Abstract
Glioblastoma multiforme (GBM), the most common brain tumor in adults, is neurologically destructive and has a dismal response to virtually all therapeutic modalities. One phenomenon that can contribute to this complexity is the presence of a relatively small subset of glioma stem cells (GSCs) within the tumor and the activation of pathways that control cellular differentiation. The Notch signaling pathway, which is responsible for maintaining a balance between cell proliferation and apoptosis, is believed to be deregulated in cancer stem cells (CSCs), leading to tumor growth through the generation or expansion of CSCs. In this study, Notch-1 small interfering RNA (siRNA) was used to silence Notch-1 gene expression in GSCs. An MTT assay demonstrated inhibitory effects on the proliferation of GSCs in vitro. Real-time PCR showed that Notch-1 expression levels were markedly decreased in GSCs transfected with Notch-1 siRNA in vitro. Notch-1 silenced GSCs engrafted on Balb/c nude mice showed a significantly greater reduction in oncogenicity than the control group (P < 0.05). Furthermore, direct intratumoral injections of Notch-1-siRNA/PEI significantly delayed the growth of pre-established tumors in nude mice (P < 0.05). These results suggest that siRNA-mediated silencing of the Notch-1 gene may represent a novel target for gene therapy of GBM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most common and primary malignant brain tumors in humans [1, 2]. Glioblastoma multiforme (GBM) is the highest grade glioma and is the most aggressive form of astrocytoma with the poorest prognosis in adults. Recent studies have shown that there are a group of cancer stem cells (CSCs) that constitute a minor fraction of the tumor mass, but may have an essential role in tumor formation and maintenance [3]. Although the existence of glioma stem cells (GSCs) remains controversial, accumulating reports suggest that gliomas contain CSCs and that these cells may contribute to therapeutic resistance and tumor angiogenesis [4, 5]. It has recently been reported that common chemotherapeutic drugs and traditional radiation therapy, predominantly target the CD133-negative population, sparring the CD133-positive population [6, 7]. Thus, conventional therapies appear to effectively remove only the better-differentiated cells, while leaving many GSCs, which may be responsible for tumor recurrence. Significant efforts have been undertaken to identify potential targets in GSCs that promote tumor maintenance and might be amenable to disruption [8].
These CD133 positive and side population cells possess some intrinsic stem cell properties as they generate differentiated tumor cells in vivo, can be further transplanted, and preferentially express some ‘‘stemness’’ genes, including Notch-1 [9]. Notch signaling is involved in cell proliferation and death which affects the development and function of many organs. Recently, the Notch signaling pathway has been shown to control stem cell self-renewal and multi-potency. There is strong evidence that Notch-1 is involved in the carcinogenesis of breast cancer [10]. In addition, Notch-1 maintains the malignant phenotype of transformed cells [11], and overexpression of Notch induces mammary tumors in mice [12].
The presence of Notch-1 in both glioma cell lines and human gliomas is critical for glioma cell survival and proliferation [13]. Although gliomas are thought to develop from GSCs, the significance of Notch-1 in GSCs is scarcely explored. In this article, we focus on the potential therapeutic role of Notch-1 in GSCs. First we cultured and identified GSCs arising from human glioblastoma cell line TJ905, and silenced the Notch-1 gene in these GSCs. An MTT assay was used to evaluate the effects of Notch-1 siRNA-mediated interference on the proliferation of GSCs in vitro. Then the oncogenicity of Notch-1-siRNA-transfected and nontransfected GSCs on nude mice were compared. Furthermore, with a polyethyleneimine (PEI) based gene-delivery system, we attempted to deliver Notch-1-siRNA directly into the GSCs engrafted tumors in order to induce a therapeutic effect in nude mice.
Materials and methods
TJ905 GSC spheres culture
Human glioblastoma multiforme cell line TJ905 (Tianjin Neurological Institute, CHN) was assayed for its ability to form glioma stem spheres by using the same methods as described previously [14]. Briefly, cells were cultured in an incubator under 37°C, 5% CO2 and saturated humidity. Cells was digested and washed twice with serum-free Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham’s medium (DMEM/F12) (Grand Island, NY, USA). Then cells were placed into serum-free medium, which contained 20 ng/ml EGF, 20 ng/ml bFGF, 10 ng/ml LIF (Rocky Hill, NJ, USA) and B-27 (×1) (Grand Island, NY, USA).
Immunofluorescence staining for identification of GSC spheres
TJ905-derived cell spheres were plated on poly-l-ornithine-coated glass coverslips. After drying at 37°C, the slides were washed thrice with phosphate buffered saline (PBS). Then the slides were fixed with paraformaldehyde for 30 min, and washed with PBS. After blocking with 5% goat/rabbit serum at 37°C for 30 min, goat anti-human CD133 (1:200) (SC-23797 Santa Cruz, CA, USA) was added, and slides were incubated in a wet chamber at 4°C overnight. Coverslips were then incubated with donkey anti-goat IgG–TRITC (SC-2094) at 37°C for 30 min. Nuclei were counterstained with DAPI (Sigma, UK). As a negative control, PBS was added instead of primary antibody. Slides were visualized under a fluorescence microscope (BX51 Olympus Co., Tokyo, Japan).
siRNA/polyethylenimine (PEI) preparation
The branched form of polyethylenimine (PEI, 25 kDa, Sigma -Aldrich) was prepared at a concentration of 0.1 mg/ml in 10 mM pH 7.2 sterile HEPES buffer. The siRNA was added to the PEI solution slowly while stirring and incubated at room temperature for 30 min to allow complex formation.
Cell transfection
Bioneer (Daejeon, Korea) synthesized the siRNA duplexes. Notch-1 siRNA sequences were as follows: r(GCACGCGGAUUAAUUUGCA)d(TT), r(UGCAAAUUAAUCCGCGUGC)d(TT). AccuTarget™ Control siRNAs (nonspecific siRNA) were also used in this study: r(CCUACGCCACCAAUUUCGU)d(TT), r(ACGAAAUUGGUGGCGUAGG)d(TT). GSCs were collected, placed into serum-free medium (containing EGF, bFGF, LIF, and B-27) and gently resuspended. Cells were plated in 12-well tissue culture plates at a density of 1 × 105 per well. Just prior to transfection, the culture medium was removed and replaced with OPTI-MEM I (Gibco, Carlsbad, CA). PEI/siRNA complexes were used for transfection. Then OPTI-MEM I was replaced with serum-free medium after 24 h. The final siRNA transfection concentration was 200 nM and the N:P ratio was 10:1 (the molar ratio of nitrogens from the PEI to the ratio of phosphates from the siRNA).
RNA extraction and real-time RT-PCR
TJ905 GSCs were collected at 48 h after transfection, and total RNA was isolated by the E.Z.N.A.™ Total RNA kit (Omega, Norcross, GA). 1 μg of total RNA from each sample was subjected to first-strand cDNA synthesis using PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara, Dalian, CHN). Reverse transcription reactions were done at 42°C for 1 h, followed by 72°C for 5 min. Real-time PCR was conducted using the ABI PRISM 7300 Sequence Detection System (Applied Biosystems) according to the following thermal cycle protocol: 94°C for 10 min, followed by 40 cycles of 94°C for 15 s and 60°C for 1 min. Bioneer provided the primers (P320437, Daejeon, Korea) used for the amplification of human Notch-1. The primers for β-actin, used as positive control, were as follows: Forward primer:5′CTGGAACGGTGAAGGTGACA3′, reverse primer:5′AAGGGACTTCCTGTAACAATGCA 3′.
Western blot analysis
TJ905 GSCs were collected at 72 h after transfection and lysed in lysis buffer. Protein concentrations were determined with BCA Protein Assay reagent (Pierce, Rockford, IL). Equal amounts of protein (40 mg per lane) were loaded. Samples were separated onto denaturing sodium dodecyl sulfate–10% polyacrylamide gels and transferred electrophoretically onto a nitrocellulose membrane. Membranes were blocked overnight at 4°C with blocking buffer [0.1 M Tris (pH = 7.5), 0.9% NaCl and 0.05% Tween-20 (TBST) containing 5% nonfat milk powder], and then incubated with rabbit anti-human Notch-1 antibody (SRP00382a, Saierbio, Tianjing, China) at a 1:500 dilution for 1 h at room temperature. After washing of the membranes, Notch-1 was detected by using Amersham ECL™ Western Blotting Detection Reagents (GE Healthcare Life Sciences).
MTT assay
Notch-1 siRNA-transfected, nonspecific siRNAs-transfected and non-transfected GCS suspensions were planted at a density of 2,000 cells/well in 96-well plates. 25 μl MTT (5 mg/ml, Omega) was added to each well at 24, 48, 72, and 96 h after transfection. After an additional 4 h incubation, the media was removed, 150 μl of DMSO (Sigma-Aldrich) was added per well for color development, the plate was mixed thoroughly for 10 min and then measured on a microplate reader at 490 nm.
Animal model
For the oncogenicity experiment, 33 four-week-old male Balb/c nude mice (Slaccas, Shanghai, China) weighing 18–22 g were randomly divided into three groups and injected subcutaneously with 1 × 106 GSCs, nonspecific siRNA-transfected GCSs or Notch-1 siRNA-transfected GSCs per mouse. One mouse from each group was sacrificed 2 weeks after transplant for histological examination.
For the tumor challenge experiment, 30 four-week-old male Balb/c nude mice were injected subcutaneously with 2 × 105 GSCs. A week after transplant, when the tumors were palpable, the mice were randomly divided into three groups. Group 1 mice were used as untreated controls that received twice weekly intratumoral injections of PEI; Group 2 mice received intratumoral injections of nonspecific siRNA/PEI; Group 3 mice received intratumoral injections of Notch-1 siRNA/PEI. Each mouse was treated with intratumoral injections of 50 μl. The final siRNA concentration was 10 μM and the N:P ratio was 10:1.
Animal observations and tumor measurements
Animals were observed daily for evaluation of tumor growth. Tumor size was measured by a caliper in two dimensions, and the volume was calculated by the formula “a × b 2/2”, where ‘a’ is the length and ‘b’ is the width of the tumor diameter. Experiments were carried out under specific pathogen-free conditions. All animals were maintained and treated in accordance with the guidelines provided by the local Experimental Ethics Committee. Mice were sacrificed at 5 weeks after inoculation for ethics reason. Tumor masses were removed within 10 min and fixed with 4% w/v formaldehyde for 24 h, dehydrated in an ascending series of ethanol solutions, cleared with toluene, and embedded in paraffin wax.
Hematoxylin and eosin staining
Paraffin-embedded specimens were cut into 4 μm sections, fixed for 24 h, and immersed in 100% ethanol for 2 min, 95% ethanol for 1 min, and 70% ethanol for 1 min, followed by a 1 min rinse in deionized water. Specimens were then placed in Harris acidified hematoxylin (Anatech, Ltd.) for 2 min, followed by a brief rinse in water, immersion in acidic alcohol for 1 min, and a final rinse in water. Slides were placed in 70% ethanol for 1 min and in eosin solution (Anatech, Ltd.) for 1.5 min, followed by serial rinsing with 95% ethanol (1 min) and 100% ethanol (3 min). Slides were mounted with Permount (BIOS, Beijing, China), and coverslips were applied.
Immunochemistry
4 μm sections were baked at 65°C for 30 min, and then deparaffinized with xylene and rehydrated. Sections were submerged into EDTA (PH = 8.0), autoclaved for antigen retrieval, and treated with 3% hydrogen peroxide, followed by incubation with 1% FBS. Notch-1 antibody (1:100 dilutions) was added and incubated overnight at 4°C. For negative controls, PBS replaced the primary antibody. Horseradish peroxidase (HRP) labeled secondary antibody in the MaxVision™ HRP-Polymer anti-mouse/rabbit IHC kit (KIT-5930 Maixin Biol, Fu Zhou, China) was applied and incubated for 30 min, followed by 5 min incubation with DAB, provided in the kit for color development. The sections were finally counterstained with hematoxylin and mounted. Results were visualized and photographed under a light microscope.
Statistical analysis
All data are presented as means ±SD. The statistical significance of differences was determined by Student’s two-tailed t-test in two groups and one-way ANOVA in multiple groups. P < 0.05 was considered statistically significant. All data were analyzed with SPSS statistical software for Windows 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Tumor spheres derived from TJ905 cells in vitro
TJ905 cells were first cultured as monolayers attached to the bottom of flasks. Small spheres comprised of about four to ten cells were observed 3 days after the medium was switched to stem cell medium. After 5 days of culture, large numbers of spherical tumor spheres were found (Fig. 1a, b). The diameter of the tumor sphere increased 5–10-fold within 2 weeks because of continuing proliferation. Tumor neurospheres could be passaged multiple times by mechanical dissociation or digestion and reseeding in fresh medium every 1–2 weeks and could be maintained for 25 weeks or more.
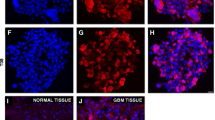
Neurosphere-like tumor spheroids derived from TJ-905 expressing CD133 and have ability of oncogenicity. TJ905 cells switched into SFM supplemented with EGF, bFGF and LIF, a large numbers of tumor spheres were found after 7 days ×40 (a) and ×400 (b). Tumor spheres were CD133 positive (c). Tumor spheres could form tumor mass when xenograft on nude mice (d) and show identical pathological characteristics to Tj905 transplanted tumor (e)
Tumor sphere cells are CD133 positive
Immunofluorescent staining was performed to verify whether the tumor spheres could express the tumor stem cell markers. As shown in Fig. 1c, the cells of the tumor spheres were positive for CD133 as viewed under a fluorescent microscope.
Tumor spheres show ability of oncogenicity
TJ905 derived glioma spheres could form a tumor mass when xenografted onto nude mice (Fig. 1d) and showed identical pathological characteristics to TJ905 transplanted tumors (Fig. 1e).
siRNA could effectively down-regulate Notch-1 expression in GSCs
We examined the transfection efficiency of the siRNA constructs by real-time RT-PCR. The nonspecific siRNA-transfected and non-transfected cells were used as negative and blank controls, respectively. In our experiment, the mRNA level of Notch-1 in Notch-1 siRNA-transfected, nonspecific siRNAs-transfected and non-transfected GSCs were 0.25 ± 0.05, 1.00 ± 0.05, and 1.03 ± 0.08, respectively (Fig. 2a). The Notch-1 siRNA was able to induce Notch-1 gene silencing when transiently transfected in GSCs. The levels of Notch-1 mRNA expression showed a remarkable decrease in the Notch-1 siRNA-transfected group (P < 0.05). Western blot analysis verified that protein levels of Notch-1 were also greatly reduced in the Notch-1 siRNA-transfected group as compared to the nonspecific siRNA-transfected and the non-transfected groups (P < 0.05; Fig. 2b, c).
Effect of Notch-1 siRNA on the expression of the Notch-1. a Expression of Notch-1 mRNA detected by real-time PCR. Total RNA was extracted from GSCs and subjected to quantitative real-time PCR as described in methods. The amount of Notch-1 mRNA relative to β-actin is shown. Results were expressed relative to non-transfected GSCs that were arbitrarily assigned a value of 1.0. Results are an average of four independent experiments. C Non-transfected control group, NS Nonspecific siRNA-transfected group, S Notch-1 siRNA-transfected groups. *P < 0.001. b Immunoblots for Notch-1 and β-actin are shown. Each band was scanned and subjected to densitometry. c Intensities of Notch-1 protein relative to β-actin are shown, *P < 0.001. Each column consists of means ±SD
siRNA targeting Notch-1 inhibits GSCs proliferation
We evaluated the effects of the Notch-1 siRNA on GSC proliferation in vitro using the MTT assay. The Notch-1 siRNA-transfected group cell viability was significantly reduced as compared to the nonspecific siRNA-transfected and no siRNA-transfected groups (P < 0.05). PEI alone is nontoxic to cell proliferation versus PBS control (online resource 1). As such, the effects of Notch-1 siRNA on GSCs proliferation were specifically due to Notch-1 inhibition.
Notch-1 siRNA down-regulate GSCs oncogenicity
We next wanted to determine whether the results obtained above could be translated into effects in vivo. Results show that nude mice xenografted with GSC suspensions developed tumors with a significantly increased volume (approximate from the second week) as compared to mice that received the Notch-1 siRNA-transfected GSC (P < 0.05) (online resource 2).
Notch-1 siRNA/PEI inhibits the tumor growth
Taking advantage of the facilitation of cellular uptake by the PEI, we then explored whether the pre-established xenografts would respond to direct exposure of Notch-1 siRNA/PEI complex in vivo. 3 weeks after tumor cell inoculation four intratumoral injections of the Notch-1 siRNA/PEI complex were given to mice. Our results showed that the tumor growth was significantly inhibited following these injections (Fig. 3a). Immunochemistry showed a comparable suppression of Notch-1 protein expression in the Notch-1 siRNA/PEI group (Fig. 3b).
Notch-1 siRNA/PEI inhibits the tumor growth in vivo. a Notch-1 siRNA/PEI causes a significant reduction in tumor volume as compared to PEI control groups (*P < 0.05). b siRNA-targeting Notch-1 leads to a decrease in Notch-1 protein by immunohistology. Representative pictures with nuclear staining are shown
Discussion
Glioblastoma multiforme, the most common primary brain tumor in adults, is neurologically destructive and has dismal responses to virtually all therapeutic modalities. It is thought that 18.5% of all CNS tumor cases and 81% of all malignant tumors are GBMs [1]. Standard therapies have had limited success in treating GBM patients. The median survival is approximately 14 months and less than 10% of these patients can survive over 5 years [15]. Since the prognosis of glioma does not meet expectations, many new therapies such as immune treatment, gene treatment and photodynamic therapy [16] have been proposed successively and a certain measure of progress has been made.
It remains a puzzle as to how the glioma cells can escape surgical removal and invade into distant brain tissues, causing patients to relapse [17]. One phenomenon that can contribute to this complexity is the presence of GSCs within the tumor. Several groups have identified unique populations within GBMs with CSC properties [18]. In this study, we isolated glioma spheres from glioblastoma cell line TJ905. These spheres exhibit stem cell properties, such as expression of CD133 and the capability of self-renewal. The spheres could induce tumor mass, which was morphologically identical to the parental tumor, but Jin et al. failed to demonstrate this point [14].
The cancer stem cell theory may alter the current paradigm in drug development. Eradication of gliomas may require the targeting and elimination of GSCs. Thus, we must devise strategies that can selectively kill these GSCs while sparing normal stem cells, such as those in the hippocampus. This represents a challenge because many pathways involved in self-renewal are shared by normal stem cells and CSCs. However, a variety of recent studies using animal models, which have targeted these pathways, including Notch-1 [11], indicate the feasibility of this approach. Human Notch genes encode transmembrane receptors that play a key role in a variety of cellular processes, including proliferation, differentiation, survival and apoptosis [19]. Notch ligands, receptors, and targets have been found in a wide range of neoplasms, including lung, breast, cervix, head/neck, renal, and pancreas carcinoma, neuroblastoma, myeloma, melanoma, choroid plexus tumor, medulloblastoma and glioma [20]. In many of these tumor types, it was shown that increased Notch activity promotes tumor growth, and blocking the Notch pathway inhibits proliferation and survival. Notch activation is initiated by its cleavage by γ- secretase to generate the Notch intracellular domain (NICD). The NICD translocates to the nucleus and binds C promoter Binding Factor 1, converting it from a transcriptional repressor to a transcriptional activator [21]. γ- secretase inhibitors (GSIs) were recently shown to have activity against breast cancers that over express Notch-1 [11, 22]. GSIs reduced neurosphere growth and clonogenicity in vitro, effectively blocking tumor growth and significantly prolonging survival in vivo [23]. Inhibition of Notch signaling is therefore a promising therapeutic avenue for a wide range of cancers. Notch activation contributes to glial cells transformation and to glioma growth, survival or both. Notch-1 was over expressed in a majority of the GBM cell lines and primary GBM samples. Furthermore inhibition of Notch-1 signaling leads to suppression of the growth of glioma cells [24]. Notch-1 may also play a role in GSC biology. In our study, silencing of Notch-1 expression could dramatically inhibited the proliferation of GSCs in vitro. Furthermore, Notch-1-silenced GSCs, which were engrafted onto Balb/c nude mice showed a significant reduction in oncogenicity as compared to the control group. This indicates that Notch-1 may contribute to GSC proliferation and oncogenicity.
Gastrointestinal toxicity has been a major concern when using GSIs, with the inhibition of Notch inducing goblet cell differentiation in intestinal transit amplifying cells, leading to severe diarrhea [25]. The use of siRNA as a therapeutic intervention for cancer is an active area of research [26]. With the aid of various delivery systems, siRNAs were intratumorally or intravenously administered to treat various cancers. Important research has been moving toward the development of polycation-based gene-delivery systems, such as PEI conjugates designed to minimize nuclease degradation, protected RNA and decrease endosomal swelling and rupture before RNA breakdown. PEI complexed with siRNA has been used in the systemic delivery to glioblastoma xenografts without generating any measurable siRNA-mediated immune response [27]. In this article, direct intratumoral injections of a Notch-1-siRNA/PEI significantly diminished pre-established tumors in nude mice. The method of direct intratumoral injections of Notch-1-siRNA/PEI not only avoids the danger from other adopted virus, but also can obtain the same appreciable results. To the best of our knowledge, our study is the first trial of not only injecting Notch-1-siRNA intra-glioma in vivo, but also using PEI to transfect Notch-1 siRNA, providing another available transfection agent for introducing siRNA into glioma cells.
Cancer therapies frequently fail because they are directed toward the wrong cellular targets [28]. CD133-positive cells represent the cellular population that confers glioma radio resistance and could be the source of tumor recurrence after radiation. Fractionated irradiation may activate the Notch-1 developmental pathway, which may cause the numbers of breast cancer initiating cells to increase [29], offering a mechanism for accelerated repopulation during radiation therapy treatment gaps. This suggests that radiation activates the Notch-1 developmental pathway. Eradication of the stem cell component of a glioma also may be essential to achieve long-lasting remission and even a cure for glioma. Advances in our knowledge of the properties of stem cells have made specific targeting and eradication of GSCs a topic of considerable interest. In this article, we discuss the properties of GSCs, outline initial therapeutic strategies against them, and present challenges for the future.
In sum, siRNA-mediated Notch-1 inhibition can be applied to the understanding of glioma progression as well as the development of experimental therapeutic strategies. To maximize the efficacy of anti-GSCs siRNA agents, it will likely be necessary to give them in combination with traditional modalities. Glioblastoma patients would benefit from aggressive multimodality therapy.
References
Porter KR, McCarthy BJ, Freels S et al (2010) Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol 12:520–527
Kleihues P, Louis DN, Scheithauer BW et al (2002) The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61:215–225 discussion 26–29
Reya T, Morrison SJ, Clarke MF et al (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Bleau AM, Hambardzumyan D, Ozawa T et al (2009) PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell 4:226–235
Qiu B, Zhang D, Wang C et al (2011) IL-10 and TGF-beta2 are overexpressed in tumor spheres cultured from human gliomas. Mol Biol Rep 38(5):3585–3591
Bao S, Wu Q, McLendon RE et al (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760
Bar EE, Chaudhry A, Lin A et al (2007) Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells 25:2524–2533
Zhou BB, Zhang H, Damelin M et al (2009) Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov 8:806–823
Patrawala L, Calhoun T, Schneider-Broussard R et al (2005) Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res 65:6207–6219
Politi K, Feirt N, Kitajewski J (2004) Notch in mammary gland development and breast cancer. Semin Cancer Biol 14:341–347
Weijzen S, Rizzo P, Braid M et al (2002) Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med 8:979–986
Dievart A, Beaulieu N, Jolicoeur P (1999) Involvement of Notch1 in the development of mouse mammary tumors. Oncogene 18:5973–5981
Purow BW, Haque RM, Noel MW et al (2005) Expression of notch-1 and its ligands, delta-like-1 and jagged-1, is critical for glioma cell survival and proliferation. Cancer Res 65:2353–2363
Jin F, Zhao L, Zhao HY et al (2008) Paradoxical expression of anti-apoptotic and MRP genes on cancer stem-like cell isolated from TJ905 glioblastoma multiforme cell line. Cancer Invest 26:338–343
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Wang C, Cao S, Tie X et al (2011) Induction of cytotoxicity by photoexcitation of TiO(2) can prolong survival in glioma-bearing mice. Mol Biol Rep 38(1):523–530
Nakada M, Nakada S, Demuth T et al (2007) Molecular targets of glioma invasion. Cell Mol Life Sci 64:458–478
Singh SK, Hawkins C, Clarke ID et al (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776
Nickoloff BJ, Osborne BA, Miele L (2003) Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene 22:6598–6608
Lai EC (2002) Keeping a good pathway down: transcriptional repression of Notch pathway target genes by CSL proteins. EMBO Rep 3:840–845
Pece S, Serresi M, Santolini E et al (2004) Loss of negative regulation by numb over Notch is relevant to human breast carcinogenesis. J Cell Biol 167:215–221
Fan X, Khaki L, Zhu TS et al (2010) NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28:5–16
Kanamori M, Kawaguchi T, Nigro JM et al (2007) Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg 106:417–427
Searfoss GH, Jordan WH, Calligaro DO et al (2003) Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional gamma-secretase inhibitor. J Biol Chem 278:46107–46116
Merkerova M, Klamova H, Brdicka R et al (2007) Targeting of gene expression by siRNA in CML primary cells. Mol Biol Rep 34(1):27–33
Grzelinski M, Urban-Klein B, Martens T et al (2006) RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum Gene Ther 17:751–766
Biswal BK, Debata NB, Verma RS (2010) Development of a targeted siRNA delivery system using FOL–PEG–PEI conjugate. Mol Biol Rep 37:2919–2926
Phillips TM, McBride WH, Pajonk F (2006) The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 98:1777–1785
Acknowledgment
This study was supported by the Nature Science Foundation of Shandong Province, China (Y2008C58).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Wang, C., Meng, Q. et al. siRNA targeting Notch-1 decreases glioma stem cell proliferation and tumor growth. Mol Biol Rep 39, 2497–2503 (2012). https://doi.org/10.1007/s11033-011-1001-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1001-1