Abstract
Keratin-associated protein is one of the major structural proteins of the hair, whose content in hair has important effect on the quality of cashmere. In order to study the relationship between HGTKAP gene expression and cashmere fineness, the quantitative real-time RT–PCR (qRT–PCR) was firstly used to detect the levels of KAP7.1, KAP8.2 gene expression in the primary and secondary hair follicles; semi-quantitative RT–PCR was used to detect whether KAP7.1, KAP8.2 gene are expressed in heart, liver, spleen, lung, kidney tissues; and in situ hybridization(ISH) to detect KAP7.1 gene expression location. qRT–PCR result showed that the expression of both KAP7.1 and KAP8.2 gene in the secondary hair follicles are significantly higher than that in the primary follicles, relative quantitative analysis obtained that KAP7.1 was 2.28 times, while KAP8.2 was 2.71 times. Semi-quantitative RT–PCR results revealed that KAP 7.1 and KAP8.2 mRNA were not detected in the heart, liver, spleen, lung and kidney tissues, demonstrating that KAP7.1 and KAP8.2 were specially expressed in hair follicles, participating in hair formation. Moreover, KAP7.1 gene has a strong expression in the cortical layer, inner root sheath of the primary follicles and the cortical layer, inner root sheath and hair matrix of the secondary hair follicles by ISH analysis. Taken together, the evidence presented here indicated that in the formation of cashmere and wool, differential expression of these two genes in the primary and secondary hair follicles may have an important role in regulating the fiber diameter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Keratin and keratin-associated protein (KAP) are the main structural proteins of hair fiber, determining the quality of hair fiber together. Hair keratin consists of represent two multi gene families, the type I (acidic) and type II (basic) families, which form the skeleton of hair, what’s more, its content and structure are more stable in the different species hair. But KAP encoded by large multigene families differs greatly, which can be classified into high sulfur KAP, ultra-high sulfur KAP and high glycine- tyrosine KAP [1–3]. Study showed that quality of hair is closely relevant to the keratin composition of hair, and also subject to genetic factors. Cashmere characteristics significantly change in cashmere goat of the different regions and different species, which is caused by different expression of keratin genes [4]. Therefore, from the level of molecular to study keratin and KAP genes are the important means to control fiber fineness and improve cashmere quality.
Hair follicles of cashmere goat generate cashmere and wool. Observation on histological structure, we can see that the hair follicle can be divided into the outer epidermis, outer and inner root sheath, hair cuticle and cortical layer from outside to inside, while medullary layer only exists in the primary follicles [5]. The formation of mature hair is the upper hair matrix cell which continuously move upward, when they leave the germinal zone, the encoding gene of the two structural protein families in the hair matrix, cuticular Layer and cortical layers were activated, this two structural protein families are keratin and KAP, which consist of the basic structure of the hair [2]. The high glycine–tyrosine (HGT) KAPs encoded by KAP6n, KAP7 and KAP8 gene families are the smallest of wool keratin, whose expression is the greatest different in cashmere goat of different species [6].To date, there are few reports on HGTKAPs. Parsons et al. [7] found that the loci between KAP6 and KAP8 are chained with fiber diameter. Zhao et al. [8] had proved that KAP8.2 is significantly correlated with cashmere fineness. Zhao et al. [9] use PCR-SSCP technique to illustrate that KAP8.1 has a remarkable correlation with cashmere fineness. Liu et al. [10] regard the exons of KAP6.1 as a candidate gene of cashmere fineness. KAP6.1, KAP7 and KAP8 were localized in chromosome 1 in the study of sheep. Moreover, function similar gene is clustered together in the chromosome [3]. The above study indicated that HGTKAPs genes are fineness-related genes. However, there is no report about the expression of HGTKAPs gene in hair follicles. Therefore, in this report we firstly took unique genetic resources in China that Liaoning new-breeding cashmere goat skin hair follicles as the research object, and KAP7.1 and KAP8.2 of HGTKAPs gene families were selected as candidate genes. Quantitative real-time PCR, semi-quantitative RT–PCR and in situ hybridization were used to detect the expression characteristics of the two genes in primary and secondary follicles, aiming at improving the cashmere quality by regulating gene expression.
Materials and methods
Goats and hair follicle’s separation
Liaoning new-breeding cashmere goat were obtained from cashmere goat farm at Wafangdian, Dalian, China. In later October, the skin, heart, liver, spleen, lungs, kidneys tissues were collected after slaughtering and quickly placed in liquid nitrogen for preservation. The separation of primary and secondary hair follicles: Firstly cutting the skin of cashmere goat into a leash with width of 0.5 cm, exposing the complete hair follicle bulb after the removal of subcutaneous fat with dissecting needle to fix leash, using forceps bluntly separating the hair follicle tissues along the direction of hair follicles growth, and then using needle to remove surrounding tissues of follicles as far as possible under the premise of the hair follicles not damaged, and then immigrating into the culture medium of follicles.
Primer design
Using cashmere goat β-actin as internal standard gene, the primers of Actin and the gene of interest, KAP7.1 and KAP8.2 were designed by Primer 5.0 software according to the gene sequences of cashmere goats KAP7.1 (AY510121), KAP8.2 (AY510123) and β-actin (AF481159) reported by GeneBank (Table 1), and were synthesized from Dalian Takara Corporation.
Total RNA extraction and reverse transcription
Trizol (Invitrogen, USA) was used to extract total RNA from skin, heart, liver, spleen, lung and kidney, primary and secondary hair follicles. 1% agarose gel electrophoresis and UV spectrophotometer were used to detect the RNA quality and concentration. The total RNA samples were placed at −80°C reservation. Ahead of RT–PCR, the extracted samples were handled with Dnase I. Then, the purified total RNA needed to be detected by agarose gel electrophoresis and UV detection one more time. Reverse transcription was performed following the RT–PCR (Takara, Japan) Kit Manufacturing instructions. cDNA products were reserved in −20°C.
Quantitative real-time PCR
The qPCR assays were performed in a PCR Thermal Cycler Dice and Real Time system (Takara, Japan). Reaction system for the 25 μl, including 12.5 μl SYBR Premix Ex TaqII (2×), 1 ul upstream and downstream primers, 2 ul RT product, 9.5 ul dH2O. qPCR amplification conditions: 95°C predegeneration 10 s; 95°C denaturation 5 s; 60°C annealing 30 s, 72°C extension, 40 cycle. Test was firstly performed in an ordinary qPCR which can optimize experimental conditions, including annealing temperature and primer concentration. β-Actin served as a reference gene, each sample was repeated three times.
Semi-quantitative RT–PCR
cDNA first chain was synthesized according to the RT–PCR Kit (Tiangen, China) instruction, RT products were used as template and designed specific primers to amplify KAP7.1, KAP8.2 and β-actin cDNA fragment of goat. The reaction system (25 ul) is as follows: 2× Master Mix 12.5 ul, RT products 2 ul, the upstream and downstream primers 1 ul, deionized water 8.5 ul; reaction conditions: 94°C predegeneration 3 min, 94°C denaturation 30 S, 56.5°C annealing 30 s, 72°C 1 min, 30 cycles, 72°C extension 5 min. PCR products were examined by 1.0% agarose gel electrophoresis.
In situ hybridization
After cloning and sequencing, RT–PCR products of KAP7.1 gene were repeated purified, generated the KAP7.1 cDNA product concentration for 104.5 ng/ul, which was used as a Digoxigenin Labeled template. Label was performed according to digoxigenin kit instruction (Roche, Germany), and the prepared probes were stored in −20°C refrigerator. Tissue section of in situ hybridization were via dewaxing, rehydration, proteinase K digestion, dry, and then pre-hybridization 1 h with 50% deionized formamide, 42°C hybridization overnight, the hybridizated section was placed in turn in 2× SSC wet box, 1× SSC, 0.25× SSC washing, after hybridization adding antibody dilution according to the 1:500, combined with antibodies order to immunological detection, using NBT/BCIP color, and eosin-stained, neutral resin Seal Sheet. Observe by microscope and take photo.
Results
Quantitative real-time PCR
Standard curve of KAP7.1 and KAP8.2 shown in Supplementary Fig. 1 which were achieved according to different concentrations standard Ct value. Standard curve showed a good linear relationship, correlation coefficient R2 > 0.98, which achieve accurate quantificative goal in a wide range, better amplification efficiency, 0.8 < E < 1.2.
Internal standard gene β-Actin was used as the homogenization correction of KAP7.1, KAP8.2 gene quantitative results in Liaoning new-breeding cashmere goat primary and secondary hair follicles (Table 2). The results showed that: KAP7.1, KAP8.2 gene were expressed in both primary and secondary hair follicles, and the expression was more active in the secondary hair follicles than that in the primary hair follicles, KAP7.1 in the second hair follicles was 2.28 times than in primary follicles; KAP8.2 was 2.71 times as high as that in primary hair follicles (Fig. 1).
Semi-quantitative RT–PCR
Semi-quantitative RT–PCR analysis of KAP7.1 and KAP8.2 gene in the different tissues of cashmere goat, results showed that no signal were seen in heart, liver, spleen, lung and kidney of Liaoning new-breeding cashmere goat (Supplementary Fig. 2). It indicated that KAP7.1, KAP8.2 gene were specifically expressed in hair follicles and played an important role in hair formation.
In situ hybridization
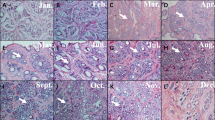
The positive results of ISH was seen based on the observation by Microscope, that was blue-violet hybridization signal, ISH results showed that KAP7.1 had a strong signal in the cortical layer of primary and secondary hair follicles (Fig. 2a, b, e). signal in the inner root sheath of in the primary hair follicles was found (Fig. 2h). Additionally, a clear signal (Fig. 2c, d) in inner root sheath and hair matrix in the secondary hair follicle were found as well, but no signal in the medulla layer of primary follicles. Each experiment had a separate control, in which no expression signal was found.
A, B the expression of KAP7.1 gene in the PF (slitting line, respectively ×100, ×400) C, D, E the expression of KAP7.1 gene in the SF (slitting line, respectively ×100, ×400) F the expression of KAP7.1 gene in the PF and SF (transverse section, ×100) G the expression of KAP7.1 gene in the SF (transverse section, ×100) H the expression of KAP7.1 gene in the PF (transverse section, ×400).
Discussion
Liaoning new-breeding cashmere goat is Chinese unique genetic resource whose characteristics are long cashmere, short wool and significantly exposing cashmere. Furthermore, cashmere yield ranked the first nation wide [11, 12]. Owing to the high quality cashmere, Liaoning new-breeding cashmere goat together with Inner Mongolia cashmere goat, are known as the most outstanding two representatives cashmere goat breed. In addition, cashmere length and yield were significantly higher than those of Inner Mongolia cashmere goat, but the cashmere fineness is thicker than that of Inner Mongolia cashmere goats. Cashmere is more valuable than wool, cashmere fineness are the determinants of price [13]. Therefore, the study of hair fiber diameter has become the most important part of Liaoning cashmere goat breeding. The experiment study fiber function associated genes, KAP7.1 and KAP8.2, aiming at controlling cashmere fineness of Liaoning new-breeding cashmere goat in the molecular level, and making cashmere quality to meet national good quality standards.
In general, cashmere goat is heterogeneous fleece composed of cashmere and wool which are respectively generated by the secondary and the primary hair follicles. The secondary hair follicle is differentiated from the primary hair follicle; but their structures and composition are very similar. However, it is surprising that there are obvious differences between wool and cashmere fineness. Previously study of human hair and different varieties of wool shows that the content of HGTKAPs (KAP6, KAP7 and KAP8) is less than 3% in human hair and the wool of sheep to 19%, and up to 30–40% in echidna quill [14], which indicates HGTKAP content changes in the different varieties of hair is relevant closely to the cashmere quality. Shu Weili et al. [15] also demonstrated that HGTKAPs can regulate the quality of hair fibers by the finding that the mutant of Australian Merino sheep has a significant reduction of HGTKAPs expression. Therefore, the research was the first analysis of the expression characteristics of KAP7.1, KAP8.2 gene by using quantitative real-time PCR in the primary and secondary hair follicles of Liaoning new-breeding cashmere goat. The results showed that both of KAP7.1, KAP8.2 have a significantly higher expression in the secondary hair follicles than that in the primary hair follicles, which presented respectively 2.28 and 2.71 times as high as those in the primary hair follicles. Therefore, we speculate that the two genes have function of regulating cashmere fineness.
In addition, the activity of hair follicles go through three regular changes including anagen, catagen and telogen in a year cycle [16]. In anagen, the villi rapidly grows and the expression of keratin genes is the most active. The previous research demonstrated that the difference of HGTKAP expression location is also the associated with the different cashmere quality [17]. Therefore, this research used ISH to analyze of the expression of KAP7.1 in hair follicles. The results showed that KAP7.1 gene has a strong signal in the cortex of the primary and secondary hair follicles in anagen, signal was also found in the inner root sheath of the primary and secondary hair follicles, all these indicate that KAP7.1 is the structural components of cortex and inner root sheath, participating in the formation of hornification and inner root sheath of villi, playing an important role in the cashmere growth and quality. In addition, hair matrix of secondary hair follicles also appeared expression signals (Fig. 2c, d) that KAP7.1 is the components of hair matrix of second hair follicles, moreover, has important implication on the growth of cashmere. But no signal in the outer root sheath (ORS), sebaceous glands and hair medulla, explained that KAP7.1 is not involved in the composition of ORS, sebaceous gland and hair medulla in hair follicle Anagen. Besides, expression pattern of KAP7.1 gene in the cashmere goat was identical to the expression in human, sheep and rabbits, which predominantly expressed in the cortex [2, 18, 19]. But the difference is that KAP7.1 in IRS of primary and secondary follicle is also expressed (Fig. 2c, d, h). This may be one of the reasons for different cashmere quality in the different species. PCR product of KAP8.2 after being sequenced varied greatly in sequence with Inner Mongolia cashmere Goats, whose causes need to be further studied. So this experiment did not detect KAP8.2 genes in ISH. Moreover, we used semi-quantitative RT-PCR to illustrate that KAP7.1, KAP8.2 were not expressed in Liaoning new-breeding cashmere goat’s heart, liver, spleen, lung and kidney, which showed KAP7.1, KAP8.2 were only expressed in the hair follicle. RT-PCR result was consistent with Rogers et al. [20] who demonstrate that a large number of KAP family members were expressed only in hair follicles.
At present, there were extensive researches on KAPs by domestic and foreign scholars. 30 KAP family members in human were located and qualitative. However, the exact function and regulation mechanism of KAPs remains unclear. Privious studies had shown that KAPs plays an important role in regulating cashmere quality, may be caused by the interaction of KAP–KAP, Keratin–KAP and Keratin–Keratin, and may be affected by other genes [18, 21, 22]. As Bawden et al. [23] who used transgenic method, illustrating when sheep K2.10 were over-expressing, the expression of KAP8 and KAP2 would be reduced; fiber quality would also change correspondingly. Jave-Suarez et al. [24] also demonstrated that the HOX13 selective regulates transcription of mice KAP16 gene cluster. The adjustment mechanism of KAP gene is key factor to achieve the cashmere growth of artificial control; and it has great significance to the cashmere goats breeding. Therefore, regulation mechanism of KAP7.1, KAP8.2 gene is worthy of our further research. In this study, we use quantitative real-time PCR, semi-quantitative RT-PCR and in situ hybridization firstly compare the expressions of KAP7.1, KAP8.2 gene in the primary and secondary follicles anagen. Two gene expressions play a regulatory role in cashmere fineness were investigated in the molecular level, laying a foundation for the study of HGTKAPs gene function in cashmere goat.
Abbreviations
- KAP:
-
Keratin-associated protein
- qRT–PCR:
-
Quantitative real-time PCR
- PCR:
-
Polymerase chain reaction
- ISH:
-
In situ hybridization
- PF:
-
Primary hair follicle
- SF:
-
Secondary hair follicle
References
Langbein L, Rogers MA, Winter H et al (2001) The catalog of human hair keratins. J Biol Chem 276:35123–35132
Rogers MA, Langbein L, Winter H et al (2002) Characterization of a first domain of human high glycine–tyrosine and high sulfur keratin-associated protein (KAP) genes on chromosome 21q22.1. J Biol Chem 277:48993–49002. doi:10.1074/jbc.M206422200
Yin J, Hu TM, Li JQ (2004) Cloning and analysis of six full-length cDNA similar to sheep KAP6-1 from cashmere goat. Acta Genet Sin 31:502–507
Jeffrey EP, Warren GB (2000) Application of proteomics for determining protein markers for wool quality traits. Electrophoresis 21:1899–1906. doi:10.1002/(SICI)1522-2683(20000501)21:9<1899:AID-ELPS1899>3.0.CO;2-R
Rogers GE (2004) Hair follicle differentiation and regulation. Int J Dev Biol 48:163–170
Powell BC, Rogers GE (1986) Hair keratin: composition, structure and biogenesis. Biol Integument 2:696–721
Parsons YM, Cooper DW (1994) Evidence of linkage between high-glycine–tyrosine keratin gene loci and wool fibre diameter in a Merino half-sib family. Anim Genet 25:105–108
Zhao J, Ren Y, Yue W (2007) Analysis of polymorphism on KAP8 gene in three goat breeds with PCR-SSCP. Biotechnology 17(5):3–6
Zhao M, Chen H, Wang X (2008) PCR-SSCP and DNA sequencing detecting two silent SNPs at KAP8.1 gene in the cashmere goat. Mol Biol Rep 36:1387–1391. doi:10.1007/s11033-008-9325-1
Liu GF, Tian KC, Zhang EP (2007) Candidate gene analysis of high quality merino sheep. Hereditas 29:70–74. doi:10.1360/yc-007-0070
Jin M, Fu ZY, Luan YY (2006) Cloning and evolution analysis of ZFX, ZFY partial gene in new-breeding cashmere goat and identification of its sex. Acta Vet Zootech Sin 37(6):530–536
Jin M, Cui YH, Fu ZY et al (2006) The correlation analysis of blood protein polymorphism with economics traits in Liaoning new-breeding cashmere goat. Hereditas (Beijing) 28(5):529–532
Li CQ, Yin J, Zhang YJ et al (2005) Comparative study on skin and hair follicles cycling between Inner Mongolia and Liaoning cashmere goats. Acta Vet Zootech Sin 36(7):674–679
Aoki N, Ito M (1997) Isolation and characterization of mouse high-glycine/tyrosine proteins. J Biol Chem 272(48):30512–30518
Shu WL, Ouyang HS, Rogers GE, Bawden CS (2009) Characterization of the structural and molecular defects in fibres and follicles of the merino felting luster mutant. Exp Dermatol 18:134–142. doi:10.1111/j.1600-0625.2008.00774.x
Cui ZF, Zhao J, Wang H (2008) Influences of different mediums on goat hair follicles cultured in vitro. Journal of Shandong Univ (Nat Sci) 43(5):1–5
Zhang JX, Yin J (2006) Expression of KAP7.1 gene in the inner mongolian cashmere goat skin. China herbivores. pp 76–77
Rogers MA, Langbein L, Praezel-Wunder S, Giehl K (2008) Characterization and expression analysis of the hair keratin associated protein KAP26.1. Br J Dermatol 159:725–729. doi:10.1111/j.1365-2133.2008.08743.x
Powell BC, Rogers GE (1997) The role of keratin proteins and their genes in the growth, structure and properties of hair. EXS 78:59–148
Rogers MA, Langbein L, Praetzel-Wunder S, Winter H, Schweizer J (2006) Human hair keratin associated proteins (KAPs). Int Rev Cytol 251:209–263. doi:10.1016/soo74-7696(06)51006-x
Kariya N, Shimomura Y, Ito M (2005) Size polymorphisms in the human ultrahigh sulfur hair keratin-associated protein 4, KAP4, gene family. J Investig Dermatol 124(6):1111–1118. doi:10.1111/j.0022-202x.2005.23662.x
Rogers MA, Winter H, Langhein L et al (2007) Characterization of human KAP24.1, a cuticular hair keratin-associated protein with unusual amino-acid composition and repeat structure. J Investig Dermatol 127:1197–1204. doi:10.1038/sj.jid5700702
Bawden CS, Powell BC, Walker SK, Rogers GE (1998) Expression of a wool intermediate filament keratin transgene in sheep fibre alters structure. Transgenic Res 7(4):273–287. doi:10.1023/A:1008830314386
Jave-Suarez LF, Winter H, Langbein L (2002) HOXC13 is involved in the regulation of human hair keratin gene expression. J Biol Chem 277(5):3718–3726
Acknowledgment
This work was supported by National Natural Science Foundation of China (No. 30571324 and 30972079), the Natural Science Fund of Liaoning Province (No. 20072151), and Science and technology plan project of Dalian (No. 2008B12NC079).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, M., Wang, L., Li, S. et al. Characterization and expression analysis of KAP7.1, KAP8.2 gene in Liaoning new-breeding cashmere goat hair follicle. Mol Biol Rep 38, 3023–3028 (2011). https://doi.org/10.1007/s11033-010-9968-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-9968-6