Abstract
It has been clear that both Oct4 and Nanog play essential roles in maintaining embryonic stem cells (ESCs) undifferentiation. However, the roles of Oct4 and Nanog in ESCs growth and apoptosis have been much less explored. In this study, we systematically examined the effects of Oct4 or Nanog knockdown on mouse ESCs (mESCs) growth and apoptosis as well as potential mechanisms. Our results show that Oct4 or Nanog knockdown induces growth arrest and apoptosis in mESCs, indicating that the two genes also play important roles in mESCs survival and growth. Moreover, upregulation in Trp53 and its downstream genes expression was detected in Oct4 or Nanog knockdown mESCs, suggesting a possible role of Trp53 in Oct4 or Nanog knockdown induced mESCs growth arrest and apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Embryonic stem cells (ESCs) are pluripotent cells derived from the inner cell mass (ICM) and are characterized by their unlimited capacity for self-renewal and contribution to all cell lineages [1].
The POU domain transcription factor Oct4 and the homeobox transcription factor Nanog are considered two crucial transcriptional factors in maintaining the undifferentiated state of ESCs [2–6]. Recent reports have showed that Oct4/Nanog target genes included not only those pluripotency or differentiation-related genes but also a large number of genes associated with cell proliferation, apoptosis and cell cycle control [7–9]. Moreover, a recent study has revealed that Oct4 was critical for survival of mouse ESCs (mESCs) through the Stat3/Survivin pathway [10]. Nanog was also reported to play a role in maintaining the proliferation rate in mESCs [11]. However, there has been no comprehensive study surrounding the effects of Oct4 or Nanog on cell growth and apoptosis in mESCs so far.
In this study, we knocked down Oct4 and Nanog in mESCs separately and systematically detected its effects on cell growth and apoptosis, including proliferation capability, cell cycle distribution, apoptosis and telomerase activity. In addition, we examined the expression of some important genes (e.g. Trp53) related to cell growth control and apoptosis. Our data not only deepen our understanding of the importance of Oct4 and Nanog on cell growth and apoptosis, but also provide clues of possible new pathways that might be involved in mESCs growth and apoptosis control.
Materials and methods
Cell culture
The mESCs line R1/E (SCRC-1036, ATCC) was maintained in a feeder-free culture system in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Logan, UT, USA) supplemented with 15% fetal bovine serum (FBS) (Invitrogen-Gibco, Grand Island, NY, USA), 2 mM l-glutamine (Invitrogen-Gibco), 0.1 mM non-essential amino acids (Invitrogen-Gibco), 0.1 mM beta-mercaptoethanol (Invitrogen, Carlsbad, CA, USA), and 1000 U/ml leukemia inhibitory factor (LIF) (Chemicon, Temecula, CA, USA). The cells were passaged every 3 to 4 days. Before cell passaging, dishes or plates were coated with 0.1% gelatin (Sigma-Aldrich, St. Louis, MO, USA) at room temperature for 1 h. Then gelatin was removed and cells were seeded into the gelatin-coated dishes or plates.
All cell cultures were maintained at 37°C with 5% CO2.
RNAi plasmid construction and transfection
RNAi plasmids were constructed by inserting three published short hairpin RNA (shRNA) sequences, including one control sequence and two sequences targeting the coding region of Oct4 and Nanog separately [12], to the Bgl II/Hind III sites of pSUPER.retro.puro (OligoEngine, Seattle, WA, USA).
One day before transfection, mESCs were seeded into 6-well plates at a density of 4.0 × 105 cells per well. The cells in each well were transfected with 4 μg of plasmid DNA using LipofectamineTM2000 (Invitrogen). Twenty-four hours after transfection, cells were cultured in medium containing puromycine (Sigma, final concentration 2 μg/ml) for 3 days to selectively remove most of the non-transfected ESCs and then were collected for detection.
RNA isolation, RT-PCR and real-time PCR analysis
Total RNA was extracted using Trizol reagent (Invitrogen) and treated with DNaseI (NEB, Ipswich, MA, USA) to remove genomic DNA and/or plasmid DNA contamination. cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (Fermentas, Burlington, Canada). Real-time PCR was performed using SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) with the iCycler System (Bio-Rad). The samples were run in duplicate, and the experiments were repeated at least three times. The mRNA levels were normalized against Gapdh levels and calculated using the cycle threshold (Ct) method. For RT-PCR analysis, the signals of amplified gene products were analyzed with the Gel-Pro analyzer (Media Cybernetics, USA) and normalized against Gapdh levels, and experiments were repeated at least three times. Details of primers used in RT-PCR and real-time PCR are listed in the Table 1.
Immunocytochemical and flow cytometry analysis of ESCs markers
For alkaline phosphatase (AKP) staining, cells were fixed with 4% paraformaldehyde (Sigma) for 15 min at room temperature, and AKP activity was detected using the Fast Red Substrate Pack (Invitrogen).
For detection of mESCs specific marker SSEA-1, cells were fixed with 4% paraformaldehyde (Sigma) for 15 min at room temperature and blocked with PBS containing 0.1% Triton X-100, 1% BSA and 10% normal donkey serum for 45 min at room temperature. After blocking, cells were incubated with diluted SSEA-1 primary antibody (1:10, MAB2155, R&D System, Minneapolis, MN, USA) overnight at 4°C followed by fluorescein isothiocyanate (FITC)-coupled anti-mouse IgM at room temperature in the dark for 1 h. Fluorescence data were collected using a FACScalibur (Becton–Dickinson, Mountain View, CA, USA). The results were expressed as the average ± standard deviation (SD) of three separate experiments and shown to be statistically significant by Student’s t test (P < 0.05).
Western blotting analysis
The cells were harvested, washed with pre-chilled PBS and lysed with RIPA buffer (50 mM Tris–Cl, pH7.2, 150 mM NaCl, 1% Triton X-100, 1% Sodium Deoxycholate, 1% SDS, 1× protease inhibitor cocktail) for 30 min on ice. The lysates were centrifuged for 15 min at top speed at 4°C and the supernatant was collected. After protein quantification by the BCA Assay Reagent (Pierce, Rockford, IL, USA), 30–50 μg of the total protein was separated by 10% SDS-PAGE and transferred to 0.45 μM polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). The membranes were incubated with blocking buffer (TBS containing 5% skimmed milk and 0.1% Tween-20) for 1 h at room temperature and followed by incubation with the following primary antibodies diluted in the same buffer: anti-Oct4 (1:1000, ab19857, Abcam, Cambridge, U.K.), anti-caspase 3 (1:500, #9661, Cell Signaling Technology, Danvers, MA, USA), anti-β-actin (1:1000, sc-47778, Santa Cruz, Santa Cruz, CA, USA). After incubation with the HRP-conjugated secondary antibody, proteins were detected using LumiGLO Peroxidase Chemiluminescent Substrate (KPL, Gaithersburg, MD, USA).
5-Bromo-2-deoxyuridine incorporation assay
5-Bromo-2-deoxyuridine (BrdU) (Sigma) was added to the medium to a final concentration of 50 μM for 4 h. Mouse anti-BrdU antibody (1:200, Sigma) was added and incubated for 1 h at room temperature. The binding of the primary antibody was detected by using FITC-conjugated secondary antibody (1:100, Invitrogen). The cell nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI, Chemicon). Cells were examined under an Olympus fluorescence microscope (IX71, Olympus, Tokyo, Japan). BrdU + and DAPI + cells were counted separately in each visual field, five or more fields were selected randomly for each group, and at least 60 cells were counted per visual field. The percentage of BrdU + cells was calculated according to this formula: BrdU + cells/DAPI + cells × 100%. The results were expressed as the average ± standard deviation (SD) of three separate experiments and shown to be statistically significant by Student’s t test (P < 0.05).
Cell cycle and apoptosis analysis
Cells were washed with pre-chilled PBS and then suspended in 70% ethanol overnight at −20°C. Before flow cytometry analysis, cells were washed with PBS twice and then stained with 50 μg/ml propidium iodide (PI) and 20 μg/ml RNase A at room temperature in the dark for 30 min. Fluorescence data were collected on using a FACScalibur (Becton–Dickinson) and the cell cycle parameters and the percentage of apoptotic cells (sub-G1 peak) were determined using the CellQuest software program (Becton–Dickinson).The results were expressed as the average ± standard deviation (SD) of three separate experiments and shown to be statistically significant by Student’s t test (P < 0.05).
Telomerase activity analysis
Telomerase activity was detected using a TRAPeze Telomerase detection kit (Chemicon) according to the manufacturer’s instructions. In brief, after addition of 10–20 μl telomerase assay lysis buffer (1 × CHAPS), the cells were lysed on ice. The lysate was incubated on ice for 30 min and then centrifuged at 12,000 rpm for 20 min at 4°C. The supernatant was collected and the protein concentration was determined by standard procedures (BCA protein assay). A volume of 0.33 μg protein equivalent was added to a 48 μl reaction solution consisting of TRAP buffer, dNTP Mix, TS primer, RP primer mix, and 2 U Taq polymerase. The PCR condition was 33 cycles of 94°C for 30 s and 59°C for 30 s. The PCR products were determined by electrophoresis in 12.5% non-denaturing polyacrylamide gel with silver staining.
At least three independent experiments showed consistent results.
Results
Oct4 or Nanog was effectively knocked down in mESCs
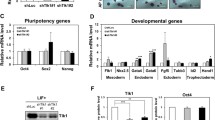
The Oct4 and Nanog expression levels were significantly decreased in Oct4 or Nanog knockdown groups (Fig. 1a, b). Besides, we detected an apparent differentiation in Oct4 or Nanog knockdown mESCs, as shown by cell morphology changes, weakened AKP staining (Fig. 1c) and decreased SSEA-1 expression (Fig. 1d). These results indicated that Oct4 or Nanog was effectively knocked down in mESCs.
Oct4 or Nanog was effectively knocked down in mESCs. a Real-time PCR analysis of Oct4, Nanog and Sox2 expression in Oct4 or Nanog knockdown mESCs and control mESCs. Oct4 or Nanog was knocked down effectively as shown by a significant reduction in their mRNA levels. b Western blotting analysis of Oct4 showed that Oct4 protein level decreased dramatically in Oct4 knockdown mESCs and significantly in Nanog knockdown mESCs. The results of c Alkaline phosphatase (AKP) staining and d SSEA-1 expression analysis indicated mESCs differentiation in Oct4 or Nanog knockdown group, confirming indirectly that Oct4 or Nanog was effectively knocked down in mESCs. (E.V. Empty vector control, N.C. sh Negative control shRNA, Oct4 sh Oct4 shRNA, Nanog sh Nanog shRNA)
Oct4 or Nanog knockdown induced cell cycle arrest and suppressed proliferation in mESCs
To evaluate the effects of Oct4 or Nanog knockdown on the growth capability of mESCs, we compared cell cycle distributions between Oct4 and Nanog knockdown cells and control cells. The percentage of cells in G0/G1 phase increased from ~30% (29.62% for empty vector control and 30.31% for negative shRNA control) to 39.21% (Oct4 knockdown group) and 32.80% (Nanog knockdown group), while the percentage of S phase cells decreased from ~40% (40.43% for empty vector control and 39.44% for negative shRNA control) to 31.87% (Oct4 knockdown group) and 37.54% (Nanog knockdown group) (Fig. 2a). This result indicated that Oct4 or Nanog knockdown induced cell cycle arrest (in G0/G1 phase) in mESCs, and Oct4 knockdown had a more significant effect than Nanog knockdown.
Oct4 or Nanog knockdown induced cell cycle arrest and suppressed proliferation in mESCs. a Cell cycle phase detection. The percentages of cells in G0/G1 phase significantly increased in Oct4 or Nanog knockdown group, and those in S phase significantly decreased. No difference was detected in the percentages of cells in G2/M phase between knockdown groups and control ones. b BrdU assay for cell proliferation. The BrdU signal was much weaker in knockdown groups than in control ones. c Cell counts in BrdU assay indicated that the percentages of BrdU + cells were significantly lower in knockdown groups (E.V. 54.85%, N.C. sh 61.04%, Oct4 sh 11.17%, Nanog sh 46.98%)
In addition, we investigated the effects of Oct4 or Nanog knockdown on mESCs proliferation using BrdU incorporation assay. We observed a much weaker fluorescence signal in knockdown groups than in control groups (Fig. 2b). Further cell counts indicated that the percentages of BrdU + cells were significantly lower in knockdown groups (11.17% for Oct4 knockdown group and 46.98% for Nanog knockdown group) than in control groups (54.85% for empty vector control and 61.04% for negative shRNA control) (Fig. 2c). This result demonstrated that Oct4 or Nanog knockdown suppressed mESCs proliferation, and Oct4 knockdown had a more significant effect than Nanog knockdown.
Oct4 or Nanog knockdown induced apoptosis in mESCs
We detected the effects of Oct4 or Nanog knockdown on mESCs apoptosis. Our results showed that the percentages of apoptotic cells in knockdown groups (6.02% for Oct4 knockdown group and 5.06% for Nanog knockdown group) were significantly higher than in control groups (2.94% for empty vector control and 2.87% for negative shRNA control) (Fig. 3a). In addition, Caspase-3 expression detection showed that the 17 kDa cleaved Caspase-3 increased obviously in the knockdown groups (Fig. 3b). Collectively, these results indicated that Oct4 or Nanog knockdown induced apoptosis in mESCs.
Oct4 or Nanog knockdown induced apoptosis in mESCs. a The percentage of apoptotic cells in Oct4 or Nanog knockdown group was significantly higher than in control groups (E.V. 2.94%, N.C. sh 2.87%, Oct4 sh 6.02%, Nanog sh 5.06%). b Western blotting analysis of Caspase-3 expression showed that the 17 kDa cleaved Caspase-3 expression level increased obviously in Oct4 or Nanog knockdown group
Oct4 or Nanog knockdown repressed telomerase activity in mESCs
We investigated the telomerase activity of mESCs after Oct4 or Nanog knockdown by TRAPeze telomerase assay. The result showed that the telomerase activity of Oct4 knockdown cells was much lower than the control cells, while the telomerase activity of Nanog knockdown cells only slightly decreased (Fig. 4).
Oct4 or Nanog knockdown repressed telomerase activity in mESCs. The frequency and intensity of PCR bands reflect the telomerase activity. As can be seen, the telomerase activity of Oct4 knockdown cells was much lower than the control cells, while the telomerase activity of Nanog knockdown cells only slightly decreased
Oct4 or Nanog knockdown upregulated the expression of several genes involved in cell growth control and apoptosis
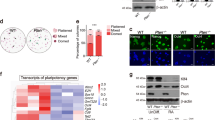
To explore potential pathways involved in the cell growth arrest and apoptosis processes induced by Oct4 or Nanog knockdown in mESCs, we examined the expression of several associated genes. Trp53 expression was upregulated by about one fold in Oct4 or Nanog knockdown groups compared to the control groups (Fig. 5). The detection on Trp53 downstream genes indicated that p21cip1 (also known as Cdkn1a), Bax and Gadd45a expression levels were also obviously upregulated, and Igf-bp3 expression was upregulated slightly, while Pcna expression showed no significant change (Fig. 5). These data suggest that the Trp53-mediated pathways might be involved in the cell growth arrest and apoptosis processes induced by Oct4 or Nanog knockdown in mESCs.
RT-PCR detection of the mRNA levels of several genes involved in cell growth control. a Gel map of the RT-PCR analysis. b Gray value analysis normalized against Gapdh. Trp53, p21cip1, Bax, Gadd45a expression were upregulated significantly, and Igf-bp3 expression was upregulated slightly, while Pcna expression showed no significant change
Discussion
Compared to the well-known roles of Oct4 and Nanog in maintaining the undifferentiated state of ESCs, the roles of Oct4 and Nanog in sustaining ESCs survival and growth seem to be ignored. So far, only limited knowledge has been obtained: Oct4 was critical for survival of mESCs through the Stat3/Survivin pathway [10], and Nanog was reported to play a role in maintaining the proliferation rate in mESCs [11]. In this study, we demonstrated that Oct4 and Nanog knockdown induced cell growth arrest and apoptosis in mESCs and Trp53-mediated pathways might be involved in these effects.
ESCs exhibit cell cycle properties entirely different from those of somatic cells, the G1 phase of both human ESCs (hESCs) and mESCs are highly abbreviated [13, 14]. Previous studies have shown that G1 elongation was likely to initiate early differentiation in hESCs [15] and upregulation of Nanog distinctly promoted the G1 to S transition in hESCs [16]. Cells with short G1 phase usually display strong proliferation capability and extension of the G1 phase is accompanied by a decline in cell proliferation capability [15]. In this study, our results demonstrated that Oct4 or Nanog knockdown repressed cell growth in mESCs as shown by an extended G0/G1 phase and inhibited cell proliferation in Oct4 or Nanog knockdown group. Moreover, Oct4 or Nanog knockdown induced apoptosis in mESCs.
The tumor suppressor Trp53 has been identified as a downstream target of Oct4 by chromatin immunoprecipitation analysis in mESCs [8] and is considered to play a central role in cell growth control and apoptosis [17]. Therefore, we examined the effect of Oct4 or Nanog knockdown on Trp53 expression. The results showed that Trp53 expression was upregulated in Oct4 or Nanog knockdown group, suggesting Trp53 might be involved in mESCs growth control and apoptosis.
Bearing this conjecture, we further detected the expression of several Trp53 downstream genes involved in cell cycle control or apoptosis pathways. Notably, a significant upregulation of p21cip1 expression was detected in Oct4 or Nanog knockdown group. The 21 kDa protein encoded by the p21cip1 gene suppresses cell growth mainly by inhibiting the G1 to S transition [18, 19] and DNA synthesis [20]. Therefore, the p21cip1 upregulation might be an important event involved in the G1 phase arrest and inhibited proliferation in Oct4 or Nanog knockdown group. Moreover, we detected an obvious Bax upregulation and a slight Igf-bp3 upregulation in Oct4 or Nanog knockdown group. The Bcl-2 associated X protein (Bax) and the insulin-like growth factor binding protein 3 (Igf-bp3) are two important modulators in the Trp53-mediated apoptosis pathways. Bax promotes cell apoptosis by competing with Bcl-2 [21], an anti-apoptosis factor, or cooperating with p53-upregulated modulator of apoptosis (PUMA) [22]. Igf-bp3 inhibits cell growth and induces apoptosis by binding with the insulin-like growth factor Igf-1 and blocking the Igf-1-involved mitogenic signal pathway [23, 24]. The upregulation of Bax and Igf-bp3 indicated that the Trp53-mediated apoptosis pathways might be involved in Oct4 or Nanog knockdown induced mESCs apoptosis.
The expression of Gadd45a, another target gene of Trp53, was also notably upregulated. Gadd45a is an important factor involved in cellular responses (such as cell cycle control and DNA repair, or apoptosis) to a variety of DNA damage. It appears to be an important component in the cellular defense network that is required for maintenance of genomic stability [25]. Hence, we conjecture that the upregulation of Gadd45a might reflect DNA damage events induced by Oct4 or Nanog knockdown in mESCs.
It is worth mentioning that Oct4 knockdown resulted in a more significant effect on mESCs growth than Nanog knockdown, suggesting that Oct4 might play a more important role in sustaining mESCs survival and growth.
In summary, our data demonstrated that Oct4 and Nanog knockdown induced cell growth arrest and apoptosis in ESCs, and the Trp53-mediated pathways might be involved in these effects.
References
Smith AG (2001) Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol 17:435–462
Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95:379–391
Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113:631–642
Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113:643–655
Hay DC, Sutherland L, Clark J, Burdon T (2004) Oct4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells 22:225–235
Hough SR, Clements I, Welch PJ, Wiederholt KA (2006) Differentiation of mouse embryonic stem cells after RNA interference-mediated silencing of Oct4 and Nanog. Stem Cells 24:1467–1475
Matoba R, Niwa H, Masui S, Ohtsuka S, Carter MG, Sharov AA, Ko MS (2006) Dissecting Oct3/4-regulated gene networks in embryonic stem cells by expression profiling. PLoS One 1:e26
Campbell PA, Perez-Iratxeta C, Andrade-Navarro MA, Rudnicki MA (2007) Oct4 targets regulatory nodes to modulate stem cell function. PLoS One 6:e553
Sharov AA, Masui S, Sharova LV, Piao Y, Aiba K, Matoba R, Xin L, Niwa H, Ko MS (2008) Identification of Pou5f1, Sox2, and Nanog downstream target genes with statistical confidence by applying a novel algorithm to time course microarray and genome-wide chromatin immunoprecipitation data. BMC Genomics 9:269
Guo Y, Mantel C, Hromas RA, Broxmeyer HE (2008) Oct-4 is critical for survival/antiapoptosis of murine embryonic stem cells subjected to stress: effects associated with stat3/survivin. Stem Cells 26:30–34
Liu N, Feng X, Fang Z, Ma F, Lu S, Lu M, Han Z (2008) Identification of genes regulated by Nanog which is involved in ES cells pluripotency and early differentiation. J Cell Biochem 104:2348–2362
Zhang JQ, Tam WL, Tong GQ, Wu Q, Chan HY, Soh BS, Lou Y, Yang J, Ma Y, Chai L, Ng HH, Lufkin T, Robson P, Lim B (2006) Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol 8:1114–1123
Becker KA, Ghule PN, Therrien JA, Lian JB, Stein JL, van Wijnen AJ, Stein GS (2006) Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol 209:883–893
Savatier P, Lapillonne H, Jirmanova L, Vitelli L, Samarut J (2002) Analysis of the cell cycle in mouse embryonic stem cells. Methods Mol Biol 185:27–33
Filipczyk AA, Laslett AL, Mummery C, Pera MF (2007) Differentiation is coupled to changes in the cell cycle regulatory apparatus of human embryonic stem cells. Stem Cell Res 1:45–60
Zhang X, Neganova I, Przyborski S, Yang C, Cooke M, Atkinson SP, Anyfantis G, Fenyk S, Keith WN, Hoare SF, Hughes O, Strachan T, Stojkovic M, Hinds PW, Armstrong L, Lako M (2009) A role for NANOG in G1 to S transition in human embryonic stem cells through direct binding of CDK6 and CDC25A. J Cell Biol 184:67–82
Grandela C, Pera MF, Wolvetang EJ (2008) p53 is required for etoposide-induced apoptosis of human embryonic stem cells. Stem Cell Res 1:116–128
Stead E, White J, Faast R, Conn S, Goldstone S, Rathjen J, Dhingra U, Rathjen P, Walker D, Dalton S (2002) Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene 21:8320–8333
Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9:400–414
Moldovan GL, Pfander B, Jentsch S (2007) PCNA, the maestro of the replication fork. Cell 129:665–679
Basu A, Haldar S (1998) The relationship between Bcl2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod 4:1099–1109
Shimizu S, Tsujimoto Y (2000) Proapoptotic BH3-only Bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate voltage dependent anion channel activity. Proc Natl Acad Sci USA 97:577–582
Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger BR, Kley N (1995) Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature 377:646–649
Rajah R, Valentinis B, Cohen P (1997) Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-beta1 on programmed cell death through a p53- and IGF-independent mechanism. J Biol Chem 272:12181–12188
Zhan Q (2005) Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat Res 569:133–143
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30570937), the National High Technology Research and Development Program of China (No. 2006AA02A102) and the Innovation Foundation for Graduate Student of Central South University of China (2340-74334000003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Tianji Chen and Juan Du contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, T., Du, J. & Lu, G. Cell growth arrest and apoptosis induced by Oct4 or Nanog knockdown in mouse embryonic stem cells: a possible role of Trp53. Mol Biol Rep 39, 1855–1861 (2012). https://doi.org/10.1007/s11033-011-0928-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-0928-6