Abstract
In this study, we conducted a genome-wide linkage analysis to identify the quantitative trait loci (QTL) that influence back fat thickness and carcass pH in an F2 intercross between Landrace and Korean native pigs. Eight phenotypes related with back fat thickness and carcass pH were measured in more than 960 F2 progeny. All experimental animals were subjected to genotypic analysis using 173 microsatellite markers located throughout the pig genome. The GridQTL program, based on the least squares regression model, was used to perform the QTL analysis. We identified 22 genome-wide significant QTL in 9 chromosomal regions (SSC1, 2, 5, 6, 7, 8, 12, 15, and 16) and 29 suggestive QTL in 16 chromosomal regions (SSC2, 3, 4, 5, 6, 7, 8, 10, 11, 12, 14, 15, 16, 17, 18, and X). On SSC5, we detected a QTL affecting back fat thickness that accounted for 4.8 % of the phenotypic variance, which was the highest test statistic (F-ratio = 50.3 under the additive model, nominal P value = 2.5 × 10−12) observed in this study. Additionally, we showed that there were significant QTL on SSC16 affecting carcass pH traits. In conclusion, the QTL identified in this study together with associated positional candidate genes could play an important role in determining the genetic structure underlying the variation of back fat thickness and carcass pH in pigs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Back fat thickness and carcass pH are important economic traits in the pork industry. Back fat thickness is one of the major determinants of the carcass lean meat yield. Decreased back fat thickness tends to be more profitable due to an increased carcass lean meat yield [1]. Carcass pH is a commonly used trait for evaluating pork quality as it is correlated with meat quality traits, such as meat color, water-holding capacity, drip loss, and sensory traits (e.g., juiciness and tenderness). Low pH affects the denaturation of muscle protein and the water-holding capacity. Hence, pork with lower pH values tends to be less desirable. Although a lot of numbers of studies that identified QTLs affecting back fat thickness and pH traits have been performed, the causal mutations for these traits are still unknown [2].
There are two types of Korean native pigs: the native pig raised on the Korean Peninsula and the Jeju native pig raised on Jeju Island. Due to being raised on an island that has been isolated for more than 1,000 years, the Jeju native pig (hereafter, the Jeju native pig is referred to as KNP) has unique genetic properties that are different from those of the pigs raised on the Korea Peninsula [3]. The coat color of KNP is black, and its feed efficiency and growth rate are low, as with most native breeds. However, it has excellent meat quality characteristics, such as solid fat structure, white colored fat, red meat color [4]. Nevertheless, studies regarding what genetic factors affect back fat thickness and meat quality traits, such as carcass pH, of KNP have not yet been performed in detail. Additionally, as in most cases of quantitative traits, back fat thickness and carcass pH are determined by a number of genetic factors; thus, it is difficult to explain phenotypic variance based only on a few known genes.
In this study, using an F2 intercross between KNP and Landrace pigs, we identified QTL that influence back fat thickness and carcass pH of the longissimus dorsi muscle (LDM).
Materials and methods
Animals and genotypic analysis
A three-generation resource population was generated and managed as described by Cho et al. [5]. Briefly, 19 purebred KNP (8 males and 11 females) were crossed with 17 purebred Landrace (8 males and 9 females). From these crosses, 91 F1 progeny and 1,106 F2 progeny (568 males and 538 females) from 79 full-sib families were produced. None of the F2 males were castrated.
A total of 173 informative microsatellite markers, covering the autosomes and X chromosome, were PCR-amplified in 1,233 pigs as described by Cho et al. [5]. Map order and genetic distance were determined using the build option in the CRIMAP software version 2.4 [6]. The total map length was 2348.8 centimorgans (cM). The sex-average autosomal linkage map was used for further QTL analysis, except for the analysis of the X chromosome.
Phenotypic analysis
F2 pigs were slaughtered in the same commercial slaughterhouse. Prior to slaughter, pigs were fasted for 24 h but with free access to water. These pigs were slaughtered based on age, i.e., the average age at the time of slaughter was 199 days. To measure muscle pH traits, the measurements were conducted during the first 24 h of refrigeration process; sampling of the LDM was performed 1 h (1HPH), 3 h (3HPH), 6 h (6HPH) and 24 h (24HPH) postmortem.
We measured back fat thickness between the 4 and 5th ribs (45RIBBFT), the 11 and 12th ribs (1112RIBBFT), and the last rib and first lumbar vertebrae (THOLUMBFT). In addition, back fat thickness (BFT) was measured according to the rules of the Korea Institute for Animal Products Quality Evaluation (KAPE): BFT = [(back fat thickness between the 11 and 12th thoracic vertebrae) + (back fat thickness between the last thoracic and first lumbar vertebrae)]/2. However, the BFT value was measured within a short time during the process of meat product quality evaluation. For this reason, the BFT value has less accuracy compared to other three back fat thickness values. Measurements of the back fat thickness traits were taken on the left half of the carcass of each animal. The thickness of fat was measured in millimeters.
Statistical and QTL analyses
Before QTL analysis, we obtained descriptive statistics and verified the normal distribution of the phenotypic data. When putative outliers were observed, we omitted them based on the ascertainment of normality using the MINITAB program (Minitab Inc., USA). When necessary, the phenotypic values were transformed by natural logarithm. Pearson correlation coefficients and the significance of each pairwise comparison of traits were also calculated using the MINITAB program.
QTL analysis for each trait was performed using the web-based program GridQTL (http://www.gridqtl.org.uk). The interval mapping model based on the least squares regression method [7] was used for QTL analysis, which included the cofactors of sex, parity, and carcass weight, along with additive and dominance regression variables for the putative QTL. Identification of QTL was based on an F-ratio test statistic that was calculated from sums of squares explained by the additive and dominance regression coefficients for the QTL. The F-ratios were calculated at 1 cM intervals through the genome. At the QTL peak, we extracted the additive and dominance coefficients of each of the F2 progeny to evaluate the significance of each additive and dominance effect using the MINITAB program. From this, we selected the final model for QTL analysis. Both the additive and the dominance regression coefficients were included in the QTL model if the effect of the dominance regression coefficient was significant, regardless of the significance level of the additive coefficient. Only the additive regression coefficient was included in the QTL model if the effect of the dominance regression coefficient was not significant. To perform QTL analysis with the X-specific linkage group, the female specific linkage map was used. Genome-wide empirical significance thresholds of the test statistic (i.e., F-ratio) were obtained by 1,000 permutations of data [8]. Genome-wide thresholds for highly significant (α = 0.01) and significant linkage (α = 0.05) were employed. Suggestive linkage was employed using a 5 % chromosome-wide threshold. The 1.5-LOD (logarithm of odds) drop method was used to estimate support intervals for identified QTL at the suggestive and significant levels of linkage [9].
Results and discussion
Eight traits related to back fat thickness and carcass pH were used to perform the genome-wide linkage analysis to map the QTL. Table 1 shows the descriptive statistics with respect to the measured traits. We identified 22 significant QTL and 29 suggestive QTL (Table 2). In all of the identified QTL affecting back fat thickness, the KNP allele was always associated with higher back fat thickness, whereas the allele from the Landrace was associated with lower phenotypic values. These results are consistent with the observed reduction of back fat thickness in Western breeds through selective breeding. As for carcass pH, Park et al. [4] reported that the pH value of the KNP was higher than that of the Landrace. At the QTL on SSC 2, 6, 10, 11, 14, 16, and 17, the effect of the KNP allele caused an increase in carcass pH. However, the KNP allele also resulted in a decrease in carcass pH at the QTL on SSC3, 5, 7, 18, and the X chromosome. A statistical analysis of the phenotypic data from the F2 population revealed that a number of the traits were significantly correlated (Supplementary Table 1). For example, 1112RIBBFT was strongly correlated with THOLUMBFT (r = 0.90). Positive and significant correlations were also observed among pH traits (r = 0.65 or higher), indicating the presence of pleiotropic loci, which may influence multiple phenotypes (e.g., QTL on SSC16 affecting 1HPH, 6HPH and 24HPH).
Back fat thickness
Back fat thickness serves as one of the most important criteria for grading carcass quality. We found significant and suggestive QTL affecting back fat thickness-related traits (BFT, 45RIBBFT, 1112RIBBFT, and THOLUMBFT) in eleven genomic regions (SSC1, 2, 4, 5, 6, 7, 8, 12, 14, 15 and X).
On SSC1, we detected several significant QTL for the four back fat thickness-related traits (100–109 cM). These QTL explained up to 2.7 % of the phenotypic variance. Furthermore, these QTL regions overlapped with the QTL regions reported in previous studies [10–13]. In the QTL region for 45RIBBFT on SSC1 (109 cM), there is a gene encoding the melanocortin receptor type 4 (MC4R), which is known to be closely related to fatness and growth [14]. In addition, studies on the association of the p.Asp298Asn mutation in the MC4R gene with fatness and growth rate were reported for various populations [15, 16]. However, there are also contradictory reports indicating the lack of an association between this mutation and fatness traits [17, 18].
On SSC2, two highly significant QTL for BFT (78 cM) and 45RIBBFT (26 cM) were detected, and they explained up to 1.9 % of the phenotypic variance. Milan et al. [19] reported a QTL affecting back fat thickness between the 3rd and 4th ribs on SSC2 in an F2 intercross between Meishan and Large White pigs. This QTL overlapped with our QTL region for 45RIBBFT. To our knowledge, the QTL on SSC2 for BFT (78 cM), 1112RIBBFT (81 cM), and THOLUMBFT (82 cM) are novel. The lipid storage droplet protein 5 gene (LSDP5; also known as perilipin 5, PLIN5) is located in this novel QTL region. PLIN5 is member of the perilipin family; this family of proteins coat intracellular lipid storage droplets and protect them from lipolytic degradation [20]. Wang et al. [21] reported that PLIN5 has a negative regulatory role in lipid droplet hydrolysis by binding and inhibiting adipose triglyceride lipase activity at the lipid droplet surface under basal conditions. PLIN5 also plays a critical role in oxidative tissues (e.g., heart and skeletal muscle) by protecting mitochondria from a rapid increase of fatty acid during lipolysis. A previous study reported that the IGF2 gene on SSC2 was associated with back fat thickness [22]. However, the genetic map of our study does not include the IGF2 locus. Thus, further studies using the map with the IGF2 locus are necessary to evaluate the effects of the IGF2 gene on back fat traits.
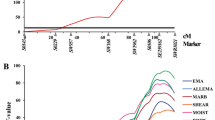
On SSC5, we identified highly significant QTL affecting the four BFT-related traits (55–60 cM). The test statistic for BFT was 50.3 (nominal P value = 2.5 × 10−12) with only an additive effect, which was the highest F-ratio observed in this study (Fig. 1a). These QTL explained up to 4.8 % of the phenotypic variance. These QTL regions overlapped with the QTL regions identified by Kim et al. [23] and Guo et al. [24].
QTL profiles for backfat thickness and carcass pH traits. The y-axis represents the F-value testing the hypothesis of a single QTL in a given position on the chromosome. Marker map with genetic distance between microsatellite markers in Kosambi cM is given on the x-axis. The thick horizontal line indicate the 1 % genome-wise significant threshold and thin horizontal line indicate the 5 % genome-wise significant threshold. a Test statistic curves for the BFT QTL on SSC5 b Test statistic curves for meat pH QTL on SSC16. Trait abbreviations are given in Table 1
On SSC6, significant QTL for 45RIBBFT (41 cM), 1112RIBBFT (41 cM) and THOLUMBFT (40 cM) were identified. These QTL explained up to 2.1 % of phenotypic variance and showed only an additive effect. They also overlapped with the QTL region reported by Fontanesi et al. [25]. The FTO obesity-associated gene has been considered a strong positional candidate gene for fatness in this region. FTO was reported to have a strong association with BMI and other obesity-related traits in humans [26]. Association of the FTO gene with intramuscular fat (IMF) deposition, feed conversion rate, BFT and marbling score have been reported in pigs [12, 25].
On SSC7, we identified significant QTL affecting BFT (64 cM) and 1112RIBBFT (62 cM). These QTL showed only an additive effect and accounted for up to 1.7 % of the phenotypic variance. Our QTL regions overlapped with the regions identified by Guo et al. [24] and Chen et al. [27]. Interestingly, Chen et al. [27] highlighted SLC39A7 as a candidate gene in one of these QTL regions. According to that study, the c.205G > A polymorphism of SLC39A7 was closely linked to SW1856 in a F2 population from a Large White and Meishan cross, and it was reported to be significantly associated with fatness.
On SSC8, highly significant QTL for BFT (41 cM) and THOLUMBFT (41 cM) were identified. These QTL explained up to 2.0 % of phenotypic variance. These particular QTL regions overlapped with the regions identified by De Koning et al. [28] and Vidal et al. [29].
On SSC12, we detected a significant QTL affecting 45RIBBFT (106 cM), and this QTL accounted for 2.1 % of the phenotypic variance. This QTL region overlapped with the region reported by Thomsen et al. [10].
Significant additive QTL for BFT (123 cM) and 45RIBBFT (117 cM) were identified on SSC15. These QTL explained up to 1.5 % of the phenotypic variance. These QTL regions also overlapped with the QTL regions identified by Thomsen et al. [10].
Meat pH
The pH of meat can impact meat color, microbial growth, and water-holding capacity (WHC). WHC, which indicates the ability of meat to retain water following physical treatment, is closely associated with meat texture and moisture percentage. We analyzed the QTL for the pH of the LDM at different time points and identified significant and suggestive QTL affecting carcass pH-related traits (1HPH, 3HPH, 6HPH, and 24HPH) in twelve chromosomal regions (SSC2, 3, 5, 6, 7, 10, 11, 14, 16, 17, 18 and X). On SSC16, significant QTL for 6HPH (67 cM) and 24HPH (71 cM) were detected. These QTL accounted for up to 1.5 % of the phenotypic variance. The significant QTL affecting carcass pH found in this study overlapped with the suggestive QTL affecting meat pH identified by Duan et al. [30].
In this study, we performed a genome-wide QTL analysis for back fat thickness and meat quality traits using an F2 intercross between KNP and Landrace pigs. Many significant QTL regions were identified for back fat thickness, but only one significant QTL was found for pH. These results indicate that the phenotypic differences between the parental lines may not be significant enough for pH-related traits. Another possible explanation for different number of significant QTL for back fat thickness and pH is that there could be different number of genetic factors with large or small effects on the two traits.
The results from this study not only detected a novel chromosomal region (i.e., QTL on SSC2 for BFT, 1112RIBBFT, THOLUMBFT) but also validated previously reported QTL (e.g., QTL on SSC5 for BFT; QTL on SSC16 for 6HPH) that were significantly associated with back fat thickness and carcass pH in pigs. The results presented herein could play an important role in investigating the genetic structure of phenotypic variation of back fat thickness and meat pH.
References
Cliplef RL, Mckay RM (1993) Carcass quality characteristics of swine selected for reduced backfat thickness and increased growth rate. Can J Anim Sci 73:483–494
Hu Z, Park C, Reecy JM (2012). Animal QTLdb Extension (V): addition of new data types and functions. In: Plant & animal genomes XX conference, Town & Country Convention Center, San Diego, 14–19 January 2012
Cho IC, Han SH, Fang M, Lee SS, Ko MS, Lee H, Lim HT, Yoo CK, Lee JH, Jeon JT (2009) The robust phylogeny of Korean wild boar (Sus scrofa coreanus) using partial D-loop sequence of mtDNA. Mol Cells 28:423–430
Park JC, Kim YH, Jung HJ, Park BY, Lee JI, Moon HK (2005) Comparison of meat quality and physicochemical characteristics of pork between Korean native black pigs (KNBP) and Landrace by market weight. J Anim Sci Technol (Kor) 47:91–98
Cho IC, Park HB, Yoo CK, Lee GJ, Lim HT, Lee JB, Jung EJ, Ko MS, Lee JH, Jeon JT (2011) QTL analysis of white blood cell, platelet and red blood cell-related traits in an F2 intercross between Landrace and Korean native pigs. Anim Genet 42:621–626
Green P, Falls K, Crooks S (1990) Documentation for CRIMAP version 2.4. Washington University School of Medicine, St. Louis
Haley CS, Knott SA, Elsen JM (1994) Mapping quantitative trait loci in crosses between outbred lines using least squares. Genetics 136:1195–1207
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Sen S, Churchill GA (2001) A statistical framework for quantitative trait mapping. Genetics 159:371–387
Thomsen H, Lee HK, Rothschild MF, Malek M, Dekkers JCM (2004) Characterization of quantitative trait loci for growth and meat quality in a cross between commercial breeds of swine. J Anim Sci 82:2213–2228
Karlskov-Mortensen P, Bruun CS, Braunschweig MH, Sawera M, Markljung E, Enfält AC, Hedebro-Velander I, Josell Å, Lindahl G, Lundström K, Von Seth G, Jørgensen CB, Andersson L, Fredholm M (2006) Genome-wide identification of quantitative trait loci in a cross between Hampshire and Landrace I: carcass traits. Anim Genet 37:156–162
Fan B, Du ZQ, Rothschild MF (2009) The fat mass and obesity-associated (FTO) gene is associated with intramuscular fat content and growth rate in the pig. Anim Biotechnol 20:58–70
Geldermann H, Cepica S, Stratil A, Bartenschlager H, Preuss S (2010) Genome-wide mapping of quantitative trait loci for fatness, fat cell characteristics and fat metabolism in three porcine F2 crosses. Genet Sel Evol 42:31
Kim KS, Larsen N, Short T, Plastow G, Rothschild MF (2000) A missense variant of the porcine melanocortin-4 receptor (MC4R) gene is associated with fatness, growth, and feed intake traits. Mamm Genome 11:131–135
Óvilo C, Fernández A, Rodríguez MC, Nieto M, Silió L (2006) Association of MC4R gene variants with growth, fatness, carcass composition and meat and fat quality traits in heavy pigs. Meat Sci 73:42–47
Fan B, Onteru SK, Plastow GS, Rothschild MF (2009) Detailed characterization of the porcine MC4R gene in relation to fatness and growth. Anim Genet 40:401–409
Park HB, Carlborg Ö, Marklund S, Andersson L (2002) Melanocortin-4 receptor (MC4R) genotypes have no major effect on fatness in a large white x wild boar intercross. Anim Genet 33:155–157
Stachowiak M, Szydlowski M, Obarzanek-Fojt M, Switonski M (2006) An effect of a missense mutation in the porcine melanocortin-4 receptor (MC4R) gene on production traits in Polish pig breeds is doubtful. Anim Genet 37:55–57
Milan D, Bidanel JP, Iannuccelli N, Riquet J, Amigues Y, Gruand J, Le Roy P, Renard C, Chevalet C (2002) Detection of quantitative trait loci for carcass composition traits in pigs. Genet Sel Evol 34:705–728
Dalen KT, Dahl T, Holter E, Arntsen B, Londos C, Sztalryd C, Nebb HI (2007) LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta 1771:210–227
Wang H, Bell M, Sreenevasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, Coleman R, Gong D, Brasaemle D, Sztalryd C (2011) Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem 286:15707–15715
Kim JJ, Rothschild MF, Beever J, Rodriguez-Zas S, Dekkers JC (2005) Joint analysis of two breed cross populations in pigs to improve detection and characterization of quantitative trait loci. J Anim Sci 83:1229–1240
Kim CW, Hong YH, Yun SI, Lee SR, Kim YH, Kim MS, Chung KH, Jung WY, Kwon EJ, Hwang SS, Park DH, Cho KK, Lee JG, Kim BW, Kim JW, Kang YS, Yeo JS, Chang KT (2006) Use of microsatellite markers to detect quantitative trait loci in Yorkshire pigs. J Reprod Dev 52:229–237
Guo Y, Lee GJ, Archibald AL, Haley CS (2008) Quantitative trait loci for production traits in pigs: a combined analysis of two Meishan x large white populations. Anim Genet 39:486–495
Fontanesi L, Scotti E, Buttazzoni L, Davoli R, Russo V (2009) The porcine fat mass and obesity associated (FTO) gene is associated with fat deposition in Italian Duroc pigs. Anim Genet 40:90–93
Scuteri A, Sanna S, Chen W, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orrú M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR (2007) Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 3:1200–1210
Chen ZG, Ma ZX, Zuo B, Lei MG, Xiong YZ (2009) Molecular characterization and association with carcass traits of the porcine SLC39A7 gene. J Anim Breed Genet 126:288–295
De Koning DJ, Pong-Wong R, Varona L, Evans GJ, Giuffra E, Sanchez A, Plastow G, Noguera JL, Andersson L, Haley CS (2003) Full pedigree quantitative trait locus analysis in commercial pigs using variance components. J Anim Sci 81:2155–2163
Vidal O, Noguera JL, Amills M, Varona L, Gil M, Jiménez N, Dávalos G, Folch JM, Sánchez A (2005) Identification of carcass and meat quality quantitative trait loci in a Landrace pig population selected for growth and leanness. J Anim Sci 83:293–300
Duan YY, Ma JW, Yuan F, Huang LB, Yang KX, Xie JP, Wu GZ, Huang LS (2009) Genome-wide identification of quantitative trait loci for pork temperature, pH decline, and glycolytic potential in a large-scale white Duroc × Chinese Erhualian resource population. J Anim Sci 87:9–16
Acknowledgments
We thank two anonymous reviewers for their valuable suggestions and comments on this article. This work was supported by a grant from the Next-Generation BioGreen 21 Program (No.PJ008016), Rural Development Administration, Republic of Korea. C. K. Yoo was supported by a scholarship from the Brain Korea 21 Program, the Ministry of Education, Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoo, CK., Lim, HT., Han, SH. et al. QTL analysis of back fat thickness and carcass pH in an F2 intercross between Landrace and Korean native pigs. Mol Biol Rep 39, 8327–8333 (2012). https://doi.org/10.1007/s11033-012-1682-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1682-0