Abstract
Systemic acquired resistance (SAR) is an inducible defense mechanism which plays a central role in protecting plants from pathogen attack. A new elicitor, PeaT1 from Alternaria tenuissima, was expressed in Escherichia coil and characterized with systemic acquired resistance to tobacco mosaic virus (TMV). PeaT1-treated plants exhibited enhanced systemic resistance with a significant reduction in number and size of TMV lesions on wild tobacco leaves as compared with control. The quantitative analysis of TMV CP gene expression with real-time quantitative PCR showed there was reduction in TMV virus concentration after PeaT1 treatment. Similarly, peroxidase (POD) activity and lignin increased significantly after PeaT1 treatment. The real-time quantitative PCR revealed that PeaT1 also induced the systemic accumulation of pathogenesis-related gene, PR-1a and PR-1b which are the markers of systemic acquired resistance (SAR), NPR1 gene for salicylic acid (SA) signal transduction pathway and PAL gene for SA synthesis. The accumulation of SA and the failure in development of similar level of resistance as in wild type tobacco plants in PeaT1 treated nahG transgenic tobacco plants indicated that PeaT1-induced resistance depended on SA accumulation. The present work suggested that the molecular mechanism of PeaT1 inducing disease resistance in tobacco was likely through the systemic acquired resistance pathway mediated by salicylic acid and the NPR1 gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants respond to pathogens in a variety of strategies to protect themselves. The treatment of plants with specific biotic or abiotic agents can result in arouse of immunity system. The resistance induced by such treatments is generally characterized by a restriction of pathogen growth and a decrease in disease severity [1]. This form of induced resistance is generally referred to as systemic acquired resistance (SAR) [2]. The development of SAR has been closely correlated with the expression of a set of nine genes called SAR gene family [3]. Five of these gene families encode the pathogenesis-related (PR) proteins [4]. Some belong to protein classes (chitinases, β-1,3-glucanases, and thaumatin-like proteins) with demonstrated in vitro antifungal activity alone or in combination [5–9]. Those pathogenesis-related (PR) proteins play a role in the resistance response and the establishment of SAR, which is characterized by the systemic accumulation of some PR proteins and salicylic acid [10]. Salicylic acid (SA) is an important signaling molecule in the local (hypersensitive reaction, HR) and systemic (SAR) plant response against pathogen attacks [11]. Several lines of evidence suggest that SA is involved in the induction of SAR and application of SA induces resistance to pathogens and activates a set of genes as biological inducers of SAR [12]. In contrast, nahG transgenic tobacco is unable to accumulate SA and fail to develop SAR, due to expression of an introduced bacterial gene encoding salicylate hydoxylase, which degrades SA to catechol [13–15]. Recently, it is found that even though SA is involved in the establishment of SAR, the mobile SAR signal is not SA, but methyl salicylate (MeSA) [16]. It is also proved that the disease resistance induced by some inducers or elicitors was dependent on SA-mediated defences [17, 18]. Salicylic-acid signaling is mediated by at least two mechanisms, first requires the NON-EXPRESSOR OF PR1 (NPR1) gene and another is independent of NPR1 [19]. The analysis of expression level of a set of genes correlated to SAR will help to clarify the mechanism of the inducer or elicitor enhancing plant disease resistance.
In our earlier work, we have purified a microbial protein elicitor PeaT1 from Alternaria tenuissima [20] and cloned the gene (GenBank No. EF030819.1). Here, we reported its mode of action in inducing systemic acquired resistance against tobacco mosaic virus in tobacco plant. The present study will provide a basis for elucidation of molecular mechanism of PeaT1 in inducing disease resistance in plants.
Materials and methods
Plant material and TMV bioassay
The tobacco genotype, N. tabacum cv. Samsun-NN, a local lesion host for TMV, and seeds of its transgenic nahG tobacco were obtained from Institute of Genetics and Developmental Biology, Chinese Academy of Sciences. Xanthi’nn’, a systemic susceptible host for TMV, and TMV strain inoculated in Xanthi’nn’ used in the present study was a kind gift from the Institute of Plant Protection and Environmental Protection, Beijing Agricultural Sciences, Beijing, P.R. China. The fully expanded leaves of four to five leave stage Samsun-NN plants were mechanically inoculated with TMV in 0.01 M phosphate buffer (pH 7.0). The concentration of TMV solution was 1.0 g diseased leaves in 5 ml 0.01 M phosphate buffer (pH 7.0). After inoculation, plants were maintained at 25°C in a growth chamber with a day/night period of 16 h/8 h. The number and the size of TMV lesions on each leaf was recorded at 3rd day post TMV infection as previously described [21]. The lesion inhibition was calculated by following formula.
Expression and purification of elicitor PeaT1
The elicitor-encoding gene peaT1 was inserted into a pET28-(a) vector (Novagen) and transformed into E. coli BL21 (DE3). The recombinant expression of His-PeaT1 was induced by 1.0 mM IPTG (sigma, USA) for 4 h. The cells were harvested by centrifugation and disrupted with an ultrasonic disruptor. Then, the expressed crude protein was extracted from the supernatant and purified with a HisTrap™ HP column (GE Healthcare, USA) under the following conditions: 20 mM sodium phosphate, 0.5 M NaCl, 10 mM imidazole (pH 7.4) for binding, and 20 mM sodium phosphate, 0.5 M NaCl, 0.5 M imidazole (pH 7.4) for elution. Afterwards, the protein was applied to a HisTrap™ Desalting column (GE Healthcare, USA). Further purification of the recombinant protein was carried out with Mono Q™ 5/50 GL column (GE Healthcare) and bound protein was eluted with a linear gradient of NaCl concentration from 50 to 500 mM. After desalting by ultrafiltration tube (Millipore), the dissolving buffer was changed to phosphate buffered saline (PBS, pH 7.3). The soluble protein concentration was determined at all steps by BCA™ Protein Assay Kit (PIERCE, USA). Bovine serum albumin (BSA) (1.0 mg/ml) was used as a standard protein.
Bioassay for PeaT1-induced disease resistance in Samsun-NN tobacco
Induction of systemic acquired resistance was assayed on Samsun-NN plants. The three lower leaves of tobacco plants were infiltrated with 20 μg/ml of PeaT1 and with water as control. In the TMV infection assay, upper non-treated (systemic) leaves were mechanically inoculated with TMV at 3rd, 5th and 7th day after PeaT1 treatment. The observations on the number and the size of TMV lesions were recorded as described earlier. Nine plants were used for the individual treatment and each treatment was repeated three times. For the size of the lesions, 20 randomly selected lesions were measured for each leaf.
Analysis of POD activities
Samsun-NN plants were treated as described in bioassay experiment. At various times after PeaT1 treatment, the upper non-treated (systemic) leaves were harvested (0.2 g of fresh weight) and immediately frozen in liquid nitrogen and then stored in −80°C. The activity of POD was assayed according to the method as described previously [22]. The three individual tobacco plants were used in single treatment and each treatment was repeated thrice. The enzyme activity was expressed as unit (U) and one unit was defined as 100 times of the absorbance value per gram of fresh foliage per minute under the assay conditions.
Quantification of lignin concentration
The samples for lignin accumulation were collected as described for POD activity assay. The lignin content was performed according to the TGA method described by Brinkmann [23]. The content of lignin was detected by the absorbance at 280 nm. Three replicates were performed for each sample and experiment was repeated three times.
TMV CP gene analysis
Three lower leaves of the Xanthi’nn’ plants were infiltrated with 20 μg/ml of PeaT1 and with water as control. The treated leaves were mechanically inoculated with TMV 5th day after treatment. The untreated upper (systemic) leaves were harvested at 5th day after TMV inoculation for RNA extraction. Absolute quantification assay of TMV coat protein (CP) gene, as a molecular marker of TMV propagation, using real-time quantitative PCR TaqMan probe technology was performed. Primers used in this experiment were: 5′-ATGTCTTACAGTATCAACTAC-3′ and 5′-CCTGGAGACCAGGACGTTGA-3′ for TMV-CP gene; TaqMan probe used in this experiment was: 5′-TGGCCACCG TTGCGTCGTCTACT-3′, TaqMan probe was tagged by FAM and TAMRA.
The total RNA was extracted with an RNAprep pure Kit (TIANGEN) according to the standard protocol, and the accumulation level of TMV CP mRNA was determined by one step method. A 20 μl volume contained 2.5 μl RT-PCR buffer, 2.5 μl ExTaq buffer, 2 μl dNTP (2.5 mM), 4 pmol of each primer, 20 pmol TaqMan probe, 1 μl RNase Inhibitor, 300 ng of total RNA, 1 μl AMV Reverse transcriptase, 0.5 μl ExTaq (TAKARA, Japan), 11 μl RNase-free H2O. The PCR was processed on an IQ-5 Real-Time System (Bio-Rad, USA) under the following program: one cycle of 45°C for 30 min, 95°C for 10 min, then 44 cycles for 95°C for 15 s, 60°C for 1 min. Standard curves were constructed using serial dilutions of the plasmid with TMV CP gene. Three technical replicates were performed at the same time for one sample and repeated twice. According to the standard curves and threshold cycle values, the concentration of TMV CP RNA was analyzed.
Real time Q-PCR analysis of genes related disease resistance
Relative quantitative real time PCR analyses were performed to measure transcript levels of PR1a, PR1b, NPR1, PAL and PDF1.2 after PeaT1 treatment. Three lower leaves of the Samsun-NN plants were infiltrated with 20 μg/ml of PeaT1 and with water as control. The untreated upper (systemic) leaves were harvested at 4th day post treatment. The reverse transcription reaction was performed as mentioned above and the PCR with proper program was performed using the reverse transcription product as template. Primers used in this experiment were shown in Table 1. A 20 μl reaction volume contained cDNA produced from about 200 ng of total RNA, 4 pmol of each primer, and 10 μl of SYBR Green Real-time PCR Master Mix (TAKARA, Japan). The PCR was processed on an IQ-5 Real-Time System (Bio-Rad, USA) under the following program: one cycle of 95°C for 30 s; then 40 cycles of 95°C for 10 s, 58°C for 15 s and 72°C for 15 s. The melting curve was established from 55 to 95°C. Standard curves were constructed using serial dilutions of the cDNA of PeaT1 treatment. Three technical replicates of each reaction were performed and β-actin, as constitutively expressed gene, was used as internal control for normalization. Threshold cycle values were used for the further analysis. Quantification of the relative changes in gene transcript level was performed using the 2−∆∆Ct method as described by Livak and Schmittgen [24].
Quantification of SA accumulation after PeaT1 treatment
In order to confirm PeaT1 involvement of SA signal transduction pathway in the disease resistance, SA accumulation was detected after PeaT1 treatment. SA extraction was performed using a modified protocol derived from Verberne et al. [25]. The upper sprayed leaves were harvested (0.2 g of fresh weight) and immediately frozen in liquid nitrogen and then stored in −80°C. The tobacco leaves (0.2 g) were grinded into a fine powder in nitrogen. One millilitre of methanol (90%) was added to the grinded plant tissues, and the resulting mixture was vortexed and sonicated for 5 min. After centrifugation at 7,500×g for 10 min, the supernatant was collected and the precipitate was re-extracted with 0.5 ml of methanol (100%) and re-centrifuged at 7,500×g for another 10 min. The supernatants from both extractions were combined and air dried in a water bath (60°C). The dried samples were resuspended in 0.25 ml of 5% trichloroacetic acid (TCA), vortexed, sonicated for 5 min, and centrifuged at 7,500×g for 10 min. The supernatants were collected and extracted in 0.8 ml of ethylacetate/cyclopentane (v/v = 1/1) by vigorous vortexing for 30 min and centrifuged at 7,500×g for 10 min. The top organic phase (including SA) was collected and air dried in a water bath (60°C). The TCA phase was hydrolyzed using 0.3 ml 4 M HCl in a water bath (80°C) for 1 h, and added 0.8 ml of 1/1 (vol) ethylacetate/cyclopentane, incubated for 30 min by vigorously vortexing and then centrifuged at 7,500×g for 10 min. The top organic phase (including SAG) was combined and dried in a water bath (60°C). The residues was dissolved in 200 μl HPLC mobile phase (0.2 M KAc, 0.5 mM EDTA, pH 5), vortexed, sonicated for 2 min, and spun through a 0.22 μm nylon filter (Costar, Greenwich, CT). To measure the amount of extracted SA, the samples were separated through a Wakosil C18 column (4.6 × 250 mm) with a particle size of 3 μm and a pore size of 80 Å at a mobile-phase flow rate of 0.8 ml/min. Fluorescent detection was performed on an HPLC spectrofluorescence detector equipped with a Xenon-mercury arc lamp at an excitation/emission wavelength of 295/405 nm. Two measurements were performed and each with triplicate samples.
Effect of PeaT1 treatment in nahG transgenic tobacco plants
To further confirm the involvement of SA in the induced resistance by PeaT1 in tobacco, the transgenic plants N. tabacum cv. nahG Samsun-NN and wild type tobacco were treated with PeaT1 and TMV was inoculated at 5th day after treatment. The number and size of TMV lesion were recorded at 3rd day post inoculation.
Data analysis
Analyses of variance were conducted using the “SPSS 16.0” program for the experiment. Significant effects of treatment were determined by the least significant difference (LSD) (ρ = 0.05).
Results
Purification of elicitor PeaT1
After His-affinity and ion exchange chromatographic purification, expressed protein showed a single band on SDS-PAGE (Fig. 1). The purification was more than 90% as determined according to the soft of BandScan.
SDS-PAGE analysis of recombinant PeaT1. The protein expressed in Escherichia coli was purified with HisTrap™ HP column and then was loaded on Mono Q™ column. The elution was carried out with a linear gradient of NaCl concentration from 50 to 500 mM. The purified protein showing single band on SDS-PAGE was stained with Coomassie Brilliant Blue R-250. Lane 1: Molecular weight marker; Lane 2: Purified PeaT1 protein
Induction of resistance to TMV in tobacco by PeaT1
The PeaT1 enhanced the systemic disease resistance and reduced the number and size of the lesions of TMV in untreated upper tobacco leaves (Fig. 2). The reduction in lesion size was 45.74% at 5th day after TMV inoculation. But there was no significant difference at 5% significance level in the lesion size among the TMV inoculations at 3rd, 5th and 7th day post treatment (Table 2). Besides, we also observed the delay of 6 h in lesions appearance on PeaT1 treated tobacco plants compared to control plants treated with water.
Stimulation of systemic resistance against tobacco mosaic virus (TMV) in PeaT1-treated plants. Leaves (three per plant) were infiltrated with 20 μg/ml of PeaT1 and with water as control. The top untreated leaves were inoculated with TMV at 5th after treatment. Samples were photographed at 3rd day after TMV inoculation. The PeaT1-treated leaf inoculated with TMV showed fewer lesions (a), and the control leaf treated with water showed more lesions (b)
Stimulation of POD activity and lignin accumulation after PeaT1 treatment
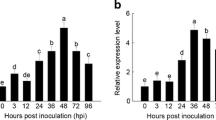
The POD activity on the upper untreated (systemic) tobacco leaves was measured at the time interval from 0 to 6 days after PeaT1 treatment. The higher POD activity appeared a peak at 4th day after treatment which showed 77.96% increase over the control (Fig. 3). Similarly, PeaT1 increased the lignin content in the untreated upper (systemic) leaves. The lignin accumulation reached to peak at 6th day after PeaT1 treatment (Fig. 3). Lignin is a major component of cell walls of plants and lignification process is a mechanism for disease resistant in plants. Therefore, the increase in lignin content suggested that PeaT1 enhanced disease resistance in tobacco.
Induction of peroxidase (POD) activity and lignin concentration in untreated (systemic) tobacco leaves after PeaT1 treatment. Three lower leaves of the Samsun-NN plants were infiltrated with 20 μg/ml of PeaT1 and with water as control. The untreated upper (systemic) leaves were selected for assay at different time intervals after treatment. Each value is an average of three independent experiments. Bar showed the mean ± standard deviation from three measurements
Effect of PeaT1 treatment on the mRNA level of TMV CP
The Taqman probe method of real-time PCR was adopted to determine TMV concentration in tobacco. The results showed that the TMV-CP RNA was decreased by 75.4% after PeaT1 treatment (Table 3). It indicated that PeaT1 treatment significantly reduced the TMV concentration in tobacco leaves. These results were consistent with the observation of virus symptoms in TMV bioassay.
Analysis of gene expression related to defense responses
The real-time quantitative PCR was employed to further quantify the expression of defense related genes. The specificity of RT-PCR products was documented with high resolution gel electrophoresis which resulted in a single product with the desired length. In addition, melting curve analysis was performed and it was found that there was only single product during 40 real-time PCR amplification cycles. The linear equations corresponding to each standard curve were worked out and the R2 value was more than 0.99 (Fig. 4). The data of the standard curves and linear equations showed the reliability and specificity of the PCR product and the system of PCR was good enough for analysis of gene expression level.
The expression level of PR1a, PR1b, NPR1, PAL, PDF1 genes after PeaT1 treatment. Real time Q-PCR analysis of relative genes expression in untreated upper (systemic) leaves of Samsun-NN plants after PeaT1 treatment at 4th day. a The melting curves; b standard curves; c relative expression level to control
The quantitative RT-PCR analysis revealed that the expression level of SA pathway related genes, PR1a, PR1b, NPR1 and PAL had differentially increased after PeaT1 treatment. There was no up-regulated expression of PDF1.2, the marker gene of Jasmonic acid (JA) and Ethylene signal transduction pathway in tobacco. Our results indicated that PeaT1 treatment induced the up-regulated expression of defense related gene and was through systemic acquired resistance mediated by salicylic acid in tobacco plant to enhance disease resistance.
Accumulation of SA in Samsun-NN tobacco plants after PeaT1 inducement
To determine SA dependent activation of the defence responses, the accumulation of SA was measured in tobacco plants. The treatment of PeaT1 resulted in an increase in SA levels and reached to peak at 24 h after treatment. However, the leaf treated with water (control) failed to show elevated SA levels (Fig. 5).
The accumulation of SA in tobacco plants treated with PeaT1. Three lower leaves of the Samsun-NN plants were infiltrated with 20 μg/ml of PeaT1 and with water as control. The treated leaves were harvested for SA assay at various time intervals after PeaT1 treatment. Bars are the mean ± standard deviation from two measurements (each with triplicate samples)
Induction of SA-dependent resistance to TMV in nahG transgenic tobacco plants
To further determine the dependence of PeaT1 on SA to induce disease resistance, the nahG transgenic tobacco plants, which are unable to accumulate SA, were examined. In nahG transgenic tobacco plants, there was no enhancement of disease resistance against TMV post PeaT1 treatment according to the number and the size of the lesions (Table 4). Furthermore, nahG plants treated with PeaT1 did not induce the expression of PR1a, PR1b (not shown). These results confirmed that PeaT1 is effective in inducing disease resistance in an SA-dependent manner.
Discussion
In present study, PeaT1 enhanced disease resistance against the viral pathogen TMV. During the induction of resistance, PeaT1 triggered POD activity, elevated lignin accumulation, reduced the TMV concentration and increased defence genes expression level in tobacco. These results indicated that a variety of disease resistance mechanisms were involved in induced resistance by PeaT1.
Several reports have suggested that POD is related to lignin and suberin synthesis, which increase the hardness of tissues, and to the production of quinones and active oxygen, which possess antibiotic properties [26–28]. Lignification has an important role in host defense against pathogen invasion and lignification is a mechanism for disease resistance in plants. In this study, we found that POD activity was increased and there was an accumulation of lignin after PeaT1 treatment. The increased activities in POD and lignin deposition have been thought to be key components in local and systemic disease resistance [29, 30]. The present results indicated that PeaT1 had already induced activation of defense related enzyme for disease resistance and produce some physical barrier against pathogen attack.
We found that TMV concentration was reduced by 75.4% in tobacco after PeaT1 treatment. It indicated that PeaT1 maybe stimulated the production of antiviral compounds and suppressed the virus replication or virus movement in tobacco plant which may be results in reduction in number and size of TMV lesions.
There was an increase in SA accumulation in PeaT1-treated tobacco leaves. The increase in endogenous SA level is sufficient to induce plant SAR [31]. So we inferred that SA signal transduction pathway was involved in the disease resistance to TMV after PeaT1 treatment.
Class 1 pathogenesis-related (PR) proteins are defense factors ubiquitously synthesized by plants in response to pathogen infections [32]. They are produced following the recognition of pathogen-derived molecules or elicitor by host plant cells and the activation of transduction pathways. Together with other defense proteins, such as β-1-3-glucanases, chitinases, and secondary metabolism enzymes including phytoalexin biosynthetic enzymes, they may contribute, directly or indirectly, to resistance to pathogen attack. The plant signaling networks governing PR-1 gene regulation during SAR are well understood [33]. In tobacco, only the subset of acidic PR-1 proteins (PR-1a, PR-1b, and PR-1c) as markers of SAR is secreted. PR-1 genes have been shown to be induced via separate signal transduction pathways and the expression of acidic PR-1 genes was activated primarily by SA-dependent pathway [34]. NPR1 is a key transcriptional co-regulator in plant defense responses. NPR1 is a regulatory gene of the SA signal pathway [35, 36]. The PAL gene is a key defence related enzyme gene due to its important role in enhancement of disease resistance and SA biosynthesis in plants [29, 37]. The plant defence gene PDF1.2 is JA-dependent gene [38]. PeaT1 induced the up-regulated expression of PR1a, PR1b, NPR1, PAL, but did not increase the expression of PDF1.2 gene. In addition, nahG transgenic tobacco had not showed the symptoms of disease resistance. It suggested the mode of action of PeaT1 was salicylic acid accumulation dependent. This study demonstrates that the disease resistance in tobacco induced by PeaT1 is through the systemic acquired resistance pathway mediated by salicylic acid and the NPR1 gene.
References
Hammond-Kosack KE, Jones JD (1996) Resistance gene-dependent plant defense responses. Plant Cell 8:1773–1791
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8:1809–1819
Ward ER, Uknes SJ, Williams SC, Dincher SS, Wiederhold DL, Alexander DC, Ahl-Goy P, Metraux JP, Ryals JA (1991) Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell 3:1085–1094
Bol JF, Linthorst HJM, Cornelissen BJC (1990) Plant pathogenesis-related proteins induced by virus infection. Annu Rev Phytopathol 28:113–138
Mauch F, Mauch-Mani B, Boller T (1988) Antifungal hydrolases in pea tissue inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase. Plant Physiol 88:936–942
Roberts WK, Selitrennikoff CP (1988) Plant and bacterial chitinases differ in antifungal activity. J Gen Microbiol 134:169–176
Vigers AJ, Roberts WK, Selitrennikoff CP (1991) A new family of plant antifungal proteins. Mol Plant Microbe Interact 4:315–323
Woloshuk CR, Meulenhoff JS, SelaBuurlage M, van den Elwn PJM, Cornelissen BJC (1991) Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell 3:619–628
Scott U, Brigitte MM, Mary M, Sharon P, Shericca W, Sandra D, Danielle C, Alan S, Eric W, John R (1992) Acquired resistance in Arabidopsis. Plant cell 4:645–656
Malamy J, Carr JP, Klessig DF, Raskin I (1990) Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250:1002–1004
Dempsey DA, Shah J, Klessig DF (1999) Salicylic acid and disease resistance in plants. Crit Rev Plant Sci 18:547–575
Kessmann H, Staub T, Hofmann C, Maetzke T, Herzog J, Ward E, Uknes S, Ryals J (1994) Induction of systemic acquired disease resistance in plants by chemicals. Annu Rev Phytopathol 32:439–459
Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J (1994) A central role of salicylic acid in plant disease resistance. Science 266:1247–1250
Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993) Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261:754–756
Shah J (2009) Plants under attack: systemic signals in defence. Curr Opin Plant Biol 4:459–464
Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318:113–115
Dong H, Delaney TP, Bauer DW, Beer SV (1999) Harpin induces systemic acquired resistance in Arabidopsis through the salicylic acid and NIM1-mediated signal transduction pathway. Plant J 20:207–215
Li Y, Zhang Z, Jia Y, Shen Y, He H, Fang R, Chen X, Hao X (2008) 3-Acetonyl-3-hydroxyoxindole: a new inducer of systemic acquired resistance in plants. Plant Biotechnol J 6:301–308
Shah J (2003) The salicylic acid loop in plant defense. Curr Opin Plant Biol 6:365–371
Zhao MZ, Yang XF, Zhang M, Yuan JJ, Qiu DW (2006) Purification and Bioactivities of a Protein from Alternaria tenuissima. Chin J Biol Control 23:170–173 (in Chinese)
Cordelier S, de Ruffray P, Fritig B, Kauffmann S (2003) Biological and molecular comparison between localized and systemic acquired resistance induced in tobacco by a Phytophthora megasperma glycoprotein elicitin. Plant Mol Biol 51:109–118
Sudhamoy M, Adinpunya M (2007) Reinforcement of cell wall in roots of Lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol Mol Plant Pathol 71:201–209
Brinkmann K, Blaschke L, Polle A (2002) Comparison of different methods for lignin determination as a basis for calibration of near-infrared reflectance spectroscopy and implications of lignoproteins. J Chem Ecol 12:2483–2501
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 25:402–408
Verberne MC, Brouwer N, Delbianco F, Linthorst HJ, Bol JF, Verpoorte R (2002) Method for the extraction of the volatile compound salicylic acid from tobacco leaf material. Phytochem Anal 1:45–50
Bowles DJ (1990) Defense-related proteins in higher plants. Annu Rev Biochem 59:873–907
Goodman RN, Kiraly Z, Wood KR (1986) The biochemistry and physiology of plant disease. University of Missouri Press, Columbia, pp 211–224
Stout MJ, Workman J, Duffey SS (1994) Differential induction of tomato foliar proteins by arthropod herbivores. J Chem Ecol 20:2575–2594
Karban R, Myers JH (1989) Induced plant responses to herbivory. Annu Rev Ecol Syst 20:331–348
Kombrink E, Somssich IE (1995) Defense responses of plants to pathogens. In: Andrews JH, Tommerup IC (eds) Advances in botanical research (incorporating advances in plant pathology), vol 21. Academic Press, London, pp 1–34
Verberne M, Muljono R, Verpoorte R (1999) Salicylic acid and biosynthesis. In: Hooykaas PJJ, Hall MA, Libbenga KR (eds) Biochemistry and molecular biology of plant hormones. Elsevier, London, pp 295–312
Van Loon LC, Van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55:85–97
Dong X (2001) Genetic dissection of systemic acquired resistance. Curr Opin Plant Biol 4:309–314
Niki T, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y (1998) Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiol 39:500–507
Mukhtar MS, Nishimura MT, Dangl J (2009) NPR1 in plant defense: It’s not over ‘til it’s turned over. Cell 137:804–806
Cao H, Bowling SA, Gordon AS, Dong X (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6:1583–1592
Mauch-Mani B, Slusarenko AJ (1996) Production of salicylic acid precursors is a major function of phenylalanine ammonialyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell 8:203–212
Guo X, Stotz HU (2007) Defense against Sclerotinia sclerotiorum in Arabidopsis is dependent on jasmonic acid, salicylic acid, and ethylene signaling. Mol Plant Microbe Interact 20:1384–1395
Acknowledgments
We thank Institute of Genetics and Developmental Biology, Chinese Academy of Sciences for providing seeds of the NahG Samsun-NN transgenic tobacco. We thank prof. Xinghong Li (the Institute of Plant Protection and Environmental Protection, Beijing Agricultural Sciences) for providing N. tabacum cv. Xanthi. nc and strains of the TMV. We also thank Sufang Zhao for her assistance in TMV bioassay experiment. This research was supported by Special Fund for Agro-scientific Research in the Public Interest (No. 200903052) and fund of State Key laboratory for Biology of Plant Disease and Insect (SKL2010OP05).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, W., Yang, X., Qiu, D. et al. PeaT1-induced systemic acquired resistance in tobacco follows salicylic acid-dependent pathway. Mol Biol Rep 38, 2549–2556 (2011). https://doi.org/10.1007/s11033-010-0393-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0393-7