Abstract
The WNT signaling is deregulated in most human colorectal cancers (CRC). Promoter methylation has been proposed as an alternative mechanism to inactivate genes in tumors. To gain insight into the methylation silencing of the WNT pathway during colorectal carcinogenesis, we examined the aberrant methylation profile of four genes, APC, Axin1, Axin2, and GSK3β in an unselected series of 112 sporadic colorectal tumors by methylation specific PCR. It has been suggested that the Axin2 C148T SNP is associated with the risk of developing certain types of cancers. To assess the contribution of Axin2 SNP to CRC susceptibility, we examined the Axin2 C148T genotype in CRC patients and 170 healthy controls by PCR-RFLP. The frequency of CRCs with at least one gene methylated was 18.75%. Promoter methylation of Axin2 and APC genes was detected in 7.1 and 11.9% of tumors, respectively. No aberrant methylation was found in Gsk3β and Axin1 gene in these tumor series. The methylation status of APC had no significant association with clinical parameters. But, promoter methylation of Axin2 was sex-related, occurring more frequently in females (P = 0.002). The frequency of Axin2 C148T genotypes were similar in patients and controls. Moreover, we observed no association between the Axin2 SNP and risk of CRC in patients stratified by age, sex, and smoking status. However, the heterozygote CT genotype was associated with a reduced CRC risk in distal patients compared with proximal patients (OR = 0.3; 95% CI 0.1–0.9, P = 0.04). Our findings indicate that Axin1 and GSK3β methylation play a minor role in colorectal carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cause of cancer-related deaths world-wide, and its incidence has also sharply increased over the last decades in Iran [1]. Molecular studies have shown that dysregulation of Wingless (WNT) signaling pathway is a crucial event in the pathogenesis of CRC with APC mutations seen in more than 80% of sporadic CRC [2, 3]. The Wnt proteins are a family of 19 conserved secreted glycoproteins that signal via seven transmembrane spanning receptors of the Frizzled family [4]. β-Catenin is the key component of the canonical WNT pathway and is regulated by a multi-protein complex consisting of glycogen synthase kinase 3β (GSK3β), AXIN, casein kinase 1α (CK1α), and APC. In the absence of a WNT signal this protein complex promotes the phosphorylation of β-catenin, which leads to its ubiquitylation and degradation by the 26S proteasome [5].
Wnt signalling pathway is turned on in nearly all colorectal carcinomas [3, 6], therefore identifying all genetic and epigenetic alterations affecting the WNT signaling pathway is important to understanding CRC tumorigenesis. The WNT pathway is deregulated by both DNA sequence changes and by promoter hypermethylation of the key components that results in transcriptional silencing [7, 8]. Epigenetic events have been hypothesized to have a complementary role with genetic alterations in colorectal tumorigenesis [9].
APC is one of the key components of Wnt pathway and its dysfunction is observed in familial polyposis coli (FAP) patients and in as many as 80% of sporadic colorectal adenomas and carcinomas [2, 3]. Two AXIN gene family members have been identified in humans, AXIN1 and AXIN2/Conductin, and both genes indicate mutations in some colorectal carcinomas [10, 11]. The Axin2 gene, a negative regulator of Wnt signaling, also acts as a tumor suppressor gene [12, 13]. Associations between polymorphisms in the Axin2 gene, including exon2 P50S, with lung [14] and breast [15] cancers have previously been shown. Although many studies have analyzed the alteration of WNT-related genes in CRC, but limited studies have addressed multiple components of the pathway in the same tumor series [16, 17]. In this study we analyzed the promoter hypermethylation of APC, GSK3β, Axin1, and Axin2 genes of WNT signaling pathway in 112 sporadic colorectal tumors. The series has also been examined for association between single nucleotide polymorphism (SNP) at codon 50 of the Axin2 gene and CRC risk.
Subjects and methods
Study population, and tumor samples
Surgically resected tumors and surrounding normal tissues from 112 patients with CRC were collected from patients who received surgical treatment at three university hospitals in Shiraz, southern Iran between the years 2003 and 2005. Ethics approval for the project was obtained from the institutional ethics committee. Tumor samples were snap-frozen immediately after surgical resection and stored at −80°C. All samples were evaluated and subjected to histological diagnosis by an expert pathologist, who also selected representative tissue sections for DNA extraction and further molecular analyses. The splenic flexure was used as the anatomical boundary to define proximal and distal CRC. Information on the age, sex, and smoking history of the patients was obtained from hospital records.
Methylation-specific PCR (MS-PCR)
DNA extracted from tumor and normal samples according to the standard phenol/chloroform method. The status of promoter methylation of the APC (promoter 1A), Gsk 3 \( \beta \), Axin1, and Axin2 genes were determined by MSP-PCR. The sequences of primers and annealing temperatures used for amplification of the promoter regions of genes are listed in Table 1. We determined the genes promoter methylation status by chemical treatment of DNA samples with sodium bisulfite and subsequent MS-PCR as previously described [18]. In every sodium bisulfite conversion reaction, DNA from peripheral blood lymphocytes and CpGenome™ Universal Methylated DNA (Milipore, CA) were included as a negative and positive control, respectively. PCR products were analyzed by electrophoresis on 2% agarose gel.
Axin2 codon 50 genotyping
Genotyping of Axin2 at codon 50 (C148T, Pro50Ser, rs2240308) of DNA from 179 controls and 110 CRC cases was performed by PCR-RFLP analysis. Genomic DNA from the samples was used as a template in PCR reactions using two Axin2 primers encompassing Axin2 exon2 where the P50S substitution is known to take place. One nucleotide at the 3′-side of the reverse primer was changed (Table 1) to create, in cases of a wild- type 148 C nucleotide in the DNA template, a Mph1103I recognition site 5′ATGCA↓T3′. The mutant allele with 148T does not contain the Mph1103I recognition sequence at the corresponding position. Mph1103I digestion of the 242 bp PCR product resulted in 218 and 24 bp fragments for the wild-type (148C) allele. Amplified fragments were digested overnight with 5U mph1103I restriction enzyme (MBI Fermentas, Vilnius, Lithuania) at 37°C. Digestion products were analyzed by electrophoresis on 10% polyacrylamide gels and stained with ethidium bromide and visualized under UV illumination.
Statistical analysis
Statistical analysis was performed using the SPSS version 16 software package (Chicago, IL). Associations between methylation of loci and clinical, biological and genotypic features were evaluated using Chi square and Fisher’s exact test as appropriate. Logistic regression was used to calculate odds ratio (OR) and 95% confidence intervals (95% CI). OR and 95% CI were adjusted for covariates, specifically including age and gender. A P-value of <0.050 was considered to be statistically significant.
Results
Distribution of selected characteristics of cases
We investigated 112 sporadic CRC tumors for promoter hypermethylation of four key genes affecting the WNT signaling pathway, including APC, Gsk 3 , Axin1, and Axin2. Single nucleotide polymorphism (SNP) at codon 50 of the Axin2 gene was also analyzed in these tumors. The CRC series investigated in this study have also been characterized previously for mutation and the methylation status of several other tumor-related genes. Therefore, this should be considered as an extension of our previous study, which could be consulted for detailed information [19].
Cases were more likely to be males (62.5%) and older than 60 years (61.6%). The incidence of distal tumors was higher than proximal tumors. Thirty patients (26.8%) had tumors in the proximal colon and 82 (73.2%) in distal parts. No statistically significant differences were found between proximal and distal cancer cases with respect to sex, age and smoking status. The majority of distal tumors was found to be well/moderately differentiated (98.6%) and was stage II or higher (93.2%).
APC2, Gsk3β, Axin1, and Axin2 gene methylation profiles
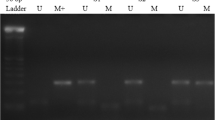
Aberrant methylation is a potential means of inactivating tumor suppressor genes, and various genes have been demonstrated to be hypermethylated and silenced in colorectal neoplasia. However, limited data is available upon the concurrent methylation of multiple genes involved in WNT signaling pathway in colorectal cancer. In this study, we investigated the promoter methylation of four genes, APC2, Gsk3β, Axin1, and Axin2, involved in CRC carcinogenesis, using methylation-specific PCR. Illustrative examples of MSP reactions for promoter methylation analysis are shown in Fig. 1. The epigenetic profile of genes involved in WNT signaling pathway in the 112 CRC patients we studied are summarized in Table 2. The frequency of tumor methylation (tumors with at least one gene methylated) was 21/112 (18.75%). We found methylation of APC and Axin2 genes in 13 (11.9%) and 8 (7.1%) of CRC patients, respectively. Aberrant promoter methylation in Gsk 3 β and Axin1 gene was not detected in the 112 CRC cases investigated. Only one of the tumors had simultaneous CpG island hypermethylation of both APC and Axin2 genes’ promoters.
Representative examples of MSP reactions for promoter methylation analysis of APC, GSK3β, Axin1, and Axin2 genes in primary CRC tumors. The presence of a visible PCR product in those lanes marked U indicates the presence of unmethylated genes; the presence of a product in those lanes marked M indicates the presence of methylated genes. C positive control, MR the 100 bp DNA size marker
Aberrant methylation of the APC promoter was not associated with significant differences of gender, age of onset, and other clinical pathological features of CRC cases.
Furthermore, APC promoter hypermethylation was not associated with aberrant methylation of other genes analyzed previously in some of these primary colorectal carcinomas [18, 19], including the cell cycle regulator p16, the DNA mismatch repair genes MLH1 and MSH2, and K-ras mutation. Finally, APC methylation was not more common in MSI positive tumors than in MSI negative CRCs.
We found no significant differences in association of methylation of Axin2 gene by age, smoking status, tumor location or stage and differentiation of tumors (Table 2). But, promoter methylation of Axin2 was sex-related, occurring more frequently in females. 87.5% of CRC tumors with Axin2 hypermethylation were found in female patients, but only 12.5% in male patients (P = 0.002).
Distribution of Axin2 C148T genotype
Samples were analyzed for the presence of a point mutation that occurs in the Axin2 gene leading to a change of proline to serine at position 50 of the AXIN2 protein. Genotyping was performed by PCR-RFLP analysis using Mph1103I restriction enzyme, as shown in Fig. 2. Genotype frequencies and odds ratios for Axin2 genotypes and colon cancer are presented in Table 3. Prevalence of the Axin2 148T allele did not differ significantly between controls (41.9%) and cases (43.2%). The distribution of Axin2 148 genotypes among both controls (CC, 30.7%; CT, 54.7%; TT, 14.5%) and cases (CC, 30.9%; CT, 51.8%; TT, 17.3%) agreed with that expected from the Hardy–Weinberg equilibrium (χ2 = 2.77, P > 0.05; χ2 = 0.34, P > 0.05, respectively).
PCR-RFLP assay for genotyping Axin2 148C/T polymorphism. For PCR-primers and reaction conditions see “Subjects and methods”. The differently sized allele-specific digestion products were separated by electrophoresis on a 10% polyacrylamide gel. UD undigested PCR product, M DNA size marker
We examined the relationship between Axin2 polymorphisms and the risk of CRC. Because of the relatively small numbers of individuals with the TT genotype, we combined individuals with Axin2 148 TT and CT genotypes. In case–control comparisons, we observed no differences in frequencies of Axin2 C148T genotypes in patients stratified by the clinicopathologic variables (Table 3). In the entire group of patients, the Axin2 CT/TT genotype was not associated with the risk of cancer incidence (Table 3).
In case–case comparisons, the heterozygote CT genotype was associated with a reduced CRC risk in distal patients compared with proximal patients (OR = 0.3; 95% CI 0.1–0.9, P = 0.04). No differences in frequencies of the Axin2 C148T genotypes were found in patients stratified by sex, smoking status, and tumor stage.
Discussion
The WNT signaling is regulated by a complex consisting of APC, AXIN, and GSK-3β. Molecular studies have shown that the Wnt signaling pathway is deregulated in over 90% of human colorectal cancers [2, 3]. Inactivation of genes involved in Wnt signaling leads to accumulation of β-catenin in the cytoplasm and nucleus, and thereby activation of WNT pathway. Loss of tumor suppressor genes expression could happen either by mutation or promoter hypermethylation [4, 5]. Although many studies have investigated the WNT components in CRC, but only a few have studied the aberrant methylation of multiple components within the same tumor series [3, 17].
To explore the contribution of aberrant methylation of components of Wnt signaling pathway to the carcinogenesis of CRC, we examined promoter methylation of four genes (APC, Axin1, Axin2, and GSK3β) in sporadic colorectal cancers. Among 112 patients with primary tumors, 7% contained Axin2 methylation and 12% APC methylation (Table 2). We found no aberrant promoter methylation of Gsk3β and Axin1 genes in this tumor series.
Promoter methylation of APC gene has been reported in a substantial proportion of sporadic CRCs [20, 21], however data pertaining to Axin1, Axin2 and GSK3β methylation in colorectal cancer are scarce. The frequencies of APC methylation have varied greatly among various studies of primary CRC tumors in different populations and have been reported to range between 0 and 62.4% [20–24]. Our findings is in line with the observation of Lin et al. [17], which found aberrant promoter methylation of APC, GSK-3ß, and Axin1 genes in 12.4, 2.2, and 0% of 185 CRC patients in Taiwan, respectively.
The reported variations in promoter hypermethylation frequencies of different tumor suppressor genes in colorectal cancer can be explained by different methods for analyzing methylation, incomplete bisulphite modification, tumor heterogeneity, and the fact that different parts of the gene promoter region in question have been analyzed. We used the same method and the same previously reported primer sets for amplification of the APC promoter 1A [20] and the GSK3β promoter [17]. We assume that differences in the genetic or environmental factors among study populations might also be involved in the methylation events that are found and reported.
Down-regulation of APC is an early event observed in colorectal carcinogenesis that leads to activation of WNT signaling. We did not analyze the APC mutational status in this group of patients. However, Esteller et al. [20] demonstrated that methylation affects only wild-type APC in 95% of sporadic CRC cases and is not observed in tumors from FAP patients who have germ-line APC mutations. Therefore, aberrant gene’s promoter methylation has been proposed as an alternative mechanism to mutation of APC inactivation in colon tumorigenesis.
In accordance with previous results [3, 20, 25], we found no correlation between APC methylation and clinico-pathological features of these tumors. Some studies have suggested that MSI was associated with promoter methylation of APC and Axin2 genes [25, 26], whereas consistent with other previous reports [3, 20], we did not observe a relationship between APC or Axin2 promoter methylation and MSI CRCs (data not shown). Kim et al. suggested that APC methylation-KRAS mutation-p16 methylation may constitute a novel phenotype in sporadic colorectal cancer [23]. Our results showed no correlation of APC methylation either with KRAS mutation or with other genes’ promoter methylation.
In our study, the frequency of tumors with methylated Axin2 gene was higher in female patients (P = 0.002) (Table 2) and a nonsignificant excess of Axin2 methylation was observed for stage 1 tumors (Table 2). Aberrant methylation of Axin2 was mainly found in 3/13 stage I tumors vs. 4/90 of more advanced tumors; P = 0.08). No other association was found between Axin2 methylation and the clinico-pathological parameters under study.
The role of GSK3β in tumorigenesis is controversial [27]. GSK3β has been proposed to function as a tumor suppressor for skin and mammary tumors, but might act as a tumor promoter for colon and pancreatic cancers. GSK3β protein overexpression has been found in human colon cancer cell lines and colorectal cancer patients [27]. Therefore, GSK3β may be a ‘‘tumor promoter” for certain types of tumors including colon cancer. Our findings indicate that Axin1 and GSK3β methylation does not play a role in CRC tumorigenesis.
Previous studies have shown that a single nucleotide polymorphism (SNP) at codon 50 of the AXIN2 gene, encoding either proline (CCT) or serine (TCT), may predispose to lung [14, 28] and breast [15] cancers. To determine if this finding could be replicated in colorectal cancer, we investigated the association between Axin2 C148T polymorphisms and the risk of cancer incidence in this group of patients.
The major genotype population in both cases and controls were the heterozygous CT (Pro/Ser) genotype (Table 3). The CC, CT and TT genotype frequencies were 30.9, 51.8, and 17.3% in colorectal cancer patients and 30.7, 54.7, and 14.5% in controls, respectively. In general, studies addressing the impact of the Axin2 Pro50Ser SNP on predisposition to colon cancer are scarce. The association of the AXIN2 polymorphism with CRC was independently investigated in two relatively small case–control cohorts from USA and Japan [14, 29]. Our results are comparable to those of Peterlongo et al. [29], but different from those of Kanzaki et al. [14], who found a CC, CT and TT genotype distribution of 47.7, 38.9 and 13.4% in CRC patients and 38.2, 47.3, and 13.6% in controls, respectively. In the study by Kanzaki et al. [14], the Axin2 SNP was found to be associated with lung cancer, but not with col-orectal and head and neck cancers. Similar to previous results, we observed no association between the Axin2 SNP and risk of colorectal cancer. In all cases, no correlation between Axin2 genotype with clinico-pathological features could be established.
Axin2 exone2 encoded domain is a potential binding site for APC [30, 31]. However, the AXIN2 P50S substitution is predicted to be benign with little effect on the structure and function of the AXIN2 protein [32]. It is noteworthy that our sample size was relatively small and the tested polymorphism in this study was also a minor genotype which calls for a larger study population to detect a significant association.
To our knowledge, this is the first study to investigate the genetic and epigenetic alterations of multiple WNT components in a cohort of Iranian colorectal cancer patients.
References
Malekzadeh R, Bishehsari F, Mahdavinia M, Ansari R (2009) Epidemiology and molecular genetics of colorectal cancer in Iran: a review. Arch Iran Med 12:161–169
Giles RH, van Es JH, Clevers H (2003) Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653:1–24. doi:10.1016/S0304-419X(03)00005-2
Thorstensen L, Lind GE, Løvig T, Diep CB, Meling GI, Rognum TO, Lothe RA (2005) Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability. Neoplasia 7:99–108. doi:10.1593/neo.04448
Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Natl Rev Mol Cell Biol 10:468–477. doi:10.1038/nrm2717
Kimelman D, Xu W (2006) β-Catenin destruction complex: insights and questions from a structural perspective. Oncogene 25:7482–7491. doi:10.1038/sj.onc.1210055
Behrens J, Lustig B (2004) The Wnt connection to tumorigenesis. Int J Dev Biol 48:477–487. doi:10.1387/ijdb.041815jb
Narayan S, Roy D (2003) Role of APC and DNA mismatch repair genes in the development of colorectal cancers. Mol Cancer 2:41. doi:10.1186/1476-4598-2-41
Hiltunen MO, Alhonen L, Koistinaho J, Myohanen S, Paakkonen M, Marin S et al (1997) Hypermethylation of the APC (adenomatous polyposis coli) gene promoter region in human colorectal carcinoma. Int J Cancer 70:644–648. doi:10.1002/(SICI)1097-0215(19970317)
Feinberg AP (2004) The epigenetics of cancer etiology. Semin Cancer Biol 14:427–432. doi:10.1016/j.semcancer.2004.06.005
Shimizu Y, Ikeda S, Fujimori M, Kodama S, Nakahara M, Okajima M, Asahara T (2002) Frequent alterations in the Wnt signaling pathway in colorectal cancer with microsatellite instability. Genes Chromosomes Cancer 33:73–81. doi:10.1002/gcc.1226
Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK et al (2000) Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat Genet 26:146–147. doi:10.1038/79859
Kikuchi A (1999) Modulation of Wnt signaling by axin and axil. Cytokine Growth Factor Rev 10:255–265
Hughes TA, Brady HJM (2005) Cross-talk between pRb/E2F and Wnt/b-catenin pathways: E2F1 induces AXIN2 leading to repression of Wnt signalling and to increased cell death. Exp Cell Res 303:32–46. doi:10.1016/j.yexcr.2004.09.014
Kanzaki H, Ouchida M, Hanafusa H, Yano M, Suzuki H, Aoe M et al (2006) Single nucleotide polymorphism of the AXIN2 gene is preferentially associated with human lung cancer risk in a Japanese population. Int J Mol Med 18:279–284
Wang X, Goode EL, Fredericksen ZS, Vierkant RA, Pankratz VS, Liu-Mares W et al (2008) Association of genetic variation in genes implicated in the beta-catenin destruction complex with risk of breast cancer. Cancer Epidemiol Biomarkers Prev 17:2101–2108. doi:10.1158/1055-9965.EPI-08-0134
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW (1997) Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275:1787–1790. doi:10.1126/science.275.5307.1787
Lin SY, Yeh KT, Chen WT, Chen HC, Chen ST, Chiou HY, Chang JG (2004) Promoter CpG methylation of tumor suppressor genes in colorectal cancer and its relationship to clinical features. Oncol Rep 11:341–348
Mokarram P, Naghibalhossaini F, Saberi Firoozi M, Hosseini SV, Izadpanah A, Salahi H et al (2008) Methylenetetrahydrofolate reductase C677T genotype affects promoter methylation of tumor-specific genes in sporadic colorectal cancer through an interaction with folate/vitamin B12 status. World J Gastroenterol 14:3662–3671. doi:10.3748/wjg.14.3662
Naghibalhossaini F, Mokarram P, Khalili I, Vasei M, Hosseini SV, Ashktorab H et al (2010) MTHFR C677T and A1298C variant genotypes and the risk of microsatellite instability among Iranian colorectal cancer patients. Cancer Genet Cytogenet 197:142–151. doi:10.1016/j.cancergencyto.2009.11.014
Esteller M, Sparks A, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA et al (2000) Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res 60:4366–4371
Fu X, Li J, Tian X, Zhang Y (2009) Hypermethylation of APC promoter 1A is associated with moderate activation of Wnt signalling pathway in a subset of colorectal serrated adenomas. Histopathology 55:554–563. doi:10.1111/j.1365-2559.2009.03411.x
Kim JC, Choi JS, Roh SA, Cho DH, Kim TW, Kim YS (2010) Promoter methylation of specific genes is associated with the phenotype and progression of colorectal adenocarcinomas. Ann Surg Oncol 17:1767–1776. doi:10.1245/s10434-009-0901-y
Lee BB, Lee EJ, Jung EH, Chun HK, Chang DK, Song SY et al (2009) Aberrant methylation of APC, MGMT, RASSF2A, and Wif-1 genes in plasma as a biomarker for early detection of colorectal cancer. Clin Cancer Res 15:6185–6191. doi:10.1158/1078-0432.CCR-09-0111
Chen SP, Chiu SC, Wu CC, Lin SZ, Kang JC, Chen YL et al (2009) The association of methylation in the promoter of APC and MGMT and the prognosis of Taiwanese CRC patients. Genet Test Mol Biomarkers 13:67–71. doi:10.1089/gtmb.2008.0045
Derks S, Postma C, Carvalho B, van den Bosch SM, Moerkerk PT, Herman JG et al (2008) Integrated analysis of chromosomal, microsatellite and epigenetic instability in colorectal cancer identifies specific associations between promoter methylation of pivotal tumour suppressor and DNA repair genes and specific chromosomal alterations. Carcinogenesis 29:434–439. doi:10.1093/carcin/bgm270
Koinuma K, Yamashita Y, Liu W, Hatanaka H, Kurashina K, Wada T et al (2006) Epigenetic silencing of AXIN2 in colorectal carcinoma with microsatellite instability. Oncogene 25:139–146. doi:10.1038/sj.onc.1209009
Luo J (2009) Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett 273:194–200. doi:10.1016/j.canlet.2008.05.045
Gunes EG, Pinarbasi E, Pinarbasi H, Silig Y (2009) Strong association between lung cancer and the AXIN2 polymorphism. Mol Med Reports 2:1029–1035. doi:10.3892/mmr_00000210
Peterlongo P, Howe LR, Radice P, Sala P, Hong YJ et al (2005) Germline mutations of AXIN2 are not associated with nonsyndromic colorectal cancer. Hum Mutat 25:498–500. doi:10.1002/humu.20189
Koch A, Weber N, Waha A, Hartmann W, Denkhaus D, Behrens J, Birchmeier W et al (2004) Mutations and elevated transcriptional activity of conductin (AXIN2) in hepatoblastomas. J Pathol 204:546–554. doi:10.1002/path.1662
Koch A, Hrychyk A, Hartmann W, Waha A, Mikeska T, Waha A et al (2007) Mutations of the Wnt antagonist AXIN2 (Conductin) result in TCF-dependent transcription in medulloblastomas. Int J Cancer 121:284–291. doi:10.1002/ijc.22675
Callahan N, Modesto A, Meira R, Seymen F, Patir A, Vieira AR (2009) Axis inhibition protein 2 (AXIN2) polymorphisms and tooth agenesis. Arch Oral Biol 54:45–49. doi:10.1016/j.archoralbio.2008.08.002
Acknowledgments
This study was part of the dissertation of Mozhdeh Zamani, submitted to Shiraz University of Medical Sciences in partial fulfillment of the requirements for the M.Sc. in biochemistry. This work was supported by a grant from the Vice Chancellor for Research, Shiraz University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naghibalhossaini, F., Zamani, M., Mokarram, P. et al. Epigenetic and genetic analysis of WNT signaling pathway in sporadic colorectal cancer patients from Iran. Mol Biol Rep 39, 6171–6178 (2012). https://doi.org/10.1007/s11033-011-1434-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1434-6