Abstract
This study intended to investigate the expression of the ZEB1 and E-cadherin proteins in lung squamous cell carcinoma (LSCC) tissues and to examine the clinicopathological correlation between protein levels and LSCC. RT-PCR and Western blot were used to examine the expression of ZEB1 and E-cadherin mRNAs and proteins in LSCC tissues as well as in adjacent normal tissues, and then analyze the relationship between the clinicopathological characteristics and the expression changes of ZEB1 and E-cadherin mRNAs in LSCC. In addition, RNAi was used to knockdown the expression of the ZEB1 gene in Human HCC827 cells; subsequently, changes in the invasive ability of the resultant cells were studied. The positive rates of ZEB1 and E-cadherin mRNAs in LSCC tissues were 69.2 and 38.5 %, respectively. They differed significantly from the corresponding positive rates in the adjacent normal lung tissues (15.4 and 80.8 %, p < 0.05). There was a negative correlation between the protein levels of ZEB1 and E-cadherin in LSCC tissues (r = -0.714, p < 0.001); in addition, it was found that ZEB1 protein expression in LSCC tissues was significantly higher than that in the neighboring normal lung tissues (p < 0.05), and its expression was also significantly higher in patients with lymph node metastases and distant metastases compared to those patients without metastatic disease (p < 0.05). On the contrary, E-cadherin expression was significantly lower in LSCC tissues than that in the neighboring normal tissue (p < 0.05). It was lower in patients with lymph node metastasis and distant metastasis compared to patients without metastatic disease (p < 0.05). However, the expression of ZEB1 and E-cadherin was independent of gender, age, tumor size, or tumor differentiation level (p > 0.05). Transfection of ZEB1 siRNA into HCC827 cells significantly reduced the ZEB1 protein level (p < 0.01) and significantly elevated E-cadherin levels (p < 0.01). Moreover, significantly less ZEB1 siRNA-transfected cells migrated through Transwell chambers in the LSCC tissue than that in the control groups (untransfected or transfected with control siRNA, p < 0.01). The expression of the ZEB1 gene in LSCC tissues is downregulated with the expression of E-cadherin. On the other hand, the expression of siRNA against ZEB1 promotes E-cadherin expression and suppresses the invasive ability conferred by E-cadherin. In conclusion, our data suggested that overexpression of the ZEB1 gene is possibly associated with the occurrence, development, invasion of LSCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite tremendous progress in treating lung cancer, the survival rate of affected patients remains poor [1]. The major cause lies in the complex biological nature of lung cancer [2–4]. As such, at diagnosis, most patients already have advanced stage disease that is often associated with metastasis and poor prognosis [5–7]. Importantly, metastasis is not only a sign of deterioration, but also a key barrier to the success of treatment [2]. Thus far, considerable exploration and investigation has been devoted to studying lung cancer metastasis, but its precise mechanisms remain elusive.
Zinc-finger E-box binding homeobox 1 (ZEB1), also known as TCF8 or deltaEF1, belongs to the zinc-finger family cluster. It is located on the short arm of human chromosome 10 and has an encoding nucleotide sequence of 3,327 bp, which generates a polypeptide of 1,108 amino acids harboring two zinc-finger clusters and a homeodomain. The N-terminal zinc-finger (NZF) cluster contains three CCHH zinc-fingers and one CCHC zinc-finger; the C-terminal cluster (CZF) harbors three CCHH zinc-fingers. The flanking zinc-finger clusters of ZEB1 may bind to the DNA containing the CACCT(G/C) and AGGTG (E-box) sequences to modulate transcription of target genes [8–12]. Studies have shown that ZEB1 can suppress the expression of many genes in a variety of cell lines. It negatively regulates the expression of IL-2, CD4, IgM and GATA-3 in hemopoietic cells [9, 13–16] and suppresses p73 gene expression in mesenchymal cells [17]. Recent reports have revealed that ZEB1 can additionally inhibit the expression of E-cadherin and, therefore, that it plays crucial roles in the occurrence, development, invasion and metastasis of several types of tumors [18–21].
E-cadherin is a transmembrane glycoprotein with a molecular weight of 120 kDa. As a member of the cadherin superfamily, E-cadherin is mainly present in human and animal epithelial cells, particularly around focal adhesions, and it plays an important role in maintaining the morphology and structural integrity of epithelial cells. Its major role is to mediate intercellular adhesion between the same types of cells. The human E-cadherin-encoding gene resides close to q22.1 of chromosome 16. The polypeptide, composed of 723–748 amino acids, contains a hydrophobic group located in the transmembrane region. Mediated by Ca2+, the extracellular N-termini in two neighboring cells interact with each other to facilitate the two E-cadherin molecules forming a homodimer, thereby promoting orderly cell arrangement. The cytoplasmic hydroxyl end binds to multiple cytoskeleton proteins including catenin, thus it plays a pivotal role in maintaining cell architecture and modulating cell adhesion [22]. The low protein level or deficiency of E-cadherin may contribute to loss of contact inhibition in tumor cells and, subsequently, to unlimited proliferation and de-differentiation of cells. In addition, insufficient E-cadherin expression causes cellular inability to form intact complexes with cytoplasmic catenin and prevents binding to the actin cytoskeleton, which results in detached intercellular connections. As such, the tumor cells are prone to migration from the primary site to neighboring tissues and blood/lymph vessels, leading to a high incidence of invasion and metastasis [22–25].
However, few studies have investigated the expression of the ZEB1 and E-cadherin genes in LSCC tissues or examined the relationship between the expression of the two proteins and LSCC invasion. In this study, we used RT-PCR and Western blot to examine the expression of the ZEB1 and E-cadherin genes in both LSCC tissues and adjacent normal lung tissues. In addition, RNA interference was employed to downregulate ZEB1 expression in Human HCC827 NSCLC cells to study changes in their invasive ability. The results of these experiments enabled us to elucidate the correlation between the two proteins (ZEB1 and E-cadherin) and the occurrence, development, invasion of LSCC, and they provide a scientific foundation for the prognosis and targeted therapy of lung cancer.
Materials and methods
Samples
Fresh surgical specimens from 52 LSCC patients treated in our hospital between September 2007 and 2010 were collected such that the carcinoma tissue and the neighboring normal tissue of each sample were frozen immediately in liquid nitrogen. The samples were from 32 males and 20 females who were aged 31 to 82 years (median = 57 years). No treatment was provided prior to the surgery. Subsequent pathological diagnosis revealed that all 52 cases were LSCC. The institutional review board of the author’s institution approved the protocol, and written informed consent was obtained from all patients before enrollment.
Major reagents
The RT-PCR kit was purchased from Gibco-BRL (Gaithersburg, MD, USA). ZEB1 siRNA was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The Human HCC827 NSCLC cell line was from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai. The Trizol kit, 1640 medium, trypsin, fetal bovine serum (FBS) and Lipofectamine 2000 transfection reagent were all purchased from Invitrogen (Carlsbad, CA, USA). Cell culture plates and Transwell chambers were purchased from Corning (Midland, MI, USA). The upstream and downstream primers of ZEB1 (NM_001174096.1) were 5′-GTGGCGGTAGATGGTAAT-3′ and 5′-AGTGGAGGAGGCTGAGTA-3′, respectively. The upstream and downstream primers of E-cadherin (NM_004360.3) were 5′-TCCAAAGCCTCAGGTCAT-3′ and 5′-CTCCTCCGAAGAAACAGC-3′, respectively. The upstream and downstream primers of GAPDH (NG_007073.2) were 5′-AATCCCATCACCATCTTCC-3′ and 5′-AGTCCTTCCACGATACCAA-3′, respectively. All primers were synthesized by Sangon Biotech (Shanghai, China). All primary antibodies were purchased from Abcam (Cambridge, MA, UK), including goat anti-ZEB1 polyclonal antibody, mouse anti-human E-cadherin polyclonal antibody and mouse anti-human GAPDH monoclonal antibody. The secondary antibodies, HRP-conjugated rabbit anti-goat polyclonal antibody and HRP-conjugated goat anti-mouse polyclonal antibody, were both from Abbiotec (Stockholm, Sweden). ECL chemiluminescent substrate was from Pierce (Rockford, MI, USA). The Bradford protein assay kit was from Bio-Rad (Richmond, CA, USA).
RT-PCR
Eighty milligrams of a carcinoma sample or adjacent normal tissue (over 5 cm away from the carcinoma tissue) was treated with 1 mL Trizol reagent and homogenized before being transferred into a 1.5 mL centrifuge tube. Subsequently, 0.2 mL chloroform was added into the tube, vortexed for 20 s, and allowed to stand for 3 min. The tube was then centrifuged at 12,000 rpm for 10 min to collect the aqueous phase of the supernatant, which was treated with 0.5 mL isopropanol, gently mixed and allowed to stand for 10 min. The tube was then centrifuged at 15,000 rpm for 10 min to remove the supernatant, 1 mL 75 % ethanol was added to gently wash the precipitate, and the mixture was spun down. After the supernatant was discarded, the tube was air-dried prior to being solubilized in 20 μL DEPC water (incubation at 65 °C to boost solubility). Afterwards, RT-PCR was performed such that the reaction volume of 20 μL contained 1 μg total RNA and was incubated at 37 °C for 1 h, inactivated at 95 °C for 5 min, kept on ice briefly, and then frozen at −20 °C. The primers for ZEB1, E-cadherin and GAPDH, synthesized by Invitrogen (Shanghai), were dissolved in ddH2O, and the aliquots were frozen at −20 °C. The PCR reaction was then performed such that 25 μL of the reaction mixture contained 5 μL of cDNA. The amplification cycle was the following: 94 °C for 3 min; 30 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s; and 72 °C for 10 min. The PCR products were examined using electrophoresis with 1.5 % agarose gel.
Western blot
Fifty milligrams of a sample frozen in liquid nitrogen was ground and treated with 0.5 mL RIPA lysis buffer [150 mM NaCl, 1 % NP40, 0.5 % sodium deoxycholate, 0.1 % SDS, 50 mM Tris (pH 7.9), 10 mM NaF, PMSF and 1× protease inhibitors (Complete cocktail tablets, Roche)]. The samples were then centrifuged at 16,000 g for 30 min to collect the supernatant and determine its protein concentration. Stacking gel (6 %) and resolving gel (12 %) were cast. For electrophoresis, 40 μg of total protein was loaded into each lane. The separated proteins were transferred (semi-dry) to a PVDF membrane (Amresco, Solon, OH, USA), which was blocked in TBS (10 mM Tris–HCl, pH 7.5, 150 mM NaCl) and supplemented with 5 % skimmed milk powder at room temperature for 1 h. The membrane was then probed with goat anti-ZEB1 polyclonal antibody (1:1,000), mouse anti-E-cadherin polyclonal antibody (1:1,000) and mouse anti-human GAPDH monoclonal antibody (1:1,000) and incubated at 4 °C overnight. The membrane was then probed with HRP-conjugated rabbit anti-goat IgG polyclonal antibody (1:2,000) and HRP-conjugated goat anti-mouse IgG polyclonal antibody (1:2,000) and subsequently incubated at 37 °C for 1 h before being washed with TBST and then subjected to ECL chemiluminescence autoradiography. The relative expression of ZEB1 and E-cadherin were expressed as gray-scale ratios of ZEB1/GAPDH and E-cadherin/GAPDH, respectively. The gray-scale (signal intensity of a band) was evaluated by QuantityOne software (Richmond, CA, USA).
The effect of ZEB1 siRNA transfection on the invasive ability of HCC827 cells
Sense and antisense ZEB1 siRNAs were transfected into human HCC827 NSCLC cells using the Lipofectamine 2000 liposomes. The resulting expression levels of the ZEB1 and E-cadherin genes were evaluated with Western blot to determine the effect of RNAi knockdown and the impact of ZEB1 downregulation on E-cadherin expression. A transwell invasion assay was performed to study the differences in the invasive ability of HCC827 cells without transfection and those transfected with control siRNA and with ZEB1 siRNA. Briefly, cells of each treatment (1 × 105 cells suspended in 200 μL 1640 medium supplemented with 10 % FBS) were inoculated into the upper chambers; the bottom chambers were supplemented with 600 μL of 1640 medium containing 10 % FBS. The plate was placed into an incubator for the appropriate time before the medium was discarded. The upper chambers were obtained and treated with sterile cotton swabs to remove cells on the membrane surface. The invading cells beneath the membrane were stained with 1 % crystal violet. Afterwards, cells that had migrated through the polycarbonate membrane were enumerated by studying eight randomly selected fields under a Leica microscope. The migrated cell numbers were used to assess metastatic capability and were expressed as the mean ± SD. Each treatment was repeated three times.
Statistical analyses
The Stata 7.0 software was used for statistical analyses, including χ 2 test, t test and Kendall rank correlation analysis. p < 0.05 was considered statistically significant.
Results
Expression of ZEB1 and E-cadherin mRNAs in LSCC tissues
The RT-PCR results showed that the positive rates of ZEB1 and E-cadherin mRNAs in LSCC tissues were 69.2 and 38.5 %, respectively, which differed significantly (p < 0.05) from the corresponding positive rates in the adjacent normal tissues (15.4 and 80.8 %). In addition, the positive rate of ZEB1 mRNA in the tissues undergoing lymph node metastasis or distant metastasis was significantly higher than that in the tissues without metastasis (p < 0.05). On the contrary, the positive rate of E-cadherin mRNA was significantly lower in the tissues experiencing lymph node metastasis or distant metastasis compared to that in the tissues without metastasis (p < 0.05). Nonetheless, no apparent correlation was detected between the expressions of ZEB1 mRNA and E-cadherin mRNA and the patient gender, age, tumor size or tumor differentiation level (p > 0.05), as shown in Table 1. Among the 36 samples of LSCC tissues with ZEB1 mRNA positive and five were E-cadherin mRNA positive. Correlation analysis showed that the expression of ZEB1 mRNA is negatively correlated with that of E-cadherin mRNA (r = -0.714, p = 0.000, Table 2).
Expression of ZEB1 and E-cadherin protein in LSCC tissues
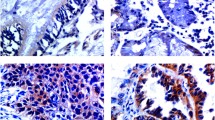
As shown in Fig. 1, the ZEB1 protein displayed a significantly higher expression in LSCC tissues than that in the adjacent normal tissues (p < 0.05); in addition, the protein level was also significantly higher in LSCC tissues from the patients who had lymph node metastases or distant metastases compared to the tissues from patients without metastases (p < 0.05). On the contrary, E-cadherin content was significantly lower in LSCC tissues than that in the neighboring normal tissues (p < 0.05); it was also significantly higher in the carcinoma tissues from the patients who had lymph node metastases or distant metastases compared to the tissues from patients without metastases (p < 0.05). The protein expression level was closely correlated with the positivity rate of mRNA among different groups (p < 0.05).
a Expression of ZEB1 and E-cadherin proteins in LSCC tissues, b changes in relative expression of ZEB1 and E-cadherin proteins in LSCC tissues (LC LSCC tissues, N adjacent normal tissue, LN positive lymph node metastasis, LN − negative lymph node metastasis, DM positive distant metastasis, DM − negative distant metastasis. *p < 0.05)
Effect of ZEB1 level on the in vitro invasive ability of human HCC827 NSCLC cells
RNAi was used to suppress the ZEB1 protein level in human HCC827 NSCLC cells, which were then examined with the Transwell invasion assay to study the subsequent change in invasive ability. The HCC827 cells were subjected to three types of treatments: no transfection, control siRNA transfection and ZEB1 siRNA transfection. Western blot showed that ZEB1 siRNA transfection significantly suppressed the ZEB1 level in the HCC827 cells (p < 0.01). Importantly, cells transfected with ZEB1 siRNA displayed a markedly lower ability to penetrate the membrane in the Transwell chambers (p < 0.01) and a significantly higher level of E-cadherin protein (p < 0.01). No apparent differences in the ZEB1 and E-cadherin levels or invasive ability of cells were detected between the two control groups (Fig. 2). These data indicated that the down-regulation of ZEB1 might be associated with the up-regulation of E-cadherin, which could inhibit the invasive ability of HCC827 cells. These data could be concluded that the negative correlation exit between the ZEB1 protein expression level and the invasive ability of HCC827 cells.
a Expression of ZEB1 and E-cadherin proteins in the individual groups of HCC827 cells, b the relative expression of ZEB1 and E-cadherin proteins in the individual groups of HCC827 cells, c the bright field images of the individual groups of HCC827 cells, d numbers of cells migrated through the Transwell chambers (A no transfection, B control transfection, C ZEB1 siRNA transfection. *p < 0.05)
Discussion
Invasion and metastasis are among the most crucial biological features of malignant tumors and are the leading causes of death among cancer patients [26]. Recently, numerous studies have been reported on the metastasis of lung cancer, although few, if any, clues on the precise mechanisms of metastasis have been elucidated. In recent years, epithelial–mesenchymal transition (EMT) has been implicated in the development of tumor invasiveness [27]. EMT refers to a phenotypic morphing of epithelial cells towards mesenchymal cells, whereby the cells acquire metastatic capacity. Such phenomena are common in the early stages of embryonic development. Recently, EMT abnormality has been closely linked to the development of epithelial tumors, including breast cancer, colon cancer, prostate cancer and lung cancer. As such, it constitutes an important mechanism of tumor invasion and metastasis [28, 29].
ZEB1/deltaEF1, Snail, Slug, SIP-1/ZEB-2 and Twist are the major transcription inhibitors of E-cadherin [30–33]. By specifically binding to its E-boxes, they are able to suppress the expression of E-cadherin. The key feature of EMT occurrence is the downregulation of E-cadherin. Eger et al. [33] reported that ZEB1 can suppress the expression of E-cadherin, which eventually initiates EMT to boost the mobility and invasiveness of epithelial cells. It has also been indicated that ZEB1 is capable of binding to the T-cadherin gene promoter and that it can inhibit its expression in gallbladder cancer cells, thereby stimulating the invasion and metastatic ability of gallbladder cancer cells [34]. Moreover, ZEB1 was also shown to be able to promote the anti-proliferative signals mediated by TGF-β [35, 36]. Our results revealed that ZEB1 is produced at a much higher level in LSCC tissues than that in normal tissues nearby (p < 0.05) and that the ZEB1 level is markedly higher in the patients had lymph node metastases or distant metastases than that in their counterparts without metastases (p < 0.05). These patterns suggested that the elevated expression of the ZEB1 gene may play a role in the occurrence, invasion of LSCC.
The downregulation of E-cadherin expression is the most crucial characteristic of EMT development. E-cadherin is a classical metastatic suppressor that belongs to the transmembrane subtype of the cadherin family. It is mainly present in epithelial cells, especially around focal adhesions, in human and animals. E-cadherin plays a key role in sustaining the architecture and structural integrity of epithelial cells, and has major activity in mediating adherence between the same types of cells [37]. The low expression or deficiency of E-cadherin may cause the loss of contact inhibition in tumor cells and, subsequently, the unlimited proliferation and dedifferentiation of cells, which causes its inability to form an intact complex with cytoplasmic catenin. Therefore, it cannot bind to the actin cytoskeleton, which results in detached intercellular connections. As such, tumor cells are highly likely to migrate from the primary site to neighboring tissues and blood/lymph vessels, leading to a high chance of invasion. Guo et al. [38] discovered that the expression of E-cadherin is markedly reduced in metastatic breast cancer tissues. Our data showed that E-cadherin expression is significantly lower in LSCC tissues than that in the adjacent normal tissues (p < 0.05) and that it is significantly lower in the tissues from patients undergoing lymph node metastasis and distant metastasis than that in the tissues from patients without metastasis (p < 0.05). These results argue that the malignant transformation of LSCC is accompanied by EMT, which enables cancer cells to acquire invasiveness.
Our results revealed a negative correlation between the expression of ZEB1 mRNA and the expression of E-cadherin mRNA in LSCC tissues, suggesting that ZEB1 might suppress the expression of the E-cadherin gene, thereby boosting the malignant phenotype of lung cancer. Putzke et al. [18] reported that ZEB1 may specifically bind to the E-boxes of E-cadherin in prostate cancer cells and downregulate the expression of E-cadherin. Such modulation enhances the invasion and metastatic ability of prostate cancer cells. To further investigate such interactions between ZEB1 and E-cadherin in LSCC tissues, this study used transfection of sense and antisense ZEB1 siRNAs into the HCC827 cells using Lipofectamine 2000 to successfully knock down the expression of the ZEB1 gene, which was followed by the increased expression of E-cadherin. This demonstrated that E-cadherin is modulated by ZEB1 in HCC827 cells. Subsequently, the Transwell chamber assay was conducted to investigate the connection between ZEB1 protein level and invasion of non-small cell lung cancer cells. The results showed that the ZEB1 siRNA-transfected HCC827 cells displayed a marked reduction in cell numbers migrating through the polycarbonate membrane and that the two control groups (no transfection and control siRNA transfection) exhibited no apparent difference in cellular invasiveness. These data might support the theory that downregulation of the ZEB1 gene could promote the expression of E-cadherin, which could reduce the invasive ability of HCC826 NSCLC cells. Hence, the ZEB1 protein level is positively correlated with the invasiveness of HCC827 cells.
In conclusion, our data indicated that the overexpression of ZEB1 and downregulation of E-cadherin are closely associated with the occurrence, invasion of LSCC. LSCC tissues are characterized by high levels of ZEB1, which further might enhance its inhibition of E-cadherin expression. Such downregulation of E-cadherin levels could promote the invasiveness of LSCC cells. As such, this study revealed key roles of ZEB1 and E-cadherin in the oncogenesis of LSCC and, therefore, provided crucial scientific evidence for gene therapy aimed at decreasing its metastatic potential.
References
Giangreco A, Groot KR, Janes SM (2007) Lung cancer and lung stem cells: strange bedfellows? Am J Respir Crit Care Med 175:547–553
Shao WL, Wang DY, He JX (2010) The role of gene expression profiling in early-stage non-small cell lung cancer. J Thorac Dis 2:89–99
Yang HH, Zhang Q, He JX, Lu WJ (2010) Regulation of calcium signaling in lung cancer. J Thorac Dis 2:52–56
Xiao DK, He JX (2010) Epithelial mesenchymal transition and lung cancer. J Thorac Dis 2:154–159
Korpanty G, Smyth E, Carney DN (2011) Update on anti-angiogenic therapy in non-small cell lung cancer: Are we making progress? J Thorac Dis 3:19–29
Shash E, Peccatori FA, Azim HA Jr (2011) Optimizing the use of epidermal growth factor receptor inhibitors in advanced non-small-lung cancer (NSCLC). J Thorac Dis 3:57–64
McKeage MJ, Jameson MB (2010) AS1404–201 study group investigators. Comparative outcomes of squamous and non-squamous non-small cell lung cancer (NSCLC) patients in phase II studies of ASA404 (DMXAA)—retrospective analysis of pooled data. J Thorac Dis 2:199–204
Yang P, Xu XY, Liu XJ, Gong JS (2011) The value of delayed 18F FDG-PET imaging in diagnosis of solitary pulmonary nodules: a preliminary study on 28 patients. Quant Imaging Med Surg 1:31–34
Stridh P, Thessen Hedreul M, Beyeen AD, Adzemovic MZ, Laaksonen H, Gillett A, Ockinger J, Marta M, Lassmann H, Becanovic K et al (2010) Fine-mapping resolves Eae23 into two QTLs and implicates ZEB1 as a candidate gene regulating experimental neuroinflammation in rat. PLoS ONE 5:e12716
Prager GW, Poettler M, Unseld M, Zielinski CC (2012) Angiogenesis in cancer: anti-VEGF escape mechanisms. Transl Lung Cancer Res 1:14–25
Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van Roy F, Berx G (2005) SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell–cell junctions. Nucleic Acids Res 33:6566–6578
Postigo AA, Depp JL, Taylor JJ, Kroll KL (2003) Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J 22:2453–2462
Hidaka T, Nakahata S, Hatakeyama K, Hamasaki M, Yamashita K, Kohno T, Arai Y, Taki T, Nishida K, Okayama A et al (2008) Down-regulation of TCF8 is involved in the leukemogenesis of adult T-cell leukemia/lymphoma. Blood 112:383–393
Postigo AA, Dean DC (1999) Independent repressor domains in ZEB regulate muscle and T-cell differentiation. Mol Cell Biol 19:7961–7971
Williams TM, Moolten D, Burlein J, Romano J, Bhaerman R, Godillot A, Mellon M, Rauscher FJ III, Kant JA (1991) Identification of a zinc finger protein that inhibits IL-2 gene expression. Science 254:1791–1794
Hsu FM, Zhang S, Chen BP (2012) Role of DNA-dependent protein kinase catalytic subunit in cancer development and treatment. Transl Cancer Res 1:22–34
Fontemaggi G, Gurtner A, Strano S, Higashi Y, Sacchi A, Piaggio G, Blandino G (2001) The transcriptional repressor ZEB regulates p73 expression at the crossroad between proliferation and differentiation. Mol Cell Biol 21:8461–8470
Putzke AP, Ventura AP, Bailey AM, Akture C, Opoku-Ansah J, Celiktas M, Hwang MS, Darling DS, Coleman IM, Nelson PS et al (2011) Metastatic progression of prostate cancer and e-cadherin regulation by zeb1 and SRC family kinases. Am J Pathol 179:400–410
Argast GM, Krueger JS, Thomson S, Sujka-Kwok I, Carey K, Silva S, O’Connor M, Mercado P, Mulford IJ, Young GD et al (2011) Inducible expression of TGFbeta, Snail and Zeb1 recapitulates EMT in vitro and in vivo in a NSCLC model. Clin Exp Metastasis 28(7):593–614
Lorenzatti G, Huang W, Pal A, Cabanillas AM, Kleer CG (2011) CCN6 (WISP3) decreases ZEB1-mediated EMT and invasion by attenuation of IGF-1 receptor signaling in breast cancer. J Cell Sci 124:1752–1758
Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, Banerjee S, Azmi AS, Miele L, Sarkar FH (2011) Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett 307:26–36
van Roy F, Berx G (2008) The cell–cell adhesion molecule E-cadherin. Cell Mol Life Sci 65:3756–3788
Rachagani S, Senapati S, Chakraborty S, Ponnusamy MP, Kumar S, Smith LM, Jain M, Batra SK (2011) Activated Kras (G12D) is associated with invasion and metastasis of pancreatic cancer cells through inhibition of E-cadherin. Br J Cancer 104:1038–1048
Erdem H, Gundogdu C, Sipal S (2011) Correlation of E-cadherin, VEGF, COX-2 expression to prognostic parameters in papillary thyroid carcinoma. Exp Mol Pathol 90(3):312–317
Saad AA, Awed NM, Abd Elkerim NN, El-Shennawy D, Alfons MA, Elserafy ME, Darwish YW, Barakat EM, Ezz-Elarab SS (2010) Prognostic significance of E-cadherin expression and peripheral blood micrometastasis in gastric carcinoma patients. Ann Surg Oncol 17:3059–3067
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN et al (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50–56
Prager GW, Poettler M, Unseld M, Zielinski CC (2012) Angiogenesis in cancer: anti-VEGF escape mechanisms. Transl Lung Cancer Res 1:14–25
Lee JM, Dedhar S, Kalluri R, Thompson EW (2006) The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172:973–981
Gemmill RM, Roche J, Potiron VA, Nasarre P, Mitas M, Coldren CD, Helfrich BA, Garrett-Mayer E, Bunn PA, Drabkin HA (2011) ZEB1-responsive genes in non-small cell lung cancer. Cancer Lett 300:66–78
Bateman G, Ricketts DN, Saunders WP (2003) Fibre-based post systems: a review. Br Dent J 195:43–48
Yu HG, Li JY, Yang YN, Luo HS, Yu JP, Meier JJ, Schrader H, Bastian A, Schmidt WE, Schmitz F (2003) Increased abundance of cyclooxygenase-2 correlates with vascular endothelial growth factor-A abundance and tumor angiogenesis in gastric cancer. Cancer Lett 195:43–51
Qian Y, Corum L, Meng Q, Blenis J, Zheng JZ, Shi X, Flynn DC, Jiang BH (2004) PI3 K induced actin filament remodeling through Akt and p70S6K1: implication of essential role in cell migration. Am J Physiol Cell Physiol 286:C153–C163
Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R (2005) DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 24:2375–2385
Adachi Y, Takeuchi T, Nagayama T, Ohtsuki Y, Furihata M (2009) Zeb1-mediated T-cadherin repression increases the invasive potential of gallbladder cancer. FEBS Lett 583:430–436
Postigo AA (2003) Opposing functions of ZEB proteins in the regulation of the TGFbeta/BMP signaling pathway. EMBO J 22:2443–2452
Nakahata S, Yamazaki S, Nakauchi H, Morishita K (2010) Downregulation of ZEB1 and overexpression of Smad7 contribute to resistance to TGF-beta1-mediated growth suppression in adult T-cell leukemia/lymphoma. Oncogene 29:4157–4169
Chung JH, Rho JK, Xu X, Lee JS, Yoon HI, Lee CT, Choi YJ, Kim HR, Kim CH, Lee JC (2011) Clinical and molecular evidences of epithelial to mesenchymal transition in acquired resistance to EGFR-TKIs. Lung Cancer 73(2):176–182
Guo BH, Feng Y, Zhang R, Xu LH, Li MZ, Kung HF, Song LB, Zeng MS (2011) Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Mol Cancer 10:10
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jiaxing Zhang and Chenhui Lu contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zhang, J., Lu, C., Zhang, J. et al. Involvement of ZEB1 and E-cadherin in the invasion of lung squamous cell carcinoma. Mol Biol Rep 40, 949–956 (2013). https://doi.org/10.1007/s11033-012-2136-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2136-4