Abstract
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 infections cause serious public health problems worldwide. The translocation intimin receptor (Tir) is responsible for adhesion and attaching and effacing lesions. In the current study, we used a mitomycin-treated mouse model to evaluate the efficacy of subcutaneous vs intranasal administration of the recombinant Tir as vaccine. Following immunization, mice were infected with E. coli O157:H7 and faces were monitored for shedding. Mice immunized intrasally with purified Tir proteins produced higher IgG and IgA titers in serum and feces, resulting in significant reductions in fecal shedding of EHEC O157 and higher a survival rate (92.9%), compared with subcutaneous or control immunizations. These results demonstrate the potential for the use of Tir proteins in mucosal vaccine formulations to prevent colonization and shedding of E. coli O157:H7. Therefore, purified Tir protects mice against EHEC challenge after intranasal immunization and is worth further clinical development as a vaccine candidate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enterohemorrhagic Escherichia coli (EHEC) causes hemorrhagic colitis (HC) and, on occasion, hemolytic-uremic syndrome (HUS), a life-threatening condition in humans [1–4]. Infection with the EHEC O157:H7 serotype is highly pathogenic and fatal. Currently, there are few effective interventions to reduce the risk of this infection. Therefore, the prevalent EHEC infections in humans have become a global public health problem [5]. While antibiotics are still the most effective treatment for O157 infection, their use promotes release of EHEC Shiga toxins (Stx), which increases the chance of complicating HUS [6, 7]. Thus, finding alternative means to controlling these infections is a high priority. Vaccination is one of the most promising approaches against EHEC O157 infection [8], although there is currently no successful vaccine that can be used clinically.
The road to successful development of an EHEC vaccine is long and tedious. At present, the most common vaccines are recombinant vector vaccines and genetically engineered vaccines that are being stringently tested [9–19]. However, these potential vaccines may cause unacceptable side effects. LPS vaccines induce the formation of antibodies which worsen the disease by promoting the release of Shiga toxins. In addition, transgenic plant vaccines induce weak immune responses, while oral plant-based vaccines are prone to the problem of immune tolerance. Antigens in bacterial ghost vaccines are complex and has many antigens that are in common with E. coli which can easily cause side effects. On the other hand, genetically engineered subunit vaccines have many advantages. For example, it is safe and suitable for mass industrial production, and the antigen structure is simple. This type of vaccine not only avoids the risk of pathogenicity that vaccines derived from whole organisms could potentially cause, but also reduces or eliminates pyrogens, allergens, immune-suppression and other virulence factors associated with live attenuated vaccines.
EHEC produces several recently described virulence determinants, which enables it to colonize the large bowel and cause disease [20, 21]. Several of its virulence factors are secreted by a type III secretion system which delivers virulence factors directly into host cells [22]. These factors include EspA, which forms filamentous structures on the bacterial surface bridging to the host cells’ surface [23]. EspB is delivered primarily into the host cell membrane, where it becomes an integral membrane protein [24]. EspB, along with EspD, probably forms a pore structure through which other bacterial effectors are transported. Tir is a bacterial molecule that uses the type III secretion system, EspA, EspB, and EspD for delivery into the host cell membrane. Once translocated into the host cell, Tir then functions as the receptor for intimin, which is an integral outer membrane protein of EHEC [25]. Tir-intimin binding attaches EHEC to the intestinal cell surface and triggers actin cytoskeletal rearrangements beneath the adherent EHEC, resulting in formation of a pedestal and a characteristic pattern referred to as an attaching and effacing (A/E) lesion [26]. It has been demonstrated that interrupting the interaction between Tir and intimin can prevent A/E lesions. The majority of present studies select intimin over Tir as a candidate antigen [27–30]. However, Tir is a strong immunogen, and compared with intimin, EspA and EspB, Tir induces an earlier antibodies response, and higher and longer duration of antibody titers.
Previous work by our laboratory [31] and others [27] have suggested that the Tir protein may be a good vaccine candidate. In this study, based on the structural and functional analysis of the EHEC O157:H7 Tir full-length cDNA sequence via pre-bioinformatics analysis [31], we constructed a recombinant plasmid pET-30a(+)-tir for production and purification of the Tir protein from transformed E. coli BL21(DE3). In the present study, a mitomycin-treated mouse E. coli O157:H7 colonization model was used and given the Tir recombinant protein via the nasal cavity and subcutaneous routes to determine the immunogenicity and protective ability of this candidate vaccine against EHEC O157:H7 challenge.
Materials and methods
Mice, bacterial strains, plasmids and media
Specific-pathogen-free (SPF) BALB/c mice at 8 weeks of age were purchased from the Animal Center of Sun Yat-Sen University. Bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were grown in Luria–Bertani (LB) broth or on LB agar (Oxoid Ltd., Basingstok, Hampshire, England) supplemented with 50 μg/ml of kanamycin as needed for selection of recombinant plasmids. LB broth or LB agar without kanamycin was used for culturing O157:H7.
Plasmid construction
The genomic DNA of EHEC O157 GZ246 was prepared using a Takara kit (Takara, Japan) according to the manufacturer’s instructions. The extracted genomic DNA was then used as the template for amplification of the Tir protein coding region using a Taq DNA polymerase PCR kit (Takara, Japan). Two primer sequences corresponding to the 5′ end of the tir gene (P1: 5′-GCACATATGATGCCTATTGGTAACCTT-3′; P2: 5′-ATACTCGAGGACGAAACGATGGGAT CC-3′) were used which introduced the Nde I and Xho I recognition sites (underlined) for subcloning.
The tir PCR product was ligated into pET-30a (+) and transformed into the E. coli strain DH5α for amplification and confirmation by sequencing. The resulting recombinant plasmid and the pET-30a (+) vector were digested with the Nde I and Xho I restriction enzymes, and the Tir coding fragment was ligated with the expression vector to form the plasmid pET-30a(+)-tir, which was again confirmed by sequencing (Invitrogen Biotechnology Co. Ltd., ShangHai).
Expression and purification of recombinant proteins
The expression vector pET-30a(+)-tir was transformed into E. coli strain BL21/DE3 and Tir protein expression was induced with 1 mM isopropyl-β-D-1-thiogalactopy-loranoside (IPTG) when the cells were grown at log phase at 37°C. After 6 h, the cells were harvested by centrifugation and washed with ice-cold PBS containing 5 mM EDTA and 2 mM PMSF. All subsequent procedures were performed at 4°C.
To purify the expressed Tir proteins, the cells were resuspended in PBS (containing 5 mM EDTA and 2 mM PMSF) and lysed by sonication on ice, followed by centrifugation at 10,000 rpm for 20 min to sediment the cell debris. The supernatant was applied to a glutathione Sepharose 4B column (Pharmacia, Sweden) to purify the Tir proteins according to the manufacturer’s instructions. The Tir samples were analyzed by electrophoresis on a 12% SDS-polyacrylamide gel and purified using Ni-IDA agarose (Novagen, Germany). The total proteins in the gels were stained with 0.25% Coomassie Blue or transferred to a nitrocellulose membrane (Millipore Co., Bedford, MA, USA) for Western blot analysis. The recombinant protein was detected on the membranes using the anti-6 × His monoclonal antibody (1:1000) as the primary antibody, and goat anti-mouse IgG conjugated with horseradish peroxidase (1:2000) (Sigma, USA) as the secondary antibody. The immunoreactive signals were developed by staining with DAB (Boster, China) as a chromogen.
Immune protection by Tir recombinant protein immunizations in mice
Immunization and challenge
In order to determine the optimal route of immunization, 8-week old BALB/c mice (Mice were given drinking water containing mitomycin (2.5 g/l) to reduce the normal bacterial flora of the mice.) were divided randomly into four groups (28 mice per group, half male and half female): (1) The intranasal immunization group was immunized with 30 μg of antigens emulsified with 3 μg Cholera toxin B subunit (CTB) and diluted up to 10 μl with PBS. (2) The intranasal mock-immunized group was given PBS with 3 μg Cholera toxin B subunit. (3) The subcutaneous immunization group was immunized with 100 μg of antigens which was emulsified with an equal amount of complete Freund’s Adjuvant (CFA) (Sigma) or Freund’s incomplete adjuvant (IFA) (Sigma). (4) The subcutaneous mock-immunized group was given PBS only. All immunizations were given three times 2 weeks apart.
The blood and fecal pellets were collected both prior to and 10 days after the last injection from 10 randomly selected mice in each group. Sera and feces samples were separated and stored at −20°C until used.
IgG and IgA determination
For measurement of anti-Tir antibodies, an enzyme-linked immunosorbant assay (ELISA) was developed. For that purpose, 96-well costar plates were coated with purified Tir protein (1 μg per well) overnight at 4°C. Serum samples were serially diluted from 1:100 to 1:25,600 and fecal pellet extracts were diluted from 1:2 to 1:64 and incubated overnight at 4°C. The plates were washed, and horseradish peroxidase goat anti-mouse IgG (1:2000) or horseradish peroxidase goat anti-mouse IgA (1:2000) conjugated to alkaline phosphatase was added to the appropriate plates. The plates were incubated at room temperature for 1 h, then freshly prepared TMB substrate solution was added for 10 min at 37°C in the dark. The reaction was stopped by the addition of 2 M H2SO4, and the absorbance was measured at 450 nm using an ELISA reader (Bio-TekEL ×800, USA). All samples were run in triplicate.
Protection against exposure to EHEC O157:H7
At 20 days after the last immunization, the immunized mice were challenged by transesophageal infusion with LD50 doses (1 × 1010 CFU/ml, 0.3 ml) of lysed EHEC O157:H7 strain EDL933, LD50 which was calculated by the Karber method. Twelve hours post-infection the mice resumed a normal diet, and clinical symptoms were observed and animal deaths were recorded daily. Bacteria in feces were detected by plating on Sorbitol MacConkey agar for colony counting, and fecal shedding of EHEC O157 was compared between the immune and non-immune groups. After 15 days, surviving mice were sacrificed and dissected for observation of pathological changes.
Histopathological examinations
Mice kidney, small intestine and colon tissue samples were fixed in 10% formalin solution, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E). Pathological changes were observed under a light microscope.
Statistical analysis
All data were processed and analyzed using the SPSS 16.0 Data Editor. Differences between groups were considered significantly different if P < 0.05. Mice survival differences between different immunization groups were performed by one-sided Fisher’s exact test.
Results
Expression and purification of recombinant proteins
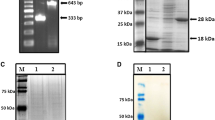
To construct an expression plasmid for the Tir protein of the E. coli strain of EHEC O157, the tir gene was obtained by PCR amplification. The expression of the recombinant Tir protein from pET-30a (+)-tir expressed in E. coli BL21(DE3) was examined by SDS-PAGE. A prominant band with an approximate molecular weight of 60 kDa appeared in the supernatant of the lysed cells after the induction but not in the control cells, suggesting that the Tir protein was successfully expressed in the bacterial cells (Fig. 1). The Tir protein was expressed with a C-terminal 6-His tag which facilitated purification over NI2+-coupled resins. The purity of the recombinant Tir proteins was determined by SDS-PAGE analysis (Fig. 1, lane 5). The identity of the purified Tir protein was further confirmed by Western blotting (Fig. 2), which showed that bacteria transformed with empty vector pET-30a (+) did not produce proteins reactive to the anti-6 × His monoclonal antibody, while IPTG-induced bacteria transformed with pET-30a (+)-tir produced a reactive product of about 60 kDa.
Expression and purification of Tir expressed from pET-30a(+)-tir analyzed by SDS-PAGE. M Protein Marker. Lane 1 PET-30a(+) transformants without IPTG induction. Lane 2 PET-30a(+)transformants with IPTG induction. Lane 3 pET-30a(+)-tir transformants without IPTG induction. Lane 4 pET-30a(+)-tir transformants with IPTG induction. Lane 5 purified soluble proteins of pET-30a(+)-tir tranformants
Expression and purification of Tir expressed from pET-30a(+)-Tir analyzed by Western blot using anti-6 × His monoclonal antibody. M Protein Marker. Lane 1 PET-30a(+) transformants with IPTG induction. Lane 2 purified soluble protein of pET-30a(+)-tir transformants. Lane 3 pET-30a(+)-tir transformants with IPTG induction
Antibody responses to immunization
We tested the antibody titers of mice sera and fecal pellets after immunization with the Tir protein. The serum IgG, IgA and fecal IgA ELISA results are summarized in Fig. 3. The mice were able to produce a high serum IgG antibody titer after both subcutaneous (6400) and intranasal (3200) immunizations. Meanwhile, the intranasal immunization induced serum IgA antibody titer (6400) was significantly higher than that of the subcutaneous immunization group (200). Fecal extracts from mice immunized intranasally produced sIgA antibody titer of 16, which was also higher than that in the subcutaneous immunization group (4).
Immune protection in mice
Survival rate of mice after bacteria challenge
After the immunized BALB/c mice were infected with transesophageal EHEC O157: H7 EDL933, the mice in each group moved slowly, appeared anorexic, lethargic, had scruffy fur, or even died on the following day. However, no hematochezia or diarrhea symptoms were observed. After the following 15 days observation period, the survival rate of both the subcutaneous control group and the intranasal control group was 50% (14/28). Meanwhile, the subcutaneously immunized group had a survival rate of 64.3% (18/28), and the intranasal immunization group had a survival rate of 92.9% (26/28). The χ2 tests and analysis determined that there was a significant difference in survival between the intranasal immunization group and the corresponding control group (P = 0.033) (Table 2). However, the subcutaneous immunization group and its control group had no significant difference (P = 0.704) (Table 3).
Fecal shedding of O157 post-infection
After challenge by EHEC O157 EDL933, fecal shedding of bacteria was detected from the immunization and control groups, the shedding results are summarized in Fig. 4. The results showed that large amounts of bacteria was shed, and the duration of the shedding in the control group lasted up to or longer than 13 days. In addition, we could not detect O157 shedding 5 days after challenge of the intranasal immunization group, while we could detect O157 shedding from the subcutaneous immunization group until 9 days later. These results showed that immunizing animals with Tir recombinant proteins reduced EHEC colonization in animal intestines, especially through the intranasal immunization route.
Histopathological examinations of immunized and challenged mice
The severity of pathological changes in the experimental mice kidneys and colons were associated with the immune protection. As shown in Fig. 5, the renal biopsies showed that the pathological changes of glomeruli, cortex and medulla, interstitial congestion of the intranasal immunization group were more mild than that of other groups. The colon biopsies also showed that in the intranasal immunization group, the pathological changes including submucosal glands and interstitial vascular congestion, inflammatory cell infiltration, and hydropic degeneration of renal tubular cells occurred to a lesser extent than in the other groups.
H&E stained histopathology sections. a Kidney tissue intranasal immunization group (left) and control group (right). b Colon tissue intranasal immunization group (left) and control group (right). c Kidney tissue Subcutaneous immunization group (left) and control group (right). d Colon tissue Subcutaneous immunization group (left) and control group (right)
Discussion
As the infection of EHEC O157:H7 is highly pathogenic and fatal, and the use of antibiotic can even promote release of EHEC Shiga toxin (Stx), the prevalence of EHEC infections in humans has become a global public health problem. Therefore, we are in dire need of a successful vaccine. Unfortunately, no effective vaccine is available for clinical use. Meanwhile whole bacterial vaccines can cause side effects because of complex antigens of EHEC. Currently, the most promising approach to a successful vaccine is development of a genetically engineered subunit vaccine by selecting a candidate antigen and determining the route of administration which can induce a strong immune response.
Since the Tir protein causes A/E lesions and its interaction with intimin is an important virulence factor, we chose Tir as a candidate antigen. Previous research has shown that many functional and immunobiological characteristics of Tir make it a good potential target for the design of a vaccine. As mentioned earlier, Tir is a strong immunogen compared with intimin, EspA and EspB. Tir induces an earlier antibody response with high titers and long duration. Because it has also been reported that that the mother’s colostrum contains pathogenic E. coli Tir specific IgA antibody, successful antibody responses to this vaccine can potentially provide protection to nursing infants as well. Furthermore, Sanches et al. found that IgG against Tir are present in infected patient sera [32].
Aside from the choice of immunogen, the route of administration is also key for developing a successful vaccine. In this study, we chose to test intranasal and subcutaneous immunizations. The result showed that with subcutaneous immunization, only high serum IgG antibody titers could be obtained, whereas intranasal immunization resulted in not only high titers of serum IgG antibody but also in high titers of IgA antibody in mice serum and feces (Fig. 3). By inoculating mice with E. coli O157:H7, we found high percentages of protected mice (92.9%) in the intranasal immunization group, significantly higher than in the subcutaneous immunization group (64.3%) and control group (50%) (Tables 2, 3). We detected fecal shedding of EHEC O157 to determine the influence of Tir immunization on the colonization in mice. The data showed that after infection with EHEC, the period of time that the mice shed bacteria in the feces of the immune group was shorter than the non-immune group. Reduction in the duration of shedding indicated that the EHEC O157 colonization of mice intestine was blocked, and the infecting bacteria were eradicated quickly. The data in this study was consistent with the one reported by Babiuk et al. [33]. In our study, the vaccine immunogen of purified Tir protein that expressed by the recombinant plasmid pET-30a(+)-tir reduced the amount and duration of shedding of EHEC O157. In addition, histological studies showed that the pathological changes in the intranasal immunization group were slighter than the other groups. Except for the intranasal immunization group, the liver surfaces of the dead mice were dull red, showing various degrees of congestion.
The subcutaneous administration approach to immunization could induce strong humoral immunity (IgG) but cannot effectively prevent intestinal infection. The reason that the subcutaneous immunization could not protect mice from EHEC challenge may be that EHEC O157 only adheres to mucosal epithelial cells, rather than invading epithelial cells. Furthermore, epithelial cells only actively transport secretory IgA antibodies but not IgG antibodies. Therefore, mucosal immunity studies have achieved increasing attention. The common mucosal immunity system (CMIS) is an important component of the body’s immune system. Mucosal immunization stimulates immune responses at remote sites, and produces sIgA prior to preventing bacterial colonization. Zhang et al. [34] challenged mice with EHEC O157: H7 outer membrane protein by the intranasal route and then detected the bacteria in mouse feces and intestinal lavage. Their results also showed that intranasal immunization can protect mice from infection which correlated with high IgA titers detected in feces and intestinal lavage.
In summary, the purified Tir protein of EHEC O157 is highly immunogenic and can induce protective immune responses via intranasal immunization. Therefore, Tir is an attractive vaccine candidate. In our future work, we will transform the recombinant plasmid into probiotic bacteria such as Lactobacillus acidophilus to establish an oral live vector vaccine which can produce intestinal immunity in humans or animals. Ruminants like cattle and sheep are the main sources of EHEC O157 infection. We hope to show that vaccination of these animals can reduce the number of EHEC O157 colonization and the duration of colonization in the intestine. Our long-term goal is to reduce transmission of EHEC O157 from ruminants to humans in order to control and prevent this infection from food sources.
References
Karmali MA, Petric M, Louie S, Cheung R (1986) Antigenic heterogeneity of Escherichia coli verotoxins. Lancet 1:164–165
Sjogren R, Neill R, Rachmilewitz D, Fritz D, Newland J, Sharpnack D, Colleton C (1994) Role of Shiga-like toxin I in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology 106:306–317
Takeda T (1993) Hemolytic uremic syndrome associated with entero-hemorrhagic Escherichia coli. Nippon Rinsho 51:198–203
Gianviti A, Rosmini F, Caprioli A, Corona R, Matteucci MC, Principato F, Luzzi I, Rizzoni G (1994) Haemolytic-uraemic syndrome in childhood: surveillance and case-control studies in Italy. Italian HUS Study Group. Pediatr Nephrol 8:705–709
Zhao X, Li Y, Wang L, You L, Xu Z, Li L, He X, Liu Y, Wang J, Yang L (2010) Development and application of a loop-mediated isothermal amplification method on rapid detection Escherichia coli O157 strains from food samples. Mol Biol Rep 37:2183–2188
Safdar N, Said A, Gangnon RE, Maki DG (2002) Risk of hemolytic uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 enteritis. JAMA 288(8):996–1001
Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI (2000) The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med 342:1930–1936
Besser RE, Griffin PM, Slutsker L (1999) Escherichia coli O157:H7 gastroenteritis and the hemolytic uremic syndrome: an emerging infectious disease. Annu Rev Med 50:355–367
Acheson DW, Levine MM, Kaper JB, Keusch GT (1996) Protective immunity to Shiga-like toxin I following oral immunization with Shiga-like toxin I B-subunit-producing Vibrio cholerae CVD 103-HgR. Infect Immun 64:355–357
Butterton JR, Ryan ET, Acheson DW, Calderwood SB (1997) Co-expression of the B subunit of Shiga toxin 1 and EaeA from enterohemorrhagic Escherichia coli in Vibrio cholerae vaccine strain. Infect Immun 65:2127–2135
Konadu EY, Parke JC, Tran HT, Bryla DA, Robbins JB, Szu SC (1998) Investigational vaccine for Escherichia coli O157 phase 1 study of O157 O-specific polysaccharide Pseudomonas aeruginosa recombinant exoprotein A conjugates in adults. J Infect Dis 177:383–387
Judge NA, Mason HS, O’Brien AD (2004) Plant cell-based intimin vaccine given orally to mice primed with intimin reduces time of Escherichia coli O157:H7 shedding in feces. Infect Immun 72:168–175
Potter AA, Klashinsky S, Li Y, Frey E, Townsend H, Rogan D (2004) Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine 22:362–369
Mayr UB, Haller C, Haidinger W, Atrasheuskaya A, Bukin E, Lubitz W, Ignatyev G (2005) Bacterial ghosts as an Oral vaccine: a single dose of Escherichia coli O157:H7 bacterial ghosts protects mice against lethal challenge. Infect Immun 73:4810–4817
Donkersgoed JV, Hancock D, Rogean D, Potter AA (2005) Escherichia coli O157:H7 vaccine field trial in 9 feedlots in Albeta and Saskatchewan. Can Vet J 46:724–728
Zhang Q, Ma Q, Li Q, Yao W, Wang C (2010) Enhanced protection against nasopharyngeal carriage of Streptococcus pneumoniae elicited by oral multiantigen DNA vaccines delivered in attenuated Salmonella typhimurium. Mol Biol Rep. doi:10.1007/s11033-010-0219-7
Zhang J, Zhang X, Zhang W, Guo Y, Guo G, Mao X, Zou Q (2010) Fusion expression and immunogenicity of Bordetella pertussis PTS1-FHA protein: implications for the vaccine development. Mol Biol Rep. doi:10.1007/s11033-010-0317-6
Seyyed ME, Majid T, Khosrow A, Hassan N, Ali M (2010) Prokaryotic expression and characterization of avian influenza A virus M2 gene as a candidate for universal recombinant vaccine against influenza A subtypes; specially H5N1 and H9N2. Mol Biol Rep 37:2909–2914
Seyyed ME, Majid T (2010) Heterologous expression, purification and characterization of the influenza A virus M2e gene fused to Mycobacterium tuberculosis HSP70359–610 in prokaryotic system as a fusion protein. Mol Biol Rep 37:2877–2883
Donnenberg MS, Kaper JB, Finlay BB (1997) Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol 5:109–114
Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S (1998) Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol 30:911–921
Hueck CJ (1998) Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 62:379–433
Knutton S, Rosenshine I, Pallen MJ, Nisan I, Neves BC, Bain C, Wolff C, Dougan G, Frankel G (1998) A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J 17:2166–2176
Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I (1998) Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol 28:143–155
Kenny B, Devinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB (1997) Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511–520
Junkal G, Gad F, Valerie FC (2005) Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect Immun 73:2573–2585
Deng W, Li Y, Hardwidge PR (2005) Regulation of type III secretion hierarchy of translocators and effectors in attaching and effacing bacterial pathogens. Infect Immun 73:2135–2146
Vlisidou I, Dziva F, La Ragione RM, Best A, Garmendia J, Hawes P (2006) Role of intimin-tir interactions and the tir-cytoskeleton coupling protein in the colonization of calves and lambs by Escherichia coli O157:H7. Infect Immun 74:758–764
Sinclair JF, Dean-Nystrom EA, Alison DO (2006) The established intimin receptor Tir and the putative eucaryotic intimin receptors nucleolin and β1 integrin localize at or near the site of Enterohemorrhagic Escherichia coli O157:H7 adherence to enterocytes in vivo. Infect Immun 74:1255–1265
Nathan TR, Benjamin LM (2007) Characterization of the binding surface of the translocated intimin receptor, an essential protein for EPEC and EHEC cell adhesion. Protein Sci 16:2677–2683
Wang L, Long BG, Fan HY, Luo J (2009) Bioinformatics analysis of the Tir gene of Enterohemorrhagic E. coli O157:H7. Chn J Trop Med 9:506–509
Sanches MI, Keller R (2000) Human colostrum and serum contain antibodies reactive to the intimin-binding region of the enteropathogenic Escherichia coli translocated intimin receptor. J Pediatr Gastroenterol Nutr 30(1):73–77
Babiuk S, Asper DJ, Rogan D, Mutwiri GK, Potter AA (2008) Subcutaneous and intranasal immunization with type III secreted proteins can prevent colonization and shedding of Escherichia coli O157:H7 in mice. Microb Pathogenesis 45:7–11
Zhang Y, Lu SQ, Zhang YZ (2004) The study for the immuno-protection of E. coli O157:H7 outer membrane protein. Natl Med J China 84:58–62
Acknowledgments
This research was supported by the Dean of the School of Public Health and Tropical Medicine, Southern Medical University (no. GW200801-039).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hong-ying Fan and Ling Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fan, Hy., Wang, L., Luo, J. et al. Protection against Escherichia coli O157:H7 challenge by immunization of mice with purified Tir proteins. Mol Biol Rep 39, 989–997 (2012). https://doi.org/10.1007/s11033-011-0824-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-0824-0