unknown. Here, we showed that SGT1 was over-expressed in gastric cancer tissues and silencing of SGT1 by siRNAs significantly inhibited the growth and colony formation of gastric cancer cells. We further showed that SGT1 could regulate Akt signaling pathway by modulating Akt ser473 phosphorylation status. Moreover, we found that SGT1 was able to regulate the stability of PHLPP1, which is the direct phosphatase for Akt ser473 phosphorylation. Immunoprecipitation assay revealed that SGT1 could enhance the binding between PHLPP1 and beta-TrCP which has been documented to be able to target PHLPP1 for destruction. Decreased PHLPP1 in SGT1 over-expressed gastric cancer cells failed to dephosphorylate Akt and resulted in increased Akt ser473 phosphorylation and amplified downstream Akt signaling. Thus, our data revealed a previously uncovered role of SGT1 in gastric cancer development, and suggested that SGT1 could be a promising anti-cancer target to against gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer represents the third type of lethal cancers with more than 700,000 deaths worldwide each year. This disease is particularly frequent in eastern Asia, and in central and eastern Europe [1]. Protein kinase B (Akt) is essential for cell growth, proliferation, and survival. Aberrant activation of Akt is one of the most common molecular events in human cancers including gastric cancer, which is believed to play an essential role in gastric cancer cell survival and chemotherapy resistance [2, 3]. Akt is activated by phospholipid binding and activation loop phosphorylation at Thr308 by PDK1 and by phosphorylation within the carboxy terminus at Ser473 [4]. Dephosphorylation of Akt inactivates this kinase and terminates the whole Akt signal pathway [5]. The lipid phosphatase PTEN is a well known phosphate which has the ability to limit Akt activation by dephosphorylating PIP3 to PIP2 [6]. Deletion or mutation of PTEN is a common observation in many types of tumors and is usually accompanied with Akt hyperactivation [7]. Combining Akt signaling pathway inhibitors with chemotherapy has successfully attenuated chemotherapeutic resistance in gastric cancer cell lines [8, 9].
Recently, PHLPP, a novel family of Ser/Thr protein phosphatases, has been reported to be able to negatively regulate Akt [10, 11]. PHLPP members directly dephosphorylate Akt and terminate the whole signaling pathway. Over-expression of PHLPP1 in glioblastoma and colon cancer cells has been reported to be able to inhibit tumorigenesis [10]. Silencing the expression of PHLPP correlates with increased metastatic potential in breast cancer cells [12]. PHLPP1 is also a short-lived protein and targeted for ubiquitin-dependent degradation by SCF-beta-TrCP complex [13].
SGT1 (suppressor of G2 allele of Skp1) was first reported to play a role in various cellular processes including kinetochore assembly and protein ubiquitination by interacting with Skp1, a component of SCF E3 ligase complex [14]. SGT1 was presented in a proinflammatory complex termed inflammasome by interacting with NLR proteins that is essential for cell response to pathogen attack [15, 16]. Silencing of SGT1 resulted in abrogation of inflammasome activity [17]. SGT1 has also been reported to be over-expressed in colorectal cancer and might give rise to promote the transcription of the gene directly subsequent to the progression of colorectal cancer cases with worsening prognosis [18]. However, the exact role of SGT1 in cancer is still unknown.
Here, we showed that SGT1 was over-expressed in gastric cancer tissues. Silencing of SGT1 by siRNAs inhibited gastric cancer cells growth and colony formation by regulating Akt ser473 phosphorylation status and downstream Akt signaling pathway. Moreover, we provided evidences that SGT1 enhanced the interaction between beta-TrCP and PHLPP1 and promoted the degradation of PHLPP1 that resulted in amplified Akt signaling in gastric cancer cells.
Materials and methods
Tissue samples
Primary gastric cancer tissues and adjacent normal gastric tissues were collected from either routine therapeutic surgery or gastrointestinal endoscopy at our department. All samples were obtained with informed consent and approved by central hospital of Fengxian district clinic institutional review board.
Cell culture
AGS and 293T cell lines were cultured in RPMI-1640 or Dulbecco’s modified Eagle’s medium supplemented with 10 % fetal bovine serum. All these cells were cultured in a 5 % CO2/95 % air at 37 °C.
Reagents
The following antibodies were purchased: anti-Akt, Akt p-308, Akt p-743, p-Erk, p38, p-p38, MEK1, MEK2 and PTEN (Cell Signaling), anti-SGT1 (Abcam), PHLPP1 (Abgent) and anti-actin (Sigma). All of the lentiviral siRNA vectors were purchased from Santa Cruz.
Colony formation assay
Each 35 mm culture dish contained a base layer consisting of 0.5 ml culture media (0.6 % agar). Cells were cultured by layering 5,000 cells in 0.5 ml culture media (0.3 % agar) over each base layer. All cells formed sufficient numbers of detectable colonies following 14 days incubation.
Plasmids and transfection
All the plasmids of this work are purchased from Addgene. All the transient transfections were conducted using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Immunoprecipitation
Cells were lysed in lysis buffer (50 mM Tris–HCl pH 7.5, 250 mM NaCl, 0.5 % Nonidet P40, and protease inhibitor cocktail) for 30 min at 4 °C. Lysates were cleared using centrifugation (13,000 rpm, 20 min), the supernatant was subjected to immunoprecipitation (IP) with appropriate antibodies overnight at 4 °C with gentle inversion. Protein A/G beads were added for additional 4 h. Beads containing immune complexes were washed with ice cold lysis buffer 4×. Precipitates were denatured in loading buffer at 100 °C for 5 min and subjected to western blot.
Western blotting
Protein extracts were equally loaded on 10 % SDS–PAGE, electrophoresed, and transferred to nitrocellulose membrane. After blocking with 5 % nonfat milk in PBS, the membranes were incubated with the indicated primary antibodies and followed by horseradish peroxidase (HRP)–linked secondary antibodies (sigma).
Statistical analysis
Values were shown as mean ± SD. Statistical differences were determined by a Student’s t test. Statistical significance is displayed as *(P < 0.05), **(P < 0.01) or ***(P < 0.001).
Results
SGT1 was over-expressed in gastric cancer samples
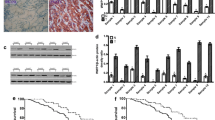
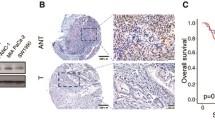
SGT1 has been reported to be over-expressed in human colorectal cancer [18]. However, whether SGT1 plays a role in gastric cancer is not known. To evaluate the expression of SGT1 in gastric cancer cells, we detected the mRNA levels of SGT1 in 30 primary gastric cancer samples and their adjacent normal tissues by way of real-time quantitative PCR. As depicted in Fig. 1a, 17 out of 30 primary gastric cancer tissues showed more than twofold of SGT1 mRNA expression levels compared with their adjacent normal tissues. We then employed Western blot with anti-SGT1 antibody to detect the protein expression level of SGT1 in those clinic samples. In consistent with the real-time PCR data, the protein level of SGT1 was much higher in gastric samples. Taken together, these results showed that SGT1 was over-expressed in gastric cancer samples (Fig. 1b and Supp.1).
SUGT1 was over-expressed in gastric cancer samples and silencing of SUGT1 inhibited gastric cancer cells growth and colony formation. a The mRNA levels of SUGT1 in gastric cancer tissues were measured by real-time PCR, normalized to the adjacent normal tissues and depicted as a fold change between gastric cancer tissues and adjacent normal tissues (more than two fold was considered over-expression). b The protein levels of SUGT1 in three paired gastric cancer tissues and their adjacent normal tissues were measured by western blot with anti-SUGT1. c The mRNA levels of SUGT1 in AGS cells infected with the indicated lentiviral siRNAs. d The protein levels of SUGT1 in AGS cells infected with the indicated lentiviral siRNAs. e Growth curves of AGS cells infected with the indicated lentiviral siRNAs. f The colony numbers of AGS cells infected with the indicated lentiviral siRNAs
Silencing of SGT1 inhibited gastric cancer cells growth and colony formation
To investigate the role of SGT1 in gastric cancer cells, we silenced the expression of SGT1 in gastric cancer cell line AGS cells by utilizing two different lentivirus-based siRNAs. The knockdown efficiency of SGT1 was measured by real-time quantitative PCR and western blot, respectively. Both siRNAs inhibited the expression of SGT1 efficiently (Fig. 1c–d). Thus, we used these siRNAs to investigate the biological roles of SGT1 in gastric cancer cells. As depicted in Fig. 1e, silencing of SGT1 significantly inhibited AGS cells growth. The colony formation ability of SGT1 silencing AGS cells was also decreased (Fig. 1f). Taken together, these data suggested that SGT1 played a role in the regulation of gastric cancer cells growth.
SGT1 regulated Akt ser473 phosphorylation in gastric cancer cells
To investigate how SGT1 regulates gastric cancer cells growth, we initiated a small western blot screen by employing signaling pathway antibodies. As shown in Fig. 2a, silencing of SGT1 significantly decreased Akt ser476 phosphorylation without affecting Akt Thr308 phosphorylation or total Akt levels. Indeed, over-expression of SGT1 in AGS cells increased Akt ser476 phosphorylation in a dose dependent manner (Fig. 2b). Moreover, stable expression of SGT1 accelerated AGS cells growth, whereas Akt inhibitor LY294002 treatment abolished this observation (Fig. 2c). These data indicated that SGT1 regulated gastric cancer cells growth by modulating AKT ser476 phosphorylation.
SUGT1 regulated AKT1 phosphorylation in gastric cancer cells. a AGS cells infected with lentiviral non-specific or SUGT1 siRNAs were subjected to western blot with indicated antibodies. b AGS cells transfected with increased dose of HA-SUGT1 plasmids for 48 h, cells were harvested and subjected to western blot with indicated antibodies. c Growth curves of AGS cells infected with the indicated lentiviral genes with or without LY294002
SGT1 regulated the stability of PHLPP1
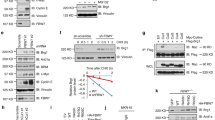
Both PTEN and PHLPP1 have been reported to be able to affect Akt phosphorylation [19–22]. To further investigate how SGT1 modulates Akt ser473 phosphorylation, we checked the protein levels of these phosphates in SGT1 silenced cells. Silencing of SGT1 did not affect the protein expression level of PTEN, but significantly increased the expression of PHLPP1 (Fig. 3a). To investigate how SGT1 regulates PHLPP1, we checked the mRNA levels of PHLPP1 in SGT1 silenced AGS cells. However, the mRNA levels of PHLPP1 were not changed in these cells (Fig. 3b). Furthermore, we over-expressed SGT1 in AGS cells and found that PHLPP1 was significantly decreased at protein level but not mRNA level (Fig. 3c and data not shown). These observations leaded us to test whether SGT1 regulates PHLPP1 at post-translational level. To test this possibility, the half-life of PHLPP1 in 293T cells transfected with PHLPP1 alone or in combination with SGT1 with or without MG132 was measured. Interestingly, over-expression of SGT1 significantly decreased the half-life of PHLPP1, whereas MG132 prevented the effect of SGT1 on PHLPP1 (Fig. 3d). These data suggested that SGT1 regulated the stability of PHLPP1 in gastric cancer cells.
SUGT1 regulated the stability of PHLPP1. a AGS cells infected with lentiviral non-specific or SUGT1 siRNAs were subjected to western blot with indicated antibodies. b mRNA levels of PHLPP1 in AGS cells infected with lentiviral non-specific or SUGT1 siRNAs. c AGS cells transfected with control or HA-SUGT1 plasmid for 48 h, cells were harvested and subjected to western blot with indicated antibodies. d The quantification of PHLPP1 half-life in 293T cells transfected with PHLPP1 alone or in combination with SUGT1 (with or without MG132)
SGT1 interacted with PHLPP1
To investigate how SGT1 regulates the stability of PHLPP1, we firstly asked whether SGT1 interacted with PHLPP1. Lysates of 293T cells transfected with HA-SGT1 or Myc-PHLPP1 or both for 48 h were subjected to HA immunoprecipitation followed by western blot with Myc antibody. Indeed, Myc-PHLPP1 was detected in HA-SGT1 immunoprecipitant (Fig. 4a). To exclude the positive false interaction caused by over-expression, endogenous SGT1 was immunoprecipitated by SGT1 antibody from AGS cells and in which endogenous PHLPP1 but not PTEN was detected (Fig. 4b). To test whether SGT1 interacted directly with PHLPP1, we further performed in vitro GST-pull down assay. Protein complexes pulled down with nickel beads from BL21 bacteria expressing GST or GST-SGT1 incubated with His-tagged PHLPP1 were subjected to western blot. His-PHLPP1 was ready to be detected in GST-SGT1 but not GST alone complexes (Fig. 4c). These data suggested that SGT1 interacted with PHLPP1 directly.
SUGT1 interacted with PHLPP1 and enhanced the binding between PHLPP1 and beta-TrCP. a Lysates of 293T cells co-transfected with HA-SUGT1 and Myc-PHLPP1 for 48 h were subjected to HA immunoprecipitation (IP) followed by western blot with the indicated antibodies. b Lysates of AGS cells were subjected to IP with SUGT1 antibody or preimmune mouse serum (IgG) followed by western blot with the indicated antibodies. c Western blot of protein complexes pulled down with nickel beads from BL21 bacteria expressing GST or GST-SUGT1 incubated with His-tagged PHLPP1. d Lysates of 293T cells co-transfected with indicated plasmids for 48 h were subjected to Myc IP followed by western blot with the indicated antibodies
SGT1 enhanced the binding between PHLPP1 and beta-TrCP
SGT1 has been reported to be able to form complexes with SCF E3 ligase [14]. It’s also documented that SCF-beta-TrCP is responsible for the efficient degradation of PHLPP1 [10]. Given the facts that SGT1 interacted directly with PHLPP1 and promoted its degradation, beta-TrCP might play a role in SGT1 mediated PHLPP1 degradation. To rule it out, Flag-PHLPP1 and Myc-beta-TrCP were co-transfected into 293T cells with or without SGT1 for 48 h, and cell lysates were subjected to Myc immunoprecipitation followed by western blot with Flag antibody. In consistent with previously study, beta-TrCP interacted with PHLPP1. Interestingly, in the present of SGT1, beta-TrCP interacted with PHLPP1 more efficiently (Fig. 4d and Supp.2), which may explain how SGT1 regulated the stability of PHLPP1.
Discussion
In the present study, we found that SGT1 was over-expressed in most cases of gastric cancer tissues. Silencing the expression of SGT1 by lentivirus-based siRNAs significantly inhibited gastric cancer cells AGS growth and colony formation. We also provided evidence that SGT1 could regulate Akt signaling pathway by modulating Akt ser473 phosphorylation status. Moreover, we found that SGT1 was able to regulate the stability of PHLPP1, which is the direct phosphatase for Akt ser473 phosphorylation. SGT1 was reported to associate with SCF complex and may play a role in protein ubiquitination and degradation. We further showed that SGT1 could enhance the binding between beta-TrCP and PHLPP1 that resulted in increased PHLPP1 degradation. The decreased PHLPP1 in turn amplified Akt ser473 phosphorylation and downstream Akt signaling that may explain the biological role of SGT1 in gastric cancer.
Mammals express two distinct paralogs of beta-TrCP including beta-TrCP1 and beta-TrCP2 with indistinguishable biochemical properties. Beta-Trcp1−/− mice have been shown to have impairment in spermatogenesis and reduced fertility without signs of gross tissue abnormalities [23]. However, Beta-Trcp1−/− MEF cells display several defects, including multipolar metaphase spindles, misaligned chromosomes and a lengthened G2–M transition [24]. It has been showed that 56 % of the colorectal cancer tissues tested had increased Beta-TrCP1 mRNA and protein levels which was associated with decreased apoptosis and poor prognosis [25]. Beta-TrCP is also overexpressed in hepatoblastomas and breast cancers [26].
Our study identified an oncogenic role of SGT1 in gastric cancer. Because beta-TrCP has been considered to be an oncogene in a serial of human cancers and SGT1 is able to enhance the binding between PHLPP1 and beta-TrCP, it will be interesting to detect the expression pattern between SGT1 and beta-TrCP in gastric cancer. It will be also essential to further investigate the exact roles of SGT1 in SCF E3 ligase complex.
In summary, our results provide new insight into how SGT1 could influence the Akt signaling pathway, which could provide a potential mechanism by which SGT1 influences gastric cancer cells growth. Ultimately, these data may provide the rationale for the development of specific SGT1 inhibitors as efficient anti-cancer drugs to against gastric cancer.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Bellacosa A, Kumar CC, Di Cristofano A, Testa JR (2005) Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res 94:29–86
Michl P, Downward J (2005) Mechanisms of disease: PI3K/AKT signaling in gastrointestinal cancers. Z Gastroenterol 43:1133–1139
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101
Qiao M, Sheng S, Pardee AB (2008) Metastasis and AKT activation. Cell Cycle 7:2991–2996
Georgescu MM (2010) PTEN tumor suppressor network in PI3 K-Akt pathway control. Genes Cancer 1:1170–1177
Li J, Yen C, Liaw D, Podsypanina K, Bose S et al (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943–1947
Lazar D, Raica M, Sporea I, Taban S, Goldis A et al (2006) Tumor angiogenesis in gastric cancer. Rom J Morphol Embryol 47:5–13
Almhanna K, Strosberg J, Malafa M (2011) Targeting AKT protein kinase in gastric cancer. Anticancer Res 31:4387–4392
Gao T, Furnari F, Newton AC (2005) PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell 18:13–24
Brognard J, Sierecki E, Gao T, Newton AC (2007) PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell 25:917–931
Qiao M, Iglehart JD, Pardee AB (2007) Metastatic potential of 21T human breast cancer cells depends on Akt/protein kinase B activation. Cancer Res 67:5293–5299
Li X, Liu J, Gao T (2009) beta-TrCP-mediated ubiquitination and degradation of PHLPP1 are negatively regulated by Akt. Mol Cell Biol 29:6192–6205
Kitagawa K, Skowyra D, Elledge SJ, Harper JW, Hieter P (1999) SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Mol Cell 4:21–33
Mayor A, Martinon F, De Smedt T, Petrilli V, Tschopp J (2007) A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol 8:497–503
Kadota Y, Shirasu K, Guerois R (2010) NLR sensors meet at the SGT1-HSP90 crossroad. Trends Biochem Sci 35:199–207
Shirasu K (2009) The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu Rev Plant Biol 60:139–164
Iwatsuki M, Mimori K, Sato T, Toh H, Yokobori T et al (2010) Overexpression of SUGT1 in human colorectal cancer and its clinicopathological significance. Int J Oncol 36:569–575
Mendoza MC, Blenis J (2007) PHLPPing it off: phosphatases get in the Akt. Mol Cell 25:798–800
Molina JR, Agarwal NK, Morales FC, Hayashi Y, Aldape KD et al (2011) PTEN, NHERF1 and PHLPP form a tumor suppressor network that is disabled in glioblastoma. Oncogene 31(10):1264–1274
Chen M, Pratt CP, Zeeman ME, Schultz N, Taylor BS et al (2011) Identification of PHLPP1 as a tumor suppressor reveals the role of feedback activation in PTEN-mutant prostate cancer progression. Cancer Cell 20:173–186
Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y et al (2011) Reciprocal feedback regulation of PI3 K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19:575–586
Guardavaccaro D, Kudo Y, Boulaire J, Barchi M, Busino L et al (2003) Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev Cell 4:799–812
Peschiaroli A, Dorrello NV, Guardavaccaro D, Venere M, Halazonetis T et al (2006) SCFbetaTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Mol Cell 23:319–329
Ougolkov A, Zhang B, Yamashita K, Bilim V, Mai M et al (2004) Associations among beta-TrCP, an E3 ubiquitin ligase receptor, beta-catenin, and NF-kappaB in colorectal cancer. J Natl Cancer Inst 96:1161–1170
Frescas D, Pagano M (2008) Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 8:438–449
Acknowledgments
We thank the anonymous reviewer for the suggestions that help to improve the work.
Conflict of interest
None of the authors has any potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ganglong Gao and Kun Tao contributed equally to this study
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, G., Kun, T., Sheng, Y. et al. SGT1 regulates Akt signaling by promoting beta-TrCP-dependent PHLPP1 degradation in gastric cancer cells. Mol Biol Rep 40, 2947–2953 (2013). https://doi.org/10.1007/s11033-012-2363-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2363-8