Abstract
As transient expression systems are effective methods for the functional characterization of genes, a transient gene expression and silencing system was developed for Betula platyphylla Suk (Chinese Birch). Firstly, the cinnamoyl-CoA reductase (CCR) gene and its promoter were isolated from Chinese Birch. The vectors for overexpression of CCR and RNAi-based silence of CCR were constructed and transformed into Agrobacterium, respectively. Overexpression and silence of the CCR gene were respectively, performed on Birch seedlings using an Agrobacterium-mediated transient expression system. The expression levels of CCR were determined using real-time PCR. The results showed that the transcripts of CCR notably increased in the Birch plants transformed with the CCR overexpression construct, and notably decreased in plants transformed with the silencing construct when compared with nontransgenic plants. These studies confirmed that this transient genetic transformation system works well on Birch plants, and can be used for the functional characterization of genes and protein production in Birch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The analyses of gene expression and regulation form the basis of study of gene function. Most of our understanding about the roles of genes in plants have been gained through the analysis of mutant phenotypes [1] or stable transformations. However, when trying to apply such approaches to the study of woody tree species, and more specifically to wood formation, these techniques appear to be of limited use due to the heterogeneity of the plant material, long generation times and a number of biochemical limitations to microscopic and molecular experiments [2]. Our current knowledge of lignin and cellulose biosynthesis at a molecular level is largely based on model plants such as Arabidopsis thaliana [2]. Additionally, the increased discovery of genes in woody tree species is dwarfing our ability to understand gene function in detail.
One of the most urgently needed tools is a reliable and efficient technique for the discovery and characterization of individual tree genes. Transient gene expression assays provide a convenient alternative to mutant phenotypes and stable transformation, as such techniques allow for the rapid analysis of gene function [3]. Compared with stable transformation, the assay can be performed in several days, and the transformation of genes can be analyzed without the generation of transgenic plants. Moreover, the transient assay allows for the expression of deleterious proteins that would disrupt plant growth and development when expressed in transgenic lines [4].
Transient gene expression technologies have facilitated studies into genetic regulation in several tree species, including Pinus sylvestris L. [5], Picea glauca [6], Populus alba L. [7], Pinus pinaster [8], and Eucalyptus globulus [2]. A. tumefaciens co-cultivation systems have demonstrated a rapid, efficient and economical assay of gene function analysis in intact plants, with minimal manual handling or specialist equipment [4]. This encouraged us to test the possibility that A. tumefaciens co-cultivation could also work well for the transient transformation of Birch. To our knowledge, Agrobacterium-mediated transformation for rapid functional gene assays has not previously been reported in this tree species.
In this study, we present an easy, efficient, and economical method for transient gene expression and silence of cinnamoyl-CoA reductase (CCR) in Birch seedlings based on A. tumefaciens-mediated transient expression procedure. The CCR gene is an important gene that is related to lignin synthesis. The transcriptional expression of CCR was determined using real-time PCR. The expression of CCR was found to increase in overexpressed plants and decrease in RNAi-based silenced plants after transformed for 48, 72 or 96 h. These results demonstrated that the Agrobacterium-mediated transient expression procedure can be used as a rapid gene assay tool to study gene regulation in Birch and to test and compare genetic constructs.
Methods
Transformation vector and cultivation of Agrobacterium tumefaciens
A cDNA library was constructed from cambium tissue of Birch, and a CCR gene was cloned [9]. To insert the CCR gene into pROKII for overexpression, the following primers were designed: forward primer engineered XbaI restriction sites: 5′-CTAGTCTAGAATGGCGACGGAGGGTGAGGT-3′, and reverse primer engineered KpnI restriction sites: 5′-CGGAGGTACCTCAAAAAATAAATCCCTTGC-3′. The CCR gene digested with XbaI and KpnI was cloned into plasmid pROKII to generate the plant overexpression vector, pROK-BpCCR. The 460-bp fragment of the CCR promoter was amplified from total DNA of Birch, for the construction of the RNAi vector. NcoI and AscI restriction sites were incorporated using CATGCCATGGCAAAAGTTGATAAAGAAATCG as the forward primer and TTGGCGCGCCGAGCAATAATTGAGATAG as the reverse primer for cloning the fragment in a sense orientation between the 35S promoter and the chalcone synthase (Chs) intron. XbaI and BamHI restriction sites were incorporated using GCTCTAGACAAAAGTTGATAAAGAAATCG as the forward and CGGGATCCGAGCAATAATTGAGATAG as the reverse primers to clone the fragment in an antisense orientation. Fragments obtained by PCR were restriction digested to produce the cohesive terminal ends, and then cloned in the sense and antisense orientations with introns harbored in between. Fragments were cloned into the RNAi plasmid vector, pFGC5941 (GenBank number. AY310901; Arabidopsis Biological Resource Center (ABRC) stock numbers CD3-447) [10], to make the plant RNAi vector, pFGC-BpCCR. The overexpression construct, the RNAi construct and the pBI121 vector were transferred into the A. tumefaciens strain EHA105 by electroporation.
Transformation of Birch seedlings
According to the optimization system which has been confirmed by our previous experiments, the monoclone of A. tumefacien harboring pBI121, pROKII-GFP, pROK-BpCCR and pFGC-BpCCR vector were respectively, inoculated in 10 ml of Luria-Bertani medium (LB) (10 g/l bacto–tryptone, 10 g/l NaCl, 5 g/l yeast extract) with antibiotics (50 μg/ml kanamycin) at 28°C for 18–24 h. Then, 1.5 ml of saturated culture was diluted with 50 ml of fresh LB medium and was grown until the OD 600 reached ~0.5. Bacterial cells were harvested by centrifugation at 4,000 rpm for 10 min. After centrifugation, the pellet of bacteria cells was resuspended into 50 ml of 1/2MS medium (contains 2.5% sucrose) at a final OD 600 of 0.8. Birch seedlings (4 week-old) growing in WPM medium (containing 2% sucrose, 0.6% agar and 0.2 mg/l NAA) were rinsed with distilled water before co-cultivation.
In clean conical flasks, three seedlings of Birch with 4–5 true leaves were immersed in 50 ml of co-cultivation medium (1/2MS, acetosyringone (As) [100 μmol l−1] with recombinant A. tumefaciens cells at a density of OD 600 = 0.8). Co-cultivation was carried out with 60 rpm at 25°C for 48, 72 and 96 h, and were harvested for GUS staining, GPF signal detection and real-time RT-PCR analysis.
Histochemical analysis of GUS
The Birch germinated seeds, seedlings and mature leaves infected following the above A. tumefaciens-mediated transient expression procedure were incubated at 37°C for 16–24 h with GUS staining solution (50 mmol l−1 Na3PO4 buffer [pH, 7.0], 10 mmol l−1 Na2EDTA [pH, 8. 0], 0. 5 mmol l−1K3 [Fe(CN)6], 0.5 mmol l−1 K4[Fe(CN)6], 0. 1% TritonX-100, 0. 8 g l−1X-Gluc), and then dehydrated by 70% (v/v) ethanol and 30% acetic acid. The GUS-stained seedlings were then imaged by a CanoScan LIDE 200 Scanner.
Imaging of GFP
The transformed leaves were placed on a glass microscope slide, dropped a drop of water and then covered with a coverslip. The GFP fluorescence was analyzed using Docuval MIC00261 (Zeiss, Jena, Germany).
Expression detection of the CCR gene by RT-PCR
To determine the effects of Agrobacterium-mediated transformation of pROK-BpCCR and pFGC-BpCCR in Birch, total RNA was isolated using the CTAB method, and was treated with DNaseI (RNase-free). The cDNA for real-time PCR analysis was generated by reverse transcription of 1 μg of total RNA in a reaction volume of 10 μl using PrimeScript® RT reagent Kit (Takara), and was then diluted to 100 μl for the PCR reaction. CCR transcript levels were monitored through RT-PCR using GAGGCAGCGGCTGCATTG as the forward and CGTAGTCATGGCCATCATCG as the reverse primers, and the α-tubulin gene (GenBank number: FG067376) was employed as an internal control.
RT-PCR reactions were carried out in 20 μl reaction volumes consisting of gene-specific primers and Power SYBR Green PCR master mix (Toyobo Co. Ltd, Osaka, Japan). The RT-PCR results were calculated using the 2−ΔΔCT method described by Kenneth [11].
Results and discussion
Infiltration optimization
We have optimized the transient expression system in Birch in previous study. Our study found that the factors, including As concentration, co-cultivation time and Agrobacterium cell density, all significantly influenced the efficiency of transient expression. The optimal conditions for Birch transient expression are 100 μM of AS, 0.8 OD 600 of Agrobacterium cells, and 3 days of co-cultivation.
Transient expression of GUS
The efficiency and versatility of the Agrobacterium-mediated transformation technique in some plant species prompted us to test the possibility to adapt a similar approach to Birch. When germinated seeds, seedlings and mature leaves of Birch were co-cultivated with A. tumefaciens carrying the pBI121 for 48 h, GUS activities in all the samples were detected (Fig. 1), which indicated that the GUS gene had been transiently expressed in the germinated seeds, whole Birch seedlings and mature leaves, giving the evidence that this transient expression system works well in Birch.
Transient expression of GFP
The transient expression in Birch was determined by visualization of GFP. The Birch leaves were transformed with 35S::GFP, and the GFP fluorescence was visualized in these leaves (Fig. 2). Confocal laser scanning revealed that the GFP signals were detected in the cells of leaves, suggesting that the GFP gene had been expressed in the leaves. These results also confirmed that this transient expression system works well in Birch.
Overexpression analysis of CCR
To analyze the efficiency of overexpression of CCR in Birch using this transient expression system, the CCR transcript levels of the whole Birch seedling transformed with the Agrobacterium harboring BpCCR, co-cultivated for 48 and 96 h, were analyzed using real-time RT-PCR. Young seedlings were purposely chosen to reduce time and space requirements, compared with the mature plants, and to avoid the problem of leaves with different ages from the same mature plant having variable transformation efficiencies [4].
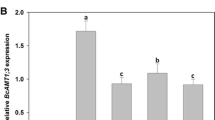
CCR expression level in transformed seedlings kept increased during the co-cultivation periods from 48 to 96 h, and reached its expression level peak at 72 h before dropping (Fig. 3). The average expression level of CCR was more than threefold than that of the control, and the highest overexpression level was over fourfold that of the control. At the same time, the mean CT value of CCR was 20.67, and that of the a-tubulin gene (the internal control) was 19.69, indicating that the endogenous level of the CCR transcripts is similar with that of the internal control a-tubulin gene. Therefore, the expression of exogenous CCR was highly improved by transient expression. These results demonstrated that this transient expression system worked well in the study of gene regulation in Birch seedlings.
The efficiencies of Agrobacterium-based transient transformation were variable from seedling to seedling, which was presumably due to an uneven distribution of Agrobacterium cells during co-cultivation and the physiological states are different among the different seedlings. Increasing the number of seedlings in an assay may allow an overall constant output to be obtained. If possible, gentle shaking of the culture medium during co-cultivation may partly help to reduce the variation of transformation efficiency between seedlings [12]. In addition to the above strategy, all the factors in our assay were kept as equal as possible and several replicates were performed, which could decrease the variation.
RNAi silencing analysis of CCR
A RNAi construct was generated and introduced into Birch seedlings by Agrobacterium-mediated transient transformation procedure, which silenced the CCR target gene. Real-time RT-PCR was used to determine the suppression of the CCR gene in the Birch seedlings. The CCR transcript was highly reduced compared with the control did (Fig. 3) and continual reduction of the CCR transcripts was observed transient transformation from 48 to 96 h.
Compared with the control samples, the transcript levels of CCR reduced above 7% during the transient transformation from 48 to 96 h, respectively, (Fig. 3). This result was consistent with previous findings in Nicotiana benthamiana, where the production of siRNAs for the target gene in the infiltrated zones started as early as 48 h after transformation, and reached a peak abundance by 120 h [13]. Our results demonstrated that the pFGC-BpCCR construct could repress the expression of CCR; therefore, this construct can be used to silence the CCR gene by stable transformation.
Transient gene expression, combined with other new technologies such as gene shuffling, VIGS and RNAi, is beginning to play an increasingly important role in plant functional genomics [14]. Gene expression had been repressed in some plants by RNAi-mediated transient silencing assay [3, 11, 15, 16]. However, to the best of our knowledge, no previous report on the use of RNAi in Birch is present in the literature.
In the present study, promoter fragments of the CCR gene were used to generate a hpRNA binary vector for the promoter region RNAi study in Birch. The construct was designed to produce hpRNA. The transcript of sense and antisense promoter fragments form complementary base pairs and intron Chs form the loop, i.e., forming a hair pin type of structure (hpRNA), with the transcripts repressed by methylation in the promoter region. Smith et al. [17] documented that this kind of RNAi vector was the most efficient for silencing the function of a particular gene. Here, we observed the significant down-regulation in the CCR transcript level in Birch seedlings (Fig. 3), which suggested that transient silencing in Birch by RNAi is effective, even in whole seedlings.
To date, the determination of gene function in the process of wood formation still largely relies on classical model systems that do not necessarily produce organized cellular files of woody tissue and require long periods of time. The Agrobacterium-mediated transient transformation procedure is becoming standard for the functional analysis of genes involved in the wood formation process, working directly in the tissue of interest and within a reasonable timeframe [2]. The procedure present in this study may provide a powerful approach that enables large-scale quantitative functional assays of candidate genes involved in the formation of wood in Birch species.
While the assay offers a number of advantages, it also comes with a few limitations that may reduce its usefulness in certain situations. For example, the ectopic protein expression ceases after 3 days. The level of transgene expression usually peaks at 60–72 h post-infiltration and declines rapidly thereafter. Besides the necrosis during co-cultivation, the expression was transient because the bacterial strain/host plant combination was inappropriate and TDNA transfer was suboptimal, post-transcriptional gene silencing (PTGS) was proposed as another limiting factor [18]. In addition, the applicability of the transient system is limited to those species that are amenable to delivery and expression of T-DNA constructs by Agrobacterium [19].
Conclusion
This study presents an easy, efficient, and economical method for transient gene expression or gene silencing approached in Birch seedlings based on A. tumefaciens co-cultivation. This method involves minimal hands-on manipulation and has no need for specialized devices, thus offering the potential of automation and large-scale quantitative analyses of gene function. The CCR was first introduced into Birch seedlings by a transient expression system. Transient expression of CCR transcripts increased in overexpressed plants and decreased in repressed plant after transformation for 48, 72 and 96 h. We demonstrated the application of this method for the expression of different constructs in Birch for functional analyses, and for the testing of constructs. These results confirmed that transient genetic transformation can be used to study gene regulation in Birch species and can provide a basic technique to test the construct quickly prior to further stable transformations in these plants.
References
Koiwa H, Bressan RA, Hasegawa PM (2006) Identification of plant stress-responsive determinants in Arabidopsis by large-scale forward genetic screens. J Exp Bot 57(5):1119–1128
Spokevicius AV, Van Beveren K, Leitch MA, Bossinger G (2005) Agrobacterium-mediated in vitro transformation of wood-producing stem segments in eucalypts. Plant Cell Rep 23(9):617–624
Bhaskar PB, Venkateshwaran M, Wu L, Ané JM, Jiang J (2009) Agrobacterium-mediated transient gene expression and silencing: a rapid tool for functional gene assay in potato. PLoS ONE 4(6):e5812
Li JF, Park E, von Arnim AG, Nebenführ A (2009) The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Methods 5:6
Aronen T, Hohtola A, Laukkanen H, Häggman H (1995) Seasonal changes in the transient expression of a 35S CaMV-GUS gene construct introduced into Scots pine buds. Tree Physiol 15(1):65–70
Ellis DD, McCabe D, Russell D, Martinell B, McCown BH (1991) Expression of inducible angiosperm promoters in a gymnosperm, Picea glauca (white spruce). Plant Mol Biol 17(1):19–27
Qiao J, Ishihara Y, Kuroda H, Sakai F, Sakai H, Komano T (1997) Transient expression of goat growth hormone gene in poplar (Populus alba L.) protoplasts: a quick method for detection of foreign gene expression in mRNA level. Biosci Biotechnol Biochem 61(9):1580–1581
Rueda-López M, Crespillo R, Cánovas FM, Avila C (2008) Differential regulation of two glutamine synthetase genes by a single Dof transcription factor. Plant J 56(1):73–85
Wang C, Wang YC, Diao GP, Jiang J, Yang CP (2009) Isolation and characterization of expressed sequence tags (ESTs) from cambium tissue of Birch (Betula platyphylla Suk). Plant Mol Biol Rep 28(3):438–449
Kerschen A, Napoli CA, Jorgensen RA, Müller AE (2004) Effectiveness of RNA interference in transgenic plants. FEBS Lett 566(1–3):223–228
Kenneth JL, Thomas DS (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆C T method. Methods 25(4):402–408
Li JF, Nebenführ A (2010) FAST technique for Agrobacterium-mediated transient gene expression in seedlings of Arabidopsis and other plant species. Cold Spring Harb Protoc 2010(5):pdb.prot5428
Koscianska E, Kalantidis K, Wypijewski K, Sadowski J, Tabler M (2005) Analysis of RNA silencing in agroinfiltrated leaves of Nicotiana benthamiana and Nicotiana tabacum. Plant Mol Biol 59:647–661
Wang HZ, Chen YP, Chen PD (2007) Plant transient expression system in functional genomics. Sheng Wu Gong Cheng Xue Bao 23(3):367–374
Mohanpuria P, Rana NK, Yadav SK (2008) Transient RNAi based gene silencing of glutathione synthetase reduces glutathione content in Camellia sinensis (L.) O. Kuntze somatic embryos. Biol Plant 52(2):381–384
Douchkov D, Nowara D, Zierold U, Schweizer P (2005) A high-throughput gene-silencing system for the functional assessment of defense-related genes in barley epidermal cells. Mol Plant Microbe Interact 18(8):755–761
Smith NA, Singh SP, Wang MB, Stoutjesdijk PA, Green AG, Waterhouse PM (2000) Total silencing by intron spliced hairpin RNAs. Nature 407(6802):319–320
Voinnet O, Rivas S, Mestre P, Baulcombe D (2003) An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 33(5):949–956
Johansen LK, Carrington JC (2001) Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol 126(3):930–938
Acknowledgments
This work has been supported by The National HighTechnology Research and Development Program(“863” Program) of China (2011AA100202), National Natural Science Foundation of China (No. 31070596) and the Fundamental Research Funds for the Central Universities (DL11EA02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, Y. & Wang, C. Gene overexpression and gene silencing in Birch using an Agrobacterium-mediated transient expression system. Mol Biol Rep 39, 5537–5541 (2012). https://doi.org/10.1007/s11033-011-1357-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1357-2