Abstract
The Matrix metalloproteinas-9 functional promoter polymorphism 1562C>T may be considered an important genetic determinant of early-onset coronary artery disease (ECAD). In this study, association between MMP-9 1562C>T allele with plasma MMP-9 activity, homocysteine and lipid–lipoproteins level and ECAD in Iranian subjects was investigated. This case–control study consisted of 53 ECAD patients (age < 55 years) and unrelated late-onsets CAD (age > 70 years) who angiographically had at least 50% stenosis. MMP-9 1562C>T polymorphism was detected by PCRRFLP, plasma MMP-9 activity, serum lipid and homocysteine levels were determined by gelatin gel zymography, enzyme assay and by HPLC, respectively. The presence of MMP-9 1562C>T allele was found to be associated with ECAD (OR = 3.2, P = 0.001). The ECAD patients with MMP-9 1562C>T allele had higher MMP-9 activity (P = 0.001), LDL-C (P = 0.045), TC (P = 0.02) and homocysteine (P = 0.01) levels than the LCAD subjects. MMP-9 1562C>T allele is a risk factor for ECAD. The carriers of this allele have high levels of MMP-9 activity, LDL-C, TC and homocysteine (P = 0.01), thus, are more likely to develop myocardial infarction and CAD at young age (less than 55 years).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular events including coronary artery disease (CAD) and stroke have been reported as one of the main causes of death and disability in developing countries such as Iran [1, 2]. A number of traditional risk factors for CAD include advanced age, sex, obesity, smoking, diabetes, hypertension, physical inactivity, elevated total plasma cholesterol, low-density lipoprotein (LDL), homocysteine, decreased high density lipoprotein (HDL), and lifestyle [3–10]. In atherosclerosis, matrix remodeling is believed to influence the migration and proliferation of cells within the plaque [11, 12]. Several groups of proteolytic enzymes, including matrix metalloproteinases (MMPs) are able to degrade components of the extracellular matrix [13, 14]. MMPs are a family of zinc-dependent enzymes that collectively degrade all of the components of the connective tissue including collagen types I–V, fibronectin, laminin, elastin and proteoglycans [13, 15].
It has been recognized that MMPs play a significant role in the progression of atherosclerosis, plaque rupture and ischemic heart disease [7, 11, 15]. MMP-9, also known as gelatinase B or 92-kDa type IV collagenase, that is secreted from macrophages in fibrous cap, has been suggested to be involved in the remodeling processes associated with atherosclerosis and plaque rupture [16, 17]. The role of MMP-9 in CAD has been substantiated by genetic studies showing that functional promoter variations of the MMP-9 gene are related to presence and severity of CAD [7, 18, 19]. Zhang et al. [20] have identified several single nucleotide polymorphisms in the MMP-9 gene including the C to T transition at position −1562 in the promoter region of the protein. The 1562T allele has a higher promoter activity than the C allele and may influence the severity and extent of coronary artery stenosis [7]. Elevated levels of MMP-9 have been reported in patients with unstable angina [21]. However, the clinical significance of MMP-9 1562T allele in early-onset of CAD is unknown. The aim of present study was to investigate whether the MMP-9 C1562T promoter gene polymorphism had any effect on the MMP-9 activity in the plasma, lipid profile, the level of homocysteine and consequently on development of the early onset of CAD in Iranian population.
Materials and methods
Subjects
All patients were selected from unrelated individuals who had undergone their first coronary angiography for evaluating the presence and extent of CAD. They were assessed and referred to the Cardiology Division of the Imam Khomini Hospital of Tehran University of Medical Sciences. Patients undergoing coronary angiography for diseases such as valvular or congenital heart disease, diabetes mellitus, kidney, thyroid, liver, and restrictive or dilated cardiomyopathy were excluded. Only patients undergoing elective angiography (in order to avoid the influence of stress situations) and had more than 50% detectable stenosis in one or more coronary arteries were included in this study.
Eligible patients were divided into two groups, early and late onset CAD. The early onset CAD consisted of 53 CAD patients (43 males and 10 females mean ages 48.16 ± 8.2 years) who presented with CAD at 55 years of age or younger. The late onset of CAD consisted of 50 patients (31 males and 19 females) who presented with CAD at 65-years of age or older with mean value of 79.43 ± 15.24 years. Informed written consent was obtained from each individual before participation. The study was approved by the Ethics Committee of the Tehran University of Medical Sciences and was in accordance with the principles of the Declaration of Helsinki II. Blood was drawn under standardized condition. Heparin used as the anticoagulant because of the inhibitory effect of EDTA on MMP-9 activity through chelating with Zn2+ in the active site of the enzyme [22].
DNA analysis
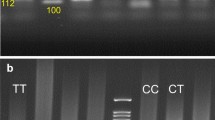
Genomic DNA was extracted from peripheral blood leukocytes using phenol chloroform extraction method [23]. Genotyping of all individuals was done without knowledge of their groups or disease. The genotyping of MMP-9 promoter at position −1562 were detected by PCR using the forward, 5′-GCC TGG CAC ATA GTA GGC CC-3′ (corresponding to base pairs −1871 to −1851) and the reverse, 5′-CTT CCT AGC CAG CCG GCA TC-3′ (corresponding to base pairs −1339 to 1319) primers, as previously described [17]. The PCR products were digested with SphI restriction endonuclease (5 U, Fermentas) and subjected to electrophoresis on a 16% polyacrylamide gel. The gels were stained with AgNO3 and photographed. The PCR product of −1562 C allele is not cleaved by SphI, while that of the −1562 T allele is cleaved by the enzyme generating 247 and 188 bp fragments [20].
Chemical analysis
MMP-9 assay
The activity of MMP-9 in the plasma was measured by gelatin gel zymography as previously has described [24]. SDS-PAGE was performed in 7% acrylamide gels containing 0.1% w/v gelatin. The gels were incubated in 2.5% (v/v) Triton X-100 for 2 h, washed several times with water and incubated overnight in 50 mM Tris–HCl, 2.5 mM CaCl2 and 0.02% NaN3, pH 8.0 at 37°C. The gels were then stained with Coomossie blue and destained. The culture media of Human HT1080 breast carcinoma cell line was used as a reference for gelatinolytic activity. This cell line constitutively expresses and releases MMP-9 into culture media. The activity of MMP-9 in the serum was quantitated by comparing the intensity of the cleared zone in the gelatin Zymogram gel that corresponds to the amount of the gelatin digested by the enzyme to that of the reference [20, 25, 26]. A linear range of MMP-9 activity of approximately 50-fold can be determined by using serial dilution of the reference enzyme (Fig. 1).
Plasma lipids
Total plasma cholesterol (TC) and triglycerides (TG) were measured by the standard enzymatic method (Pars Azmon kit, Iran), using an automated RA-1000 (Technician, USA). The plasma LDL-C and HDL-C levels were measured using commercially available enzyme assay kits (Pars Azmon kit, Iran).
Measurement of homocysteine
Plasma homocysteine levels were measured according to the method of Ubbink et al. [27] using high performance liquid chromatography (Shimadzu LC 20A, Shimadzu, Kyoto, Japan). After pretreatment of standards/samples with thiobarbituric acid the mixture was extracted with n-butanol. About 20 μl of butanol layer was injected to RP C18 column using isocratic elution with methanol/water (1:1 v/v) at a flow rate of 1 ml/min. The elution was monitored at 512 nm using excitation wavelength of 385 nm.
Statistical analysis
The allelic frequencies were calculated by the gene counting method. The χ2 test was used to verify the agreement of the observed genotype frequencies with those expected according to the Hardy–Weinberg equilibrium. The genotypes and MMP-9 allele frequencies in patients with early onset CAD were compared to those with late onset CAD using χ2 test. Odds ratios (OR) were calculated as estimates of relative risk for disease and 95% confidence intervals obtained by SPSS logistic regression. The correlation values of plasma MMP-9 activity, HDL, LDL, TC, TG and homocysteine level with the MMP-9 polymorphism between two groups were calculated using linear regression and an unpaired t test. A two-tailed Student’s t test and ANOVA analysis were used to compare quantitative data. Statistical significance was assumed at the P < 0.05.
The SPSS statistical software package version 16 was used for the statistical analysis.
Results
The gender, MMP-9 activity, lipid parameters (TG, TC, HDL-C and LDLC), plasma homocysteine concentration and distribution of genotypes and MMP-9 allele frequencies found in patients with early (ECAD) and late (LCAD) onset of CAD are reported in Tables 1 and 2. Distributions of the MMP-9 genotypes (χ2 = 9.9, df = 2, P = 0.002) and alleles (χ2 = 11.5, df = 1, P = 0.001), were significantly different in ECAD patients compared to the LCAD individuals by Hardy–Weinberg equilibrium. The MMP-9 T allele frequency for ECAD and LCAD were 33 and 13%, respectively (χ2 = 10, df = 1, P = 0.001). However, no MMP-9 T/T genotype was detected in patients with LCAD.
Overall distribution of the MMP-9 genotypes and alleles were significantly different in male (χ2 = 19.5, df = 1, P < 0.001; χ2 = 8.2, df = 1, P = 0.004) and female (χ2 = 16.3, df = 1, P < 0.001; χ2 = 4.2, df = 1, P = 0.041) individuals with ECAD compared with those with LCAD. Distribution of the C/T+T/T genotype and T allele in male subjects were significantly different among the two groups studied. Interestingly, MMP-9 C/C genotype was not found in female subjects with ECAD (Table 3).
The ECAD subjects had significantly higher homocysteine (17.7 ± 8.4 vs. 14.7 ± 3.7, P = 0.022), lipid concentration, (HDL-C, LDL-C, TC, and TG) and MMP-9 activity in the plasma (95.6 ± 61 vs. 44 ± 24, P = 0.001) than LCAD subjects (Table 1). As shown in Table 4, the presence of the MMP-9 alleles had also significant effect on these parameters in both group of subjects. Comparing plasma MMP-9 activity, LDL-C, and homocysteine levels between corresponding alleles in ECAD were found scientifically in T allele carrier than that of the LCAD. The ECAD patients with the T allele and C/T+T/T genotype had significantly higher MMP-9 activity (P < 0.001) and TG (P = 0.016, P = 0.017) concentrations in comparison with the C alleles and C/C genotype carriers. The level of lipid parameters, homocysteine and plasma MMP-9 activity in two genders in ECAD and LCAD are demonstrated in Table 5. The levels of LDL-C (P = 0.007), HDL-C (P = 0.018), TC (P = 0.004) and plasma MMP-9 activity (P < 0.001) were significantly higher in male subjects with ECAD than those with LCAD. However, except for plasma MMP-9 activity (P < 0.001), in female subjects with ECAD the levels of lipid parameters and homocysteine were not significantly different from those of LCAD subjects. Interestingly, comparing both genders, we found that female subjects, in general, had higher levels of lipid, homocysteine and plasma MMP-9 activity than male subjects (Table 5).
The sex-adjusted OR for all subjects with ECAD, with (C/T+TT) genotype of the MMP-9 was 3.7 (1.6–8.5, P = 0.002). OR of MMP-9 T allele was found to be 3.3 (1.6–6.7, P = 0.001) in ECAD subjects (Table 6). OR for MMP-9 (C/T+TT) genotype and T allele in two genders in ECAD and LCAD are demonstrated in Table 6. OR of MMP-9 T allele and (C/T+TT) genotype were found to be 3.6 (1.5–9, P = 0.006) and 14.3 (3.7–54, P < 0.001), respectively in male ECAD subjects and 3.56 (1.1–12.4, P = 0.047) in female with was found (Table 6).
Discussion
Coronary artery disease is the major cause of death in developing countries, such as Iran [28]. While little is known about the clinical significance of circulating matrix metalloproteinas-9 (MMP-9) in early-onset CAD (ECAD), MMP-9 functional promoter polymorphism 1562C>T is considered an important genetic determinant of ECAD. In this study, we compared the frequency of the MMP-9 1562C>T gene polymorphism and its association with MMP-9 activity, homocysteine and plasma lipids concentration in subjects with ECAD or LCAD in Iranian subjects. We have found that the MMP-9 1562C>T gene polymorphism increases the risk of ECAD by 3.3 fold in this population of patients. The presence of MMP-9 (C/T+T/T) genotype was associated with a 3.7 (95% CI: 1.6- 8.5, P = 0.002) fold increase in risk of ECAD compared to MMP-9 C/C genotype.
The distribution of the MMP-9 genotypes in the ECAD subjects was significantly different from that of the LCAD patients (χ2 = 9.9, df = 2, P = 0.002). The frequency of the MMP-9 T allele was found to be higher in ECAD subjects than in the LCAD patients (33 vs. 13%, P = 0.001). This data indicate that individuals carrying the 1562 T allele of the MMP-9 gene are predisposed to developing early CAD.
As shown in Table 4, ECAD patients who carry MMP-9 1562T allele have significantly higher MMP-9 activity in the plasma than LCAD patients and those who carry MMP-9 1562C allele. These findings support the notion that genetic variation in MMP-9 gene is associated with higher gelatinolytic activity, which in turn may influence large artery lesion, predisposing the individuals with MMP-9 T allele to an early onset of coronary atherosclerosis. This is supported by the previous studies demonstrating that MMP-9 T allele is associated with complicated coronary lesions and carriers of the T allele had greater levels of MMP9 mRNA and protein and stiffer large arteries [18, 19, 29, 30]. Our data is further supported by Zhang et al.’s observation that 1562C/T polymorphism in the promoter region of MMP-9 exerts an allelic effect on MMP-9 promoter strength, such that the T allelic promoter has a higher transcriptional activity than the C allelic promoter, which is likely to be attributed to binding a transcriptional repressor to the C allele [7, 18].
Interestingly, our studies have also demonstrated that the ECAD patients with MMP-9 T allele had higher levels of LDL-C, TC, and homocysteine levels that the LCAD group. LDL-C, TC and risk factors for CAD [1, 25, 31–36]. In addition, our study indicated that there is a high frequency of MMP-9 T allele in females (Table 3). Females in general have higher levels of lipid, homocysteine and plasma MMP-9 activity than male subjects (Tables 4, 5), suggesting that females are more susceptible to ECAD and myocardial infarction that male.
The mechanism whereby the MMP-9 allele contributes to CAD and atherosclerosis is unknown. Atherosclerosis is apparently initiated in response to arterial endothelial injury, which allows increased permeability to lipid, monocytes and lipid-laden macrophages. The MMP-9 secreted from macrophages apparently helps medial smooth muscle to migrate into the intima by degrading extracellular matrix [37]. The present study showed that the role of the MMP-9 allele as a risk factor for ECAD is not only due to its association with a high serum MMP-9 activity but also by its association with a high level of the atherogenic LDL-C, TC and homocysteine. Future studies are required to evaluate the significance of high serum gelatinolytic activity in genetically susceptible individuals in the development of premature arterial lesion and/or increased vascular risk.
Conclusion
The major finding of the present case–control study is that MMP-9 T allele is associated with increased risk of developing CAD in patients younger than 55 years old in the Tehran population of Iran. This study also indicated that carriers of MMP-9 T allele have distinct elevated plasma MMP-9 activity, homocysteine and lipid profile, suggesting that these individuals are susceptible to early onset coronary atherosclerosis and myocardial infarction especially at early ages (less than 55 years old).
References
Kharrazi H, Vaisi Raygani A, Sabokroh AR, Pourmotabedd T (2006) Association between apolipoprotein E polymorphism in coronary artery disease patients in Kermanshah, in west of Iran. Clin Biochem 39:613–616
Vaisi-Raygani A, Tavilani H, Rahimi Z, Zahrai M, Sheikh N, Aminian M, Pourmotaabed T (2009) Serum butyrylcholinesterase activity and phenotype associations with lipid profile in stroke patients. Clin Biochem 42:210–214
Vaisi-Raygani A, Rahimi Z, Nomani H, Tavilani H, Pourmotaabed T (2007) The presence of apolipoprotein ε4 and ε2 alleles augments the risk of coronary artery disease in Type 2 diabetic patients. Clin Biochem 40:1150–1156b
Abilleira S, Bevan S, Markus HS (2006) The role of genetic variants of matrix metalloproteinases in coronary and carotid atherosclerosis. J Med Genet 43:897–901
Vaisi-Raygani A, Rahimi Z, Tavilani H, Pourmotaabed T (2010) Butyrylcholinesterase K variant and the APOE-e4 allele work in synergy to increase the risk of coronary artery disease especially in diabetic patients. Mol Biol Rep 37:2083–2091
Li Y, Sun DL, Duan YN, Zhang XJ, Wang N, Zhou RM, Chen ZF, Wang SJ (2010) Association of functional polymorphisms in MMPs genes with gastric cardia adenocarcinoma and esophageal squamous cell carcinoma in high incidence region of North China. Mol Biol Rep 37(1):197–205
Morgan AR, Zhang B, Tapper W, Collins A, Ye S (2003) Haplotypic analysis of the MMP-9 gene in relation to coronary artery disease. J Mol Med 81:321–326
Rahimi Z, Vaisi-Raygani A, Pourmotaabed T (2011) Association between apolipoprotein ε4 allele, factor V Leiden, and plasma lipid and lipoprotein levels with sickle cell disease in Southern Iran. Mol Biol Rep 38(2):703–710
Zhi H, Wang H, Ren L, Shi Z, Peng H, Cui L, Ma G, Ye X, Feng Y, Shen C, Zhai X, Zhang C, Zen K, Liu N (2010) Functional polymorphisms of matrix metallopeptidase-9 and risk of coronary artery disease in a Chinese population. Mol Biol Rep 37(1):13–20
Rahimi Z, Felehgari V, Rahimi M, Mozafari H, Yari K, Vaisi-Raygani A, Rezaei M, Malek-Khosravi S, Khazaie H (2011) The frequency of factor V Leiden mutation, ACE gene polymorphism, serum ACE activity and response to ACE inhibitor and angiotensin II receptor antagonist drugs in Iranians type II diabetic patients with microalbuminuria. Mol Biol Rep 38(3):2117–2123
Kelly D, Cockerill G, Thompson M, Khan S, Samani NJ, Squire IB (2007) Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study. Eur Heart J 28:711–718
Li H, Xu H, Liu S (1994) Toll-like receptors 4 induces expression of matrix metalloproteinase-9 in human aortic smooth muscle cells. Mol Biol Rep 38:1419–1423
Gibbons GH, Dzau VJ (1994) The emerging concept of vascular remodelling. N Engl J Med 330:1431–1438
Ayşegül B, Veysi GH, Muzaffer M, Irfan D, Azra A, Hulyam K (2010) Is a single nucleotide polymorphism a risk factor for lung cancer in the matrix metalloproteinase-2 promoter? Mol Biol Rep 38:1469–1474
Galis ZS, Khatri JJ (2002) Matrix metalloproteinases in vascular remodeling and atherogenesis. The good, the bad, and the ugly. Circ Res 90:251–262
Nanni S, Melandri G, Hanemaaijer R, Cervi V, Tomasi L, Altimari A, Van Lent N, Tricoci P, Bacchi L, Branzi A (2007) Matrix metalloproteinases in premature coronary atherosclerosis: influence of inhibitors, inflammation, and genetic polymorphisms. Transl Res 149(3):137–144
Loftus IM, Naylor AR, Goodall S (2000) Increased matrix metalloproteinase-9 activity in unstable carotid plaques: a potential role in acute plaque disruption. Stroke 31:40–47
Zhang B, Ye S, Herrmann SM, Eriksson P, de Maat M, Evans A, Arveiler D, Luc G, Cambien F, Hamsten A, Watkins H, Henney AM (1999) Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation 99:1788–1794
Pollanen PJ, Karhunen PJ, Mikkelsson J (2001) Coronary artery complicated lesion area is related to functional polymorphism of matrix metalloproteinase 9 gene: an autopsy study. Arterioscler Thromb Vasc Biol 21:1446–1450
Zhang B, Henney A, Eriksson P, Hamsten A, Watkins H, Ye S (1999) Genetic variation at the matrix metalloproteinase-9 locus on chromosome 20q12.2-13.1. Hum Genet 105:418–423
Kai H, Ikeda H, Yasukawa H (1998) Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol 32:368–372
Kim H, Dalal S, Young E (2000) Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin Invest 106:857–866
Sambrook J, Russell DW (2001) Preparation and analysis of Eukaryotic genomic DNA. In: Argentine J, Irwin N (eds) Molecular cloning: a laboratory manual, vol 1. Cold spring Harbor Laboratory Press, Cold Spring Harbor (protocol 6.1)
Kleiner D, Stetler-Stevenson W (1994) Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem 218:325–329
Cho A, Reidy MA (2002) Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res 91:845–851
Ferrand PE, Parry S, Sammel M (2002) A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in Africans Americans. Mol Hum Reprod 8(5):494–501
Ubbink JB, Hayward Vermaak WJ, Bissbort S (1991) Rapid high-performance liquid chromatographic assay for total homocysteine levels in human serum. J Chromatogr 565:441–446
Vaisi-Raygani A, Rahimi Z, Entezami H, Kharrazi H, Bahrhemand F, Tavilani H, Rezaei M, Kiani A, Nomanpour B, Pourmotabbed T (2008) Butyrylcholinesterase K variants increase the risk of coronary artery disease in the population of western Iran. Scand J Clin Lab Invest 68(2):123–129
Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L, AtheroGene Investigators (2003) Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 107:1579–1585
Medley TL, Cole TJ, Dart AM, Gatzka CD, Kingwell BA (2004) Matrix metallaproteinase-9 genotype influences large artery stiffness through effects on aortic gene and protein expression. Arterioscler Thromb Vasc Biol 24:1479–1484
Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Sutherland P, Wilson PWF, Vasan RS (2004) Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation 109(23):2850–2856
Ranucci M, Ballotta A, Frigiola A, Boncilli A, Brozzi S, Costa E, Mehta RH (2009) Preoperative homocysteine levels and morbidity and mortality following cardiac surgery. Eur Heart J 30:995–1004
Pasali D, Marinkovi N, Grskovi B, Ferencak G, Bernat R, Stavljeni-Rukavina A (2009) C-reactive protein gene polymorphisms affect plasma CRP and homocysteine concentrations in subjects with and without angiographically confirmed coronary artery disease. Mol Biol Rep 36(4):775–780
Madani H, Rahimi Z, Manavi-Shad M, Mozafari H, Akramipour R, Vaisi-Raygani A, Rezaei M, Malek-Khosravi S, Shakiba E, Parsian A (2011) Plasma lipids and lipoproteins in children and young adults with major β-thalassemia from western Iran: influence of genotype. Mol Biol Rep 38(4):2573–2578
Ashok Kumar M, Subhashini NG, SaiBabu R, Ramesh A, Cherian KM, Emmanuel C (2010) Genetic variants on apolipoprotein gene cluster influence triglycerides with a risk of coronary artery disease among Indians. Mol Biol Rep 37(1):521–527
Liang S, Pan M, Geng HH, Chen H, Gu LQ, Qin XT, Qian JJ, Zhu JH, Liu CF (2009) Apolipoprotein E polymorphism in normal Han Chinese population: frequency and effect on lipid parameters. Mol Biol Rep 36(6):1251–1256
Robertson L, Grip L, Mattsson Hulte L, Hulthe J, Wiklund O (2007) Release of protein as well as activity of MMP-9 from unstable atherosclerotic plaques during percutaneous coronary intervention. J Internal Medici 262:659–667
Acknowledgment
This research was supported by Tehran University of Medical Sciences and Health Services. Grant no. 18898.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saedi, M., Vaisi-Raygani, A., Khaghani, S. et al. Matrix metalloproteinas-9 functional promoter polymorphism 1562C>T increased risk of early-onset coronary artery disease. Mol Biol Rep 39, 555–562 (2012). https://doi.org/10.1007/s11033-011-0770-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-0770-x