Abstract
A MADS box gene AGL20/SOC1 is a main integrator in Arabidopsis flowering pathway whose structure and function are highly conserved in many plant species. A soybean MADS box gene GmGAL1 (G lycine max A GAMOUS L ike 1) as a homolog of AGL20/SOC1, was cloned from soybean cultivar Kennong18 and its function was investigated in transgenic Arabidopsis lines. Sequence comparisons showed that the closest homolog gene to GmGAL1 is AGL20/SOC1 in Arabidopsis and VuSOC1 in Vigna unguiculata. We investigated the expression level of GmGAL1 using quantitative real-time PCR, and the result showed that GmGAL1 was regulated by a circadian mechanism and its expression oscillated at a cycle of 24 h. The expression level of GmGAL1 was fluctuated in diverse tissues/organs and developmental stages. Considering its expression can be detected in different tissues throughout the life cycle and its protein localized in cytoplasm in Arabidopsis protoplasm, we proposed that GmGAL1 may be a multifunctional gene in the context of the soybean development. Ectopic expression of GmGAL1 in Arabidopsis enhanced flowering under long-day condition and partially rescued soc1 late flowering type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MADS-box genes belong to a large multifunctional family which controls the identity of the apex meristem, development of lateral organs, and flowering time of plants [1–8]. In Arabidopsis, AGAMOUS-like (AGL) genes belong to a sub-family of MADS-box genes which play important roles especially in regulating flowering pathways. For example, FLOWERING LOCUS C (FLC, AGL25) acts as a repressor of flowering [9–12]. APETALA1 (AP1, AGL7) promotes flowering and controls the identity of meristem and sepals [13, 14] and AGL12 regulates both flowering time and root development [15].
Another AGL gene, SUPPRESSOR OF OVEREXPRESSION OF CONSTANS (SOC1), is a critical integrator of different flowering pathways which promotes flowering in Arabidopsis [16–20]. Its expression was detected in several organs include leaves, root, steam apex, etc. [19, 21]. In development stages, mRNA abundance of SOC1 increases after seed germination and further accelerates in flower meristem during flower organs transition [19, 21]. Ectopic expression of the SOC1 gene is sufficient to induce the early flowering in both long-day and short-day conditions [22], while lose-of-function of SOC1 results in late flowering. Although soc1 mutant show obviously late flowering time phenotype, it’s flower organs develop almost normally because it is functionally redundant with AGL24 and SHORT VEGETATIVE PHASE (SVP) in the control of flower organ identity [21, 23].
SOC1 genes are widely expressed in various tissues. Another SOC1 like gene ETL (Eucalyptus TM3 Like, Eucalyptus globules ssp. bicostata), is also expressed in both vegetative and reproductive organs, including shoot meristems, roots, and floral organ primordia [24]. In monocotyledons, genes similar to SOC1/TM3 are also broadly expressed and regulate floral transition or floral development, such as OsSOC1 (Oryza sativa) [17, 25], ZmMADS1 (Zea may) [26] and TrcMADS1 (Trillium camtschatcense) [27].
Although SOC1 genes are highly conserved and have similar expression patterns among dicots and monocots, their functions can be divergent in Angiosperms [22]. Recent studies have uncovered some interesting functions of SOC1s. For example, the constitutive expression of UNS (UNSHAVEN), an ortholog gene of SOC1 in Petunia hybrid, results in ectopic trichome formation on floral organs and the reversion of petals into organs with leaf-like features, besides early flowering phenotype [28]. Therefore, further research is required to uncover the functional divergence of SOC1s among plant species.
The time of flowering significantly affects the yield of soybean. As a short-day plant, flowering of soybean is sensitive to day length which makes soybean an important model plant for photoperiod research [29]. Previously, we have cloned and analyzed a MADS-box gene GmGAL2 from soybean [30]. In this work, another soybean MADS-box gene GmGAL1 (G lycine max A GAMOUS L ike 1) was cloned by rapid-amplification of cDNA ends (RACE). The expression patterns of GmGAL1 in soybean at different stage were investigated. We further demonstrated that ectopic expression of soybean GmGAL1 accelerated flowering in Arabidopsis and partially rescued soc1 late flowering phenotype.
Materials and methods
Plant materials
The soybean cultivar used in this study was Glycine max L. “KN 18”. The seeds were sown in pots (diameter 18 cm, height 16 cm) with mixed field soil, turf soil, and vermiculite with a ratio of 2:2:1. The Arabidopsis Col-0 and Ler were used as Wild-type plants. soc1-1 was in the Ler background. The soybean and Arabidopsis grown condition were as described previously [30].
RNA preparation and gene cloning
For RNA preparation, the leaves of soybean were harvested in long-day and short-day conditions (16 h/8 h, light/dark, light was provided from 8:00 a.m. to 0:00 a.m.; 8 h/16 h, light/dark, light was provided from 8:00 a.m. to 16:00 p.m. everyday) at 0:00, 4:00, 8:00, 12:00 a.m., 16:00 and 20:00 p.m. Different soybean organs were sampled at different stages respectively. The fully expanded new leaves at unifoliolate or trifoliolate stages were sampled respectively for GmGAL1 expression analysis. Samples were collected from more than three soybean plants. RNA was prepared with Trizol (Invitrogen) and reversed transcribed to cDNA with M-MLV (Invitrogen).
We detected a set of ESTs that showed high sequence similarity to AGL20 (AGAMOUS-LIKE 20, accession number NP_182090) by blast AGL20 DNA sequence against the NCBI nucleotide collections (nr/nt) (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&BLAST_PROGRAMS=megaBlast&PAGE_TYPE=BlastSearch&SHOW_DEFAULTS=on&LINK_LOC=blasthome). Among them, BQ611561.1 and BU926043.1 were used as the candidate sequences for cloning the full length GmGAL1 gene [30]. The resultant clones were then sequenced for confirmation.
RT-PCR and quantitative PCR (q-PCR)
RT- and q-PCR were performed with the primers shown in Table 1 according to the manufacturer’s instructions (ABI). Total cDNA was used as the template for RT- and q-PCR. All experiments were replicated in triplicate. The expression level of the GmACT11 or AtUBQ gene was used as the internal control for target genes in soybean or Arabidopsis respectively (http://rsb.info.nih.gov/ij/).
Constructing expression vectors and plant transformation
The ORF of the GmGAL1 gene (primers shown in Table 1) was cloned into Entry clone pDONR201 by BP clonase (Invitrogen), and transferred to destination vector by LR clonase (Invitrogen). The resultant expression vector was a binary vector in which GmGAL1 was driven by the CaMV 35S promoter. It was transferred into Arabidopsis Col-0 via floral dipping approach mediated by Agrobacterium strain GV3101 (pMP 90RK) [31].
Sub-cellular localization of GmGAL1
Confocal microscopy was performed with a Leica TCS SP5 laser scanning confocal microscope. The well-expanded rosette leaves of Col-0 plants grown for 4 weeks in short-day conditions were collected for the protoplast isolation. Protoplasts was transformed with 10 μg of plasmid DNA (prepared using the Axygen Plasmid Midi Kit, http://www.axygen.com/) and incubated at 22°. After 12–16 h of transformation, the sub-cellular localization of GFP:GmGAL1 fusion proteins in protoplasts were observed with the confocal laser scanning microscope.
Phylogenetic analysis
Phylogenetic analysis of protein sequences was carried out using the amino acid sequence alignment generated by CLUSTAL-W. A Neighbor-Joining tree was built using the software of MEGA version 3.1. Support for the tree was assessed using the bootstrap method with 1000 bootstrap replicates. The numbers at each node represent the bootstrap support (percentage).
Results
Cloning of GmGAL1 gene
We detected a set of ESTs that showed high sequence similarity with SOC1 (AGL20, TAIR:AT2G45660) by BLAST search in NCBI database. Based on the sequence of ESTs, specific RACE primers for the full-length candidate gene were designed (Table 1). Using the RACE approach, we cloned a MADS box gene, GmGAL1 (Glycine max Agamous-Like1) and sequence alignment analysis indicated that GmGAL1 was a homolog gene of Arabidopsis AGL family (Fig. 1). Full length cDNA of GmGAL1 (Genebank ID: DQ355801) was 801 bp including 630 bp of coding DNA sequence (CDS) which coded 209 amino acids. As shown in Fig. 1a, GmGAL1 had a MADS-MEF2-like domain at N terminal and a K-box domain at middle of the protein. GmGAL1 protein sequence had the highest similarity (93% identity) to VuSOC1 of Vigna unguiculata (data not shown) and it had 69.9% identity with AtAGL20 (Fig. 1b–d). Phylogenetic analysis (www.ncbi.nlm.nih.gov/blast) showed that GmGAL1 had relatively lower sequence similarity with the AGL20 homologs in monocots and other species of gymnosperm, suggesting that GmGAL1 and AGL20 belong to the same dicot SOC1 family (Fig. 2a, b).
Alignments of GmGAL1 and AGL family and SOC1 homologs in other plant species. Numbers on the branches were bootstrap values. a Alignment of GmGAL1 and AGL family genes in Arabidopsis. b Alignment of GmGAL1 and SOC1 homologs in different plant species. Dicotyledon, monocotyledon and gymnosperm were in the same branch separately
Characteristic of GmGAL1 expression at vegetative and reproductive stages of soybean
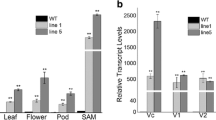
SOC1 expressed in multiple organs including leaves, roots and stem meristems in Arabidposis. GmGAL1 also expressed in several organs and at different development stages in soybean (Fig. 3a–c). The GmGAL1 mRNA abundance was low in unifoliolates of 7 and 14 day old seedings and increased a little bit in 21 day old ones. But in 28 day seedings, mRNA abundance of GmGAL1 increased rapidly in unifoliolates (Fig. 3a). These results suggested that the expression level of GmGAL1 at vegetative stage gradually increase according to development period and reach the highest level near to flowering. Using fully expanded unifoliolates and the first to the fourth trifoliolates as detection templates, we obtained the same result as using unifoliolates (Fig. 3b). Comparing to vegetative stage, the highest expression level appeared in stem of flowering phase. Its expression was low in flower and then became high in pod at reproductive stage (Fig. 3c).
GmGAL1 expression pattern in different development stages of Glycine max. a GmGAL1 mRNA abundance in unifoliolate of 7, 14, 21 and 28 day old samples. b GmGAL1 mRNA abundance in unifoliolate, 1st (T1), 2nd (T2), 3rd (T3) and 4th (T4) trifoliolate. c GmGAL1 mRNA abundance in different organs/tissues at flowering stage. FU unifoliolate at flowering, FR root at flowering, FSt stem at flowering, FT1–FT4 1–4 trifoliolate, FF flower at flowering. All leaves used are fully open. Error bars standard error
Circadian clock analysis
The GmGAL1 circadian clock was obtained by checking the mRNA abundance in fully open unifoliolates of soybean adapted with 24 h period of either short-day or long-day, followed by 48 h continuous light conditions (Fig. 4). In long-day adapted condition, GmGAL1 expression increased gradually, peaks at 8 h after light, decreased slowly till at dusk and peaked again at 8 h after light of next period. This circadian cycle oscillated at a cycle of about 24 h and continued in subsequent 1 day of continuous light. But at the third day of continuous light, the peak showed up 4 h earlier in the period, indicating the oscillating period of GmGAL1 become shorter in continuous light condition. The similar results showed in short-day and short-day to continuous light conditions.
The expression of GmGAL1 in response to either long-day (16 h light/8 h darkness) or short-day (8 h light/16 h darkness) condition for 24 h, followed by 48 h continuous light (LD-LL or SD-LL). Dark gray darkness, light gray light instead of darkness. All fully open unifoliolates were collected from more than three soybean plants. Error bars standard error
GmGAL1 promotes flowering in Arabidopsis
To study the function of GmGAL1 in soybean development, we over-expressed GmGAL1 via the CaMV-35S promoter in Arabidopsis. The transgenic plants were analyzed by RT-PCR (Fig. 5) to confirm the presence and expression level of the transferred gene. Two transgenic lines with ectopic expression of GmGAL1 showed earlier flowering phenotype compared with the wild-type control and the expression level of GmGAL1 was positively correlated with the flowering severity of flowering phenotype (Fig. 5). To further recapitulate its function, we over-expressed GmGAL1 in soc1-1 mutant. As shown in Fig. 6, GmGAL1-OX in soc1-1 mutant partially rescued the late flowering phenotype.
The sub-cellular localization of GmGAL1
We next examined the sub-cellular localization of GmGAL1 through an expression vector containing GFP: GmGAL1 fusion gene driven by CaMV-35S promoter which was constructed and introduced into protoplast of Arabidopsis. After 12–16 h transformation, the result showed GmGAL1 localized in cytoplasm as shown in Fig. 7.
GmGAL1 sub-cellular localization in cell. The construct was transformed into protoplasm of Arabidopsis and fluorescence visualized by confocal microscope. a The GFP:GmGAL1 fusion proteins were localized in the cytoplasm as green signal shown. b Bright field image of GFP:GmGAL1. c Merge of a and b. The red signal was from the autofluorescence of chloroplasts. The scale bars at the bottom represented 10 μm. (Color figure online)
Discussion
In this study, we cloned an AGL20/SOC1 homolog from soybean, GmGAL1. Its sequence shows high similarity to AGL20/SOC1 genes in many plants and it can be classed into the AGL20 group of dicots. The sequence of GmGAL1 is most similar to SOC1 protein in Arabidopsis (with 69.9% identity) and Legume plant (V. unguiculata, with 93% identity). These data suggest that SOC1 might originate from the same ancestor, but evolve independently in dicots, monocots, gymnosperm; long-day plants, and short-day plants. Thus, SOC1 has different functions between long-day and short-day plants, although they share some conserved functions. The sequence of GmGAL1 shows typical characters of MADS-box proteins, such as a highly conserved MADS-box at the N-terminus and a K-box in the middle of the MADS-box. These data suggest that GmGAL1 is a MADS-box gene and a putative SOC1 homolog in soybean.
SOC1, as a member of MADS-box proteins, its main function is as the integrator of multiple flowering signals. It has additionally interesting functions which have been uncovered by recent research [32]. But it is also proved that SOC1 evolves to become functional divergence in many species [24, 25, 27, 28, 33, 34]. It is speculated that SOC1/TM3-like genes in dicots are widely expressed in various tissues and the regulatory functions of these genes may be more diversified [22]. Our results of GmGAL1 expression in different development stages and organs are probably an evidence of this speculation. Soybean is a typical short-day plant and sensitive to photoperiod. mRNA and protein of GmCRY1/CRY2 show obvious circadian clock [29] and putative central clock genes GmLCL1, GmLCL2 (Glycinemax LHY/CCA1 Like1 and 2) and GmTOC1 also oscillate with circadian rhythms in several conditions [35]. As a main direct target gene of CO, we also detect that GmGAL1 gene expresses in a circadian manner of 24 h. In continuous light condition, the circadian period becomes shorter than 24 h (Fig. 4). Previously study has proved that the loss function of CCA1 or LHY in Arabidopsis conferring a shorter oscillator period [36] implies one of the possible explanations for our observation: the function of the central oscillator of soybean cultivar (KN18) used in this report may be as lesion as that of cca1 or lhy mutant of Arabidopsis and cannot keep its regular 24 h period when the conditions changed from light–dark cycle to continue light. These results imply that GmGAL1 is regulated by clock genes.
Because SOC1 is functionally redundant with AGL24 and SVP in control of flower organ identity [23], no obvious morphology changes are detected in SOC1 deletion mutant, although SOC1 plays important role in flowering pathway. In consistent, overexpression of GmGAL1 in wild-type Arabidopsis or in soc1 mutant accelerates flowering (Figs. 5, 6) but the transgenic lines show no obviously morphological change. These results suggest that the major role of GmGAL1 gene is to play as a critical integrator of flowering pathway, which is similar as its homolog gene SOC1 in Arabidopsis.
SOC1 localizes in cytoplasm and then in nuclear when it combines with AGL24 [37]. We don’t obtain the homolog of AGL24 gene from Glycine max, but the localization of GmGAL1 in cytoplasm of protoplast is similar to the SOC1 localization in Arabidopsis [37].
In summary, GmGAL1 is a homolog of Arabidopsis thaliana SOC1/AGL20 in soybean. It has conserved, but not identical, functions on plant development compared with its homolog in other plants.
References
Bodt DS, Raes J, Van de Peer Y, Theissen G (2003) And then there were many: MADS goes genomic. Trends Plant Sci 8:475–483
Foo E, Ross JJ, Davies NW, Reid JB, Weller JL (2006) A role for ethylene in the phytochrome-mediated control of vegetative development. Plant J 46:911–921
Irish V (2003) The evolution of floral homeotic gene function. Bioessays 25:637–646
Kater M, Dreni L, Colombo L (2006) Functional conservation of MADS-box factors controlling floral organ identity in rice and Arabidopsis. J Exp Bot 57:3433–3444
Messenguy F, Dubois E (2003) Role of MADS box proteins and their cofactors in combinatorial control of gene expression and cell development. Gene 316:1–21
Rijpkema A, Gerats T, Vandenbussche M (2007) Evolutionary complexity of MADS complexes. Curr Opin Plant Biol 10:32–38
Robles P, Pelaz S (2005) Flower and fruit development in Arabidopsis thaliana. Int J Dev Biol 49:33–43
Saedler H, Huijser P (1993) Molecular biology of flower development in Antirrhinum majus (snapdragon). Gene 135:239–243
Hepworth S, Valverde Ravenscroft FD, Mouradov A, Coupland G (2002) Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J 21:4327–4337
Poduska B, Humphrey T, Redweik A, Grbic V (2003) The synergistic activation of FLOWERING LOCUS C by FRIGIDA and a new flowering gene AERIAL ROSETTE 1 underlies a novel morphology in Arabidopsis. Genetics 163:1457–1465
Rouse DT, Sheldon CC, Bagnall DJ, Peacock WJ, Dennis ES (2002) FLC, a repressor of flowering, is regulated by genes in different inductive pathways. Plant J 29:183–191
Swarup K, Alonso-Blanco C, Lynn JR, Michaels SD, Amasino RM, Koornneef M, Millar AJ (1999) Natural allelic variation identifies new genes in the Arabidopsis circadian system. Plant J 20:67–77
Sessions A, Yanofsky MF, Weigel D (2000) Cell–cell signaling and movement by the floral transcription factors LEAFY and APETALA1. Science 289:779–782
Wagner D, Sablowski RWM, Meyerowitz EM (1999) Transcriptional activation of APETALA1 by LEAFY. Science 285:582–584
Tapia-López R, García-Ponce B, Dubrovsky JG, Garay-Arroyo A, Pérez-Ruíz RV, Kim SH, Acevedo F, Pelaz S, Alvarez-Buylla ER (2008) An AGAMOUS-related MADS-box gene, XAL1 (AGL12), regulates root meristem cell proliferation and flowering transition in Arabidopsis. Plant Physiol 46:1182–1192
Kim S, Kim SR, An CS, Hong YN, Lee KW (2001) Constitutive expression of rice MADS box gene using seed explants in hot pepper (Capsicum annuum L.). Mol Cells 12:221–226
Lee S, Kim J, Han JJ, Han MJ, An G (2004) Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in rice. Plant J 38:754–764
Moon J, Suh SS, Lee H, Choi KR, Hong CB, Paek NC, Kim SG, Lee I (2003) The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J 35:613–623
Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288:1613–1616
Sheldon C, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES (1999) The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11:445–458
Borner R, Kampmann G, Chandler J, Gleibner R, Wisman E, Apel K, Melzer S (2000) A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J 24:519–599
Lee Jungeun LI (2010) Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 61:2247–2254
Liu C, Xi WY, Shen LS, Tan CP, Yu H (2009) Regulation of floral patterning by flowering time genes. Dev Cell 16:711–722
Decroocq V, Zhu XM, Kauffman M, Kyozuka J, Peacock WJ, Dennis ES, Llewellyn DJ (1999) A TM3-like MADS-box gene from Eucalyptus expressed in both vegetative and reproductive tissues. Gene 228:155–160
Tadege M, Sheldon CC, Helliwell CA, Upadhyaya NM, Dennis ES, Peacock WJ (2003) Reciprocal control of flowering time by OsSOC1 in transgenic Arabidopsis and by FLC in transgenic rice. Plant Biotechnol J1:361–369
Heuer S, Hansen S, Bantin J, Brettschneider R, Kranz E, Lorz H, Dresselhaus T (2001) The maize MADS box gene ZmMADS3 affects node number and spikelet development and is co-expressed with ZmMADS1 during flower development, in egg cells, and early embryogenesis. Plant Physiol 127:33–45
Nakamura T, Song IJ, Fukuda T, Yokoyama J, Maki M, Ochiai T, Kameya T, Kanno A (2005) Characterization of TrcMADS1 gene of Trillium camtschatcense (Trilliaceae) reveals functional evolution of the SOC1/TM3-like gene family. J Plant Res 118:229–234
Ferrario S, Busscher J, Franken J, Gerats T, Vandenbussche M, Angenent GC, Immink RG (2004) Ectopic expression of the petunia MADS box gene UNSHAVEN accelerates flowering and confers leaflike characteristics to floral organs in a dominant-negative manner. Plant Cell 16:1490–1505
Zhang Q, Li HY, Li R, Hu RB, Fan CM, Chen FL, Wang ZH, Liu Xu, Fu YF, Lin CT (2008) Association of the circadian rhythmic expression of GmCRY1a with a latitudinal cline in photoperiodic flowering of soybean. Proc Natl Acad Sci USA 105:21028–21033
Xu JH, Zhong XF, Zhang QZ, Li HY (2010) Overexpression of the GmGAL2 gene accelerates flowering in Arabidopsis. Plant Mol Biol Rep 28:704–711
Steven J, Andrew FB (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T (2008) Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat Genet 40:1489–1492
Cseke LJZJ, Podila GK (2003) Characterization of PTM5 in aspen trees: a MADS-box gene expressed during woody vascular development. Gene 318:55–67
Tan F, Swain SM (2007) Functional characterization of AP3, SOC1 and WUS homologues from citrus (Citrus sinensis). Physiol Plantarum 131:481–495
Liu H, Wang HG, Gao PF, Xv JH, Xv TD, Wang JS, Wang BL, Lin CT, Fu YF (2009) Analysis of clock gene homologs using unifolioates as target organs in soybean (Glyciine max). J Plant Physiol 166:278–289
Lu SX, Knowles SM, Andronis C, Ong MS, Tobin EM (2009) CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL function synergistically in the circadian clock of Arabidopsis. Plant Physiol 150:834–843
Lee J, Oh M, Park H, Lee I (2008) SOC1 translocated to the nucleus by interaction with AGL24 directly regulates LEAFY. Plant J 55:832–843
Acknowledgments
This work was supported in part by the Chinese national “863” Program (2006AA10Z107), Ministry of Agriculture, major projects transgenic (2009ZX08004-010B) and the National Natural Science Foundation of China (Grant No. 31171352).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhong, X., Dai, X., Xv, J. et al. Cloning and expression analysis of GmGAL1, SOC1 homolog gene in soybean. Mol Biol Rep 39, 6967–6974 (2012). https://doi.org/10.1007/s11033-012-1524-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1524-0