Abstract
Bacterial biosensors can measure pollution in terms of their actual toxicity to living organisms. A recombinant bacterial biosensor has been constructed that is known to respond to toxic levels of Zn2+, Cd2+ and Hg2+. The zinc regulatory gene zntR and zntA promoter from znt operon of E. coli have been used to trigger the expression of GFP reporter protein at toxic levels of these ions. The sensor was induced with 3–800 ppm of Zn2+, 0.005–4 ppm of Cd2+ and 0.001–0.12 ppm of Hg2+ ions. Induction studies were also performed in liquid media to quantify GFP fluorescence using fluorimeter. To determine the optimum culture conditions three different incubation periods (16, 20 and 24 h) were followed. Results showed an increased and consistent fluorescence in cells incubated for 16 h. Maximum induction for Zn2+, Cd2+ and Hg2+ was observed at 20, 0.005 and 0.002 ppm, respectively. The pPROBE-zntR-zntA biosensor reported here can be employed as a primary screening technique for aquatic heavy metal pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic pollution by heavy metals is posing a major threat to both humans and ecological balance through bioaccumulation, resulting in poisoning of the food chain [1–4]. Identification and quantification of heavy metals for pollution monitoring normally requires expensive equipment and often needs substantial sample pretreatment. Also such methods cannot distinguish between biologically available and unavailable fractions. Heavy metals in the aquatic environment can only have an impact on living organisms to the extent they are bioavailable. Hence a test of bioavailability is important in bioremediation, waste dumping, waste treatment optimization, environmental impact assessment.
Employing designer microorganisms (Biosensors) for environmental monitoring is a good alternative in this regard. Biosensors refer to the organisms that have been genetically engineered to produce a measurable signal in response to a specific chemical or physical agent in their environment. When whole cell biosensors are employed for this purpose, detection of the target compound is possible in pico molar concentrations and is directly linked to its toxicity [5]. Metal responsive biosensors contain two essential genetic elements, a ‘metal inducible promoter’ and a ‘reporter gene’. In E. coli, it has been shown that the znt operon codes for proteins that are required for tolerance to Zn2+ and Cd2+ [6]. The product of zntR is a regulatory protein that binds zntA promoter in the absence of heavy metals. The product of zntA is an ATPase involved in the active efflux of Zn2+, Cd2+, Pb2+, Co2+ and Ni2+ from E. coli [7]. In the presence of metal ions the conformation of ZntR changes allowing the RNA polymerase to transcribe zntA [8, 9]. The zntA gene can be replaced by a reporter gene to design a recombinant heavy metal biosensor. Ivask et al. [10] used this strategy to develop a luminescent biosensor. However, use of luciferase gene as reporter makes the endpoint measurement complex as it requires the addition of substrate for luminescence. With the advent of new technologies like fluorescent in situ hybridization or scanning confocal laser microscopy for which bioluminescence is not compatible, the green fluorescent protein (GFP) is increasingly being used to construct whole-cell biosensors [11–17].
In the recombinant biosensor reported here zntR gene-zntA O/P-GFP gene cassette has been introduced on a plasmid vector into E. coli DH5α. Simple fluorescence measurements are well correlated to metal concentrations and can be effectively used for first level screening of aquatic broad spectrum heavy metal pollution.
Materials and methods
Construction of the biosensor plasmid
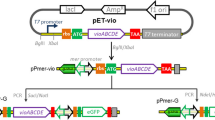
The zntA gene promoter and zntR regulatory gene of E. coli znt operon were amplified from E. coli DH5α genomic DNA and cloned upstream to the GFP gene in pPROBE-KT vector. Briefly, the zntA promoter was amplified from E. coli DH5α genomic DNA using zntA-F1 (5′-ATATCCCGGGGAGCCACTATCGCCGACGCTTCC-3′) and zntA-R1 (5′-TTAAGGTACCCATGGCATCCTCCGGTTAAGTTT-3′) primers having linkers for Sma I and Kpn I sites to facilitate directional cloning. The PCR reaction mix included 40 ng of genomic DNA, 10 pmol each of forward and reverse primers, 200 μmol each of dNTPs, 0.75 U Taq polymerase, 1.5 mM MgCl2, 1× assay buffer in a total volume of 25 μl. The thermocycler was programmed for 35 cycles of 94 °C for 45 s/58 °C for 45 s/72 °C for 45 s for denaturation, annealing and extension, with initial denaturation at 95 °C for 5 min and final extension at 72 °C for 8 min. The 193 bp product amplified was purified by extraction from 1.5 % agarose gel (QIAquick Gel extraction kit, Qiagen). The PCR product and pPROBE-GFP vector were digested sequentially with Sma I and Kpn I restriction endonucleases (NEB, UK), ligated using T4 DNA ligase and transformed into DH5α competent cells using standard protocols [18]. The recombinants were grown on LB plates containing 50 μg/ml kanamycin and the plasmid was named pPROBE-zntA.
The regulatory element zntR was amplified using primers zntR-F1 (5′-AATTGCATGCATGTATCGCATTGGTGAGCT-3′) and zntR-R1 (5′-ATATTCTAGATCAACAACCACTC TTAACGC-3′) having linkers for Sph I and Xba I sites, respectively. PCR reaction mix was prepared as discussed above. The thermocycler was programmed as discussed previously except for annealing temperature, which was kept at 60 °C. The 446 bp product amplified was purified by extraction from 1.5 % agarose gel (Gel extraction kit, Qiagen) and cloned into pPROBE-zntA plasmid using Sph I and Xba I restriction endonucleases as above. The recombinant clone is named pPROBE-zntR-zntA (Fig. 1) and the insert sequence was confirmed by sequencing.
Induction of the bacterial biosensor
The expression of GFP was induced by streaking pPROBE-zntR-zntA biosensor on LB agar-kanamycin plates containing different concentrations of inducing heavy metal ions, Zn2+ (3, 6, 20, 100, 200, 400, 600, 800 mg/l), Cd2+ (0.005, 0.01, 0.1, 0.5, 1.0, 2.0, 3.0 and 4.0 mg/l) and Hg2+ (0.001, 0.002, 0.004, 0.008, 0.016, 0.03, 0.06 and 0.12 mg/l). The heavy metal stock solutions of ZnCl2, CdCl2 and HgCl2 were prepared in double distilled water and filter sterilized. Appropriate quantities of these stock solutions were either spread on LB agar (fresh sterile plastic petri plates were used) or added to LB broth to the selective media to obtain the desired inducing ion concentration. Liquid media was prepared in specially cleaned glass test tubes. The test tubes were immersed in 0.1 % HNO3 at 50 °C for 24 h and rinsed with deionized water and dried.
Fluorescence microscopy
The expression of GFP in biosensor bacteria plated on LB-kan agar with different metal ions was observed through fluorescence microscopy. For this, the culture was allowed to grow for 18 h at 37 °C and fluorescence was recorded using the Zeiss fluorescence stereozoom microscope at 25×. For GFP detection, a bandpass GFP filter set with excitation range 450–490 nm and emission range 500–550 nm was used. The image was recorded directly on a computer using the Carl Zeiss AxioCam MRC camera and Axiovision 21 Software Version 4.3.
Fluorimetric analysis
The GFP expression at each inducing ion concentration was quantified using fluorimeter. Briefly, the expression of GFP was induced in broth culture of the biosensor by adding Zn2+ Cd2+ and Hg2+ ions separately at the above mentioned concentrations. To obtain the optimum culture conditions for biosensor assay, the GFP expression was quantified in cultures incubated for 16, 20 and 24 h separately. The cultures were incubated at 37 °C with shaking in an orbital shaker and fluorescence was quantified using the Berthold Fluorimeter, MikroWin 2000. For GFP detection, a GFP filter set with excitation range 480 nm and emission range 530 nm was used. The cell density was measured using a spectrophotometer and normalized across the samples. The GFP fluorescence was quantified directly using Mithras Software 2000.
Results and discussion
In the present study, the Zn2+ responsive elements, zntR [9] and zntA [8] were used as receptor elements controlling the expression of GFP reporter gene for engineering a bacterial biosensor responsive to toxic levels of Zn2+ and Cd2+. This receptor system has been used earlier by Ivask et al. [10] to develop a bacterial biosensor, but with luciferase gene as the reporter element. In this study, GFP protein has been used as reporter owing to its high stability, easy scoring and lack of need to add any substrate compared to luminescence-based system [19]. GFP based whole cell biosensors have been developed for arsenic [11], cadmium [13, 14], mercury [15], lead [16] and uranium [17]. However, most of these biosensors developed so far are specific to a single heavy metal. Since, the aquatic environment often is contaminated with more than one heavy metal, it is imperative to have a broad spectrum bacterial biosensor that can detect the presence of more than one heavy metal simultaneously. This will drastically decrease the number of biosensors to be employed for the screening of a water body for the presence of heavy metal pollutants.
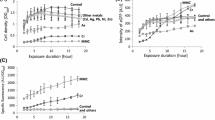
Here, we report the development of a recombinant broad spectrum-based bacterial biosensor, pPROBE-zntR-zntA. This biosensor construct was tested for inducible expression of GFP on LB-kan media containing 3, 6, 20, 100, 200, 400, 600 or 800 ppm (mg/l) of Zn2+ ion, 0.005, 0.01, 0.1, 0.5, 1.0, 2.0, 3.0 or 4.0 ppm of Cd2+ ion and 0.001, 0.002, 0.004, 0.008, 0.016, 0.03, 0.06 and 0.12 ppm of Hg2+ ion while the media without any inducer ion served as control. GFP fluorescence was seen in cells induced with Zn2+, Cd2+ or Hg2+ ions while there was no GFP expression in controls. The induction studies were also performed in liquid media in order to quantify the GFP fluorescence using a fluorimeter. To determine the optimum culture conditions for better performance, the biosensor was subjected to three different incubation periods viz, 16, 20 and 24 h. Results revealed that an incubation period of 16 h gave increased and consistent fluorescence compared to 20 and 24 h. The reduction in fluorescence beyond 16 h of incubation period could be attributed to the half life of GFP protein and cell death due to senescence [20]. In cells exposed to Zn2+, an incubation period of 24 h resulted in a sudden drop in fluorescence beyond 200 ppm for unknown reason. The maximum fluorescence was recorded at 20, 0.005 and 0.002 ppm for Zn2+, Cd2+ and Hg2+ ions, respectively (Figs. 2, 3, 4). However, Ivask et al. [10] reported the maximum induction at 650 ppm in case of zinc while it is 0.1 and 0.2 ppm in case of cadmium and mercury, respectively. This shows that the use of GFP as reporter protein increased the sensitivity of the bacterial biosensor. To test the effect of inducing metal ions on the growth of sensor bacteria, a growth curve analysis was performed by exposing bacteria to different concentrations of Zn2+, Cd2+ and Hg2+ ions. Results showed a decline in cell density beyond 200, 0.5 and 0.016 ppm of Zn2+, Cd2+ and Hg2+ ions, respectively (Fig. 5).
The co-inducibility of the biosensor with Zn2+, Cd2+ and Hg2+ ions is in agreement with earlier studies of Binet and Poole [21] and Ivask et al. [10] who reported the induction of the znt resistance system (zntR and promoter of zntA) with Zn2+ Cd2+ and Hg2+ions. It has been previously observed that ZntA protein of znt operon exports, in addition to Zn2+, also other di-valent ions such as Cd2+, Pb2+, Co2+ and Ni2+ from the cell [7, 22]. However, it is noteworthy that the concentrations of Hg2+, Cd2+ and Zn2+ needed for the induction of the zntR-zntA based sensor differ remarkably, the amount of Hg2+ being 700 and the amount of Cd2+ being 450 times lower than the respective concentration for Zn2+. Most importantly, the concentrations of mercury, cadmium and zinc inducing the biosensor are in good correlation with the toxicities of these metals. For example, for crustacean Daphnia magna the 24 h LC50 values for Hg2+ (0.01 mg/l) and for Cd2+ (0.64 mg/l; 24) are 760 and 12 times lower than for Zn2+ (7.6 mg/l; 25). The correlation of the sensitivities with toxicities of the metals is quite expected as the ‘sensing’ elements of the current biosensors originate from living organisms.
It can be concluded that the bacterial biosensor, pPROBE-zntR-zntA offers a simple method to assess toxic concentrations of Zn2+, Cd2+ and Hg2+. This is especially because sub-lethal levels of these heavy metals can be accurately predicted by measuring fluorescence of liquid cultures using a fluorescence reader. This broad spectrum-based biosensor can be employed for primary screening of naturally polluted water once the methodology is standardized. Therefore, the future challenge lies in using such biosensors to develop portable, inexpensive, single-use tools for monitoring environmental toxicity.
References
Mukhopadhyay MK, Konar SK (1985) Effects of copper, zinc and iron mixture on fish and aquatic ecosystem. Environ Ecol 3:58–64
Kumar A, Mathur RP (1991) Bioaccumulation kinetics and organ distribution of lead in a fresh water teleost, Colisa fasciatus. Environ Technol 12:731–735
Mohan D, Choudhary A (1991) Zinc accumulation in a few tissues of fish, Puntius sophore (Ham.) after sublethal exposure. J Nat Conserv 3:205–208
Wepener V, van Vuren JHJ, du Preez HH (2001) Uptake and distribution of copper, iron and zinc mixture in gill, liver and plasma of a freshwater teleost, Tilapia sparrmanii. Water SA 27:99–108
Tecon R, Meer JR (2008) Bacterial biosensors for measuring availability of environmental pollutants. Sensors 8:4062–4080
Rensing C, Mitra B, Rosen BP (1997) The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc Natl Acad Sci USA 94:14326–14331
Beard SJ, Hashim R, Membrillo-Hernandez J, Hughes MN, Poole RK (1997) Zinc(II) tolerance in Escherichia coli K-12: evidence that the zntA gene (o732) encodes a cation transport ATPase. Mol Microbiol 25:883–891
Blencowe DK, Samantha JM, Morby AP (1997) Preliminary characterization of zntA, a gene which encodes a Zn (II)/Cd (II)-export protein in Escherichia coli. Biotechnol et alia 2:1–6
Outten CE, Outten FW, O’Halloran TV (1999) DNA distortion mechanism for transcriptional activation by ZntR, a Zn (II)-responsive MerR homologue in Escherichia coli. J Biol Chem 274:37517–37524
Ivask A, Virta M, Karhu A (2002) Construction and use of specific luminescent recombinant bacterial sensors for the assessment of bioavailable fraction of cadmium, zinc, mercury and chromium in the soil. Soil Biol Biochem 34:1439–1447
Liao VHC, Ou KL (2005) Development and testing of a green fluorescent protein–based bacterial biosensor for measuring bioavailable arsenic in contaminated groundwater samples. Environ Toxicol Chem 24:1624–1631
Siddiki MSR, Kawakami Y, Ueda S, Maeda I (2011) Solid phase biosensors for arsenic or cadmium composed of a trans factor and cis element complex. Sensors 11:10063–10073
Wu CH (2009) Optimization of a whole-cell cadmium sensor with a toggle gene circuit. Biotechnol Prog 25:898–903
Raja CE, Selvam GS (2011) Construction of green fluorescent protein based bacterial biosensor for heavy metal remediation. Int J Environ Sci Technol 8:793–798
Priyadarshi H, Alam A, Gireesh-Babu P, Das R, Kishore P, Kumar S, Chaudhari A (2012) A GFP-based bacterial biosensor with chromosomally integrated sensing cassette for quantitative detection of Hg(II) in environment. J Environ Sci 24:963–968
Chakraborty T, Gireesh-Babu P, Alam A, Chaudhari A (2008) GFP expressing bacterial biosensor to measure lead contamination in aquatic environment. Curr Sci 94:800–805
Hillson NJ, Hu P, Andersen GL, Shapiro L (2007) Caulobacter crescentus as a whole-cell uranium biosensor. Appl Environ Microbiol 73:7615–7621
Sambrook J, Russell DW, Janssen KA, Irwin N (2001) Molecular cloning: a laboratory manual. Cold Spring harbor Laboratory, Cold Spring Harbor
Li YF, Li FY, Ho CL, Liao VHC (2008) Construction and comparison of fluorescence and bioluminescence bacterial biosensors for the detection of bioavailable toluene and related compounds. Environ Pollut 152:123–129
Shaner NC, Steinbach PA, Tsien RY (2005) A guide to choosing fluorescent proteins. Nat Methods 2:905–909
Binet MR, Poole RK (2000) Cd(II), Pb(II) and Zn(II) ions regulate expression of the metal-transporting P-type ATPase ZntA in Escherichia coli. FEBS Lett 473:67–70
Gatti D, Mitra B, Rosen BP (2000) Escherichia coli soft metal ion translocating ATPases. J Biol Chem 275:34009–34012
Acknowledgments
The authors would like to acknowledge Dr. W. S. Lakra, Director, CIFE and Dr. Dilip Kumar, Former Director, CIFE for providing necessary facilities, ICAR for JRF fellowship to the first author, Dr. Kshitish Majumdar and Dr. S. Ayyappan for their valuable help, advice and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gireesh-Babu, P., Chaudhari, A. Development of a broad-spectrum fluorescent heavy metal bacterial biosensor. Mol Biol Rep 39, 11225–11229 (2012). https://doi.org/10.1007/s11033-012-2033-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2033-x