Abstract
Docosahexaenoic acid (DHA) is one kind of ω-3 polyunsaturated fatty acids (PUFAs) and plays an important role in lipid metabolism. In this research, mice were daily intragastric administrated with DHA for 3 weeks. Subcutaneous adipose tissue and liver were separated every week, RNA was extracted. Peroxisome proliferator-activated receptor (PPARγ), Sterol regulatory element binding protein-1c (SREBP-1c), Fatty acid synthetase (FAS), Hormone sensitive lipase (HSL) and triglyceride hydrolase TGH genes expression were detected by quantitative PCR. Data showed that, DHA up-regulated PPARγ, HSL and TGH in adipose tissue, but it had no effect on SREBP-1c and FAS expression. However, in liver there were some differences in regulating these genes. PPARγ, SREBP-1c and FAS were down-regulated, HSL was up-regulated and TGH had no change. These results indicated that DHA played different regulating roles in lipid metabolism in different tissues. In adipose tissue, DHA increased the expression of lipogenesis and lipolysis genes. In liver lipogenesis genes were decreased, but lipolysis genes were increased by DHA. In conclusion, DHA could reduce body fat mass through regulating lipogenesis and lipolysis genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has become one of the most popular diseases in recent years. Researches on how to prevent obesity and cure relative diseases caused by obesity become very hot. DHA is an essential ω-3 PUFAs, and plays an important role in growth and functions as an important composition of nervous tissue and biomembrane [1, 2]. Many researches found that PUFAs can regulate lipid mobilization in many kinds of animals [3–5]. Dietary DHA not only affected tissue DHA concentration but also modified the expression of genes related to fatty acid metabolism [6]. ω-3 PUFAs impact lipid and sugar mobilization through regulating some transcription factors and enzymes. As transcription factors, PPARγ and SREBP-1c are known to regulate expression of many enzymes. SREBP-1c up-regulates the expression of FAS, and promotes fat synthesis [7–10]. Supplementation with long chain ω-3 PUFAs in human diet produced a decrease in fasting plasma triglyceride (TG) (−35%) and accompanied by a remarkable increase in the concentration of LPL mRNA in adipose tissue (+55%) [4]. ω-3 PUFAs also has positive therapeutic effect on cardiovascular diseases and hypertriglyceridemia [11–13]. Recent studies mainly focused on lipogenesis, however, there was few research on lipolysis. In our study, DHA was intragastric administrated to mice for 3 weeks, lipogenesis and lipolysis genes were analyzed. Results showed that DHA can decrease lipogenesis and increase lipolysis. It can provide both-around information for the treatment of obesity and other relative diseases.
Materials and methods
Animals
Male Kunming mice (2 weeks old) were purchased from the Fourth Military Medical University (Xi’an, China). These mice were maintained in a temperature-controlled room (25°C) on a 12-h light/dark cycle. The animals were fed a standard rodent chow diet, and had free access to food and water for 1 week of an acclimatization period. The mice were then divided into three groups randomly, control group (control), low concentration group (low) and high concentration group (high), each group had 15 mice, and each group had five repetition. Low and high concentration groups were daily intragastric administrated 6.25 and 12.5 g/kg DHA (Sigma) respectively, and control group was daily intragastric administrated 0.9% sodium chloride for 3 weeks. Every week five mice of each group were weighed after one-night fast and then killed by decapitation. Subcutaneous adipose tissue and liver were surgically removed and frozen in liquid nitrogen.
Gene cloning
Total RNA was extracted from adipose tissue and liver using Trizol reagent (Invitrogen, USA). First strand cDNA was prepared with RevertAidTM First Strand cDNA Synthesis Kit (Fermentas). Primers of β-actin, PPARγ, SREBP-1c, FAS, HSL and TGH genes were designed (Table 1). PCR reaction conditions were summarized in Table 1. The 50 μl PCR reaction contained 2 μl tissue-specific cDNA, 4 μl MgCl2 (25 mmol/l), 0.5 μl Taq DNA polymerase (Fermentas), 5 μl dNTPs (2.5 mmol/l), 5 μl 10× buffer and 1 μl of each primer (10 μmol/l). Product was examined by agarose gel electrophoresis (AGE).
Real-time quantitative PCR
We measured the expression of PPARγ, SREBP-1c, FAS, HSL, and TGH mRNA from samples of adipose tissue and liver by real time quantitative PCR. The 20 μl real-time reaction system contained 12.5 μl SYBR Premix EX Taq (Takara, Japan), 0.5 μl Forward Primer (10 μmol/l), 0.5 μl Reverse Primer (10 μmol/l), 1 μl cDNA, 10.5 μl ddH2O. Reactions were incubated in an TP800 Real-time System (Takara, Japan) for 10 s at 95°C, followed by 32 cycles of 95°C for 5 s and 60°C for 30 s, 95°C at 15 s, 60°C at 30 s, 95°C at 15 s.
Using real-time quantitative PCR to analyze the expression of these genes, the method of 2−ΔΔCt was also used. Relative quantification of gene expression was evaluated by utilizing the comparative critical threshold (Ct). The Ct values for each gene reaction were subtracted from the respective Ct value of the β-actin control, resulting in the ΔCt value. The largest ΔCt value was arbitrarily used as a constant that was subtracted from all other ΔCt values to determine ΔΔCt value. Fold changes were then generated for each gene by calculating 2−ΔΔCt.
Data analysis
Software SPSS 13.0 was used for statistical analysis. The expression of genes was analyzed with one-way ANOVA and LSD multiple comparison. Results were considered statistically significant if P < 0.05 (*) and extremely significant if P < 0.01 (**). All data from samples were shown as means ± standard error (SEM).
Results and analysis
DHA affected average daily gain (ADG) of mice
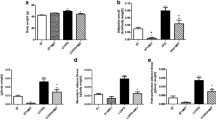
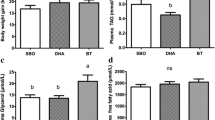
To investigate the effect of DHA on mice body weight, ADG was used to describe it. Every week before mice were killed, body weight (BWn) was weighed. ADG was obtained by formula (BWn − BWn−1)/7. Data showed that ADG had a significant decrease with time and dose increasing (P < 0.01) (Fig. 1). In control group, the ADG decreased gradually, it might be caused by sodium chloride.
Effect of DHA on lipogenesis genes mRNA expression in adipose and liver
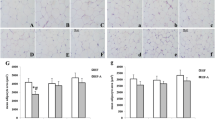
To examine the potential effect of DHA on lipogenesis, real-time PCR was used to determine the expression level of PPARγ, SREBP-1c and FAS, which were seen as lipogenesis genes. The β-actin was used as the internal standard to correct for small RNA loading differences. Results showed that in adipose tissue PPARγ mRNA expression increased significantly with time (Fig. 2) and DHA dose increasing (150, 152 and 127% compared with control for every week) (P < 0.05), however, SREBP-1c didn’t change in adipose tissue (P > 0.05) (Fig. 3). Compared with control group, there was no significant difference of FAS expression neither in low concentration group nor in high concentration group (P > 0.05) (Fig. 4). In liver DHA down-regulated PPARγ mRNA expression (51, 54 and 60% compared with control for every week) (P < 0.05). There was no time-dependent effect in previous 2 weeks, but in the 3rd week it had a significant decrease (P < 0.01) (Fig. 5). On the other hand, SREBP-1c (22, 27 and 31% compared with control for every week) (P < 0.05) and FAS (11, 21 and 28% compared with control for every week) (P < 0.05) were both down-regulated by DHA, and in the 3rd week in high concentration group SREBP-1c and FAS expressed the lowest level (P < 0.01) (Figs. 6, 7).
Effect of DHA on lipolysis genes mRNA expression in adipose and liver
To examine the potential effect of DHA on lipolysis, HSL and TGH were chosen as lipolysis genes because they are important genes in lipid hydrolysis process. β-actin was also used as the internal standard. The expression levels of these two genes at different time and doses were analyzed. Data showed that in mice adipose tissue DHA can up-regulate the expression of HSL (63, 69 and 82% compared with control for every week) and TGH (64, 69 and 76% compared with control for every week), and the level achieved the maximum in the 3rd week (P < 0.01) (Figs. 8, 9). The level change also depended on DHA dose (Figs. 8, 9). HSL increased significantly (41, 42 and 39% compared with control for every week) in liver after the treatment of DHA (P < 0.01) (Fig. 10).These findings indicated that DHA can up-regulate the expression of HSL both in adipose tissue and liver. However, TGH expressed no difference in liver among groups and weeks (P > 0.05) (Fig. 11).
Discussion
The PPARs were the nuclear receptors family, eicosanoids and fatty acids can regulate gene transcription through PPARs. PPARγ is one member of this family, and plays important roles in regulating lipogenesis and some diseases relative with obesity [14]. Human isolated adipocytes were treated with eicosapentaenoic acid (EPA) and DHA for 6 h, a significant increase in PPARγ mRNA was observed in the presence of EPA, but DHA had no significant change [15]. Our data showed that when treated mice with DHA for 3 weeks, PPARγ expression increased significantly compared with control group in adipose. And the significant increase appeared in the third and the 2nd week in low and high concentration, respectively. These results indicated that there were different effects in treating concentrations, slow effect in the low treatment and quick effect in the high. Adipose tissue was obtained from pigs fed diet containing 10% safflower oil (SO) for 12 weeks, the abundance of PPARγ mRNA was increased fourfold by SO compared with the control diet [16]. Antonella Trombetta [17] found that A549 human lung-adenocarcinoma cells were treated with arachidonic (AA) and DHA, PPARγ expression was up-regulated at 48 h. Dietary n-3 PUFAs decreased adipose tissue mass and suppressed the development of obesity in rodents by targeting a set of key regulatory transcription factors involved in both adipogensis and lipid homeostasis in mature adipocytes [18]. We found that PPARγ expression was down-regulated by DHA in liver, it was possible to have close correlation with lipogenesis in liver. However, there was no difference in weeks in low group, in the high there was a significant decrease in the 3rd week. Maybe DHA would not produce a marked effect unless it accessed to a certain concentration.
Except PPARγ, SREBP-1c is another transcriptional factor which plays important role in adipose deposition. Teruyo Nakatani [19–21] found that feeding fish oil decreased mice body weight and fat mass in a dose-dependent manner, in parallel with PPARα activation and a decrease of SREBP-1 mRNA in liver. When rat hepatocytes in monolayer culture were treated with albumin-bound 20:4(n-6) or 20:5(n-3) the half-life of total SREBP-1 mRNA was reduced by 50%. And the decay of SREBP-1c mRNA was more sensitive to PUFA than that of SREBP-1a [22, 23]. In our research, the expression of SREBP-1c in liver had a decrease comparing with the control (P < 0.01), but it was not a dose-dependent manner. We also found a time-dependent manner of SREBP-1c in liver (P < 0.01). Another research reported that SREBP-1c level was suppressed by PUFA in liver and hepatocytes [24], however, in adipose and 3T3-L1 adipocytes there was no difference. This indicates that the effects of PUFA on SREBP-1 gene expression are tissue-specific [25]. The same result was also found in pig by Hsu [6]. As the same, in our study DHA didn’t affect SREBP-1c expression in adipose (P > 0.05). In the 3rd week it had a decrease in the low (0.01 < P < 0.05), perhaps it would have to reach an exact concentration and time to effect the gene expression. In corpulent JCR:LA-cp rats, both the olive oil and menhaden oil diets reduced expression of SREBP-1c and FAS, with concomitant reductions in hepatic triglyceride content, lipogenesis, and expression of enzymes related to lipid synthesis [10]. The nuclear concentrations of hepatic SREBP-1 was 50% lower (P < 0.05) in rats that consumed a single PUFA-supplemented meal than the fat-free diet alone. This was paralleled by 63% reductions in the expression of FAS, which is the SREBP-1 target gene [26]. In obese mice PUFA markedly decreased the mature form of SREBP-1 protein and thereby reduced the expression of FAS in the liver [27]. Our data indicated the same results, in liver FAS expressed a lower level than the control, and had a dose-independent manner from the 2nd week. FAS activity was markedly lower in the liver but not in the adipose tissues of rats fed containing fish oil diet [5]. Treating bovine mammary cells with 75 μmol/l trans-10, cis-12 CLA for 48 h resulted in reductions in mRNA abundance for acetyl CoA carboxylase, FAS, and stearoyl CoA desaturase, but there was no reduction in SREBP-1 mRNA or precursor protein, whereas cis-9, trans-11 CLA had no effect on these genes [28]. This suggested that different PUFAs or cell type may have different effect. Therefore, it can explain our different result for FAS expression in adipose.
HSL is an intracellular neutral lipase that is capable of hydrolyzing triacylglycerols, diacylglycerols, monoacylglycerols, and cholesteryl esters, as well as other lipid and water soluble substrates. Its expression is highest in adipose tissue [29, 30]. In recent studies, TGH and ATGL are the two other enzymes in lipolysis besides HSL [31, 32]. Inhibiting TGH activity in primary rat hepatocytes could result in a dramatic decrease in secretion of TGs and secretion of cholesteryl ester and phosphatidylcholine was substantially decreased [33]. Mice were fed normal diet and high fat diet for 15 weeks, TGH and HSL expression had no difference in adipose between the two diets [34]. DHA can up-regulate HSL and TGH in adipose found in our study indicates that PUFA and normal fat has different effects on lipolysis. Feeding mice diets enriched in fatty acids for 3 weeks did not affect hepatic TGH expression, though a 3-week diet enriched in fatty acids and cholesterol increased hepatic TGH expression 2-fold [35]. This finding is coincidence with our conclusion in hepatic TGH expression. All these results suggest that TGH is not directly affected by DHA in liver, which pathway it influences is unknown. We also found DHA could up-regulate hepatic HSL in a dose-independent manner. It’s supposed that DHA affects lipolysis through regulating HSL expression or activity and it needs further researches to elucidate the mechanisms.
References
Tanaka K, Shimizu T, Ohtsuka Y, Yamashiro Y, Oshida K (2007) Early dietary treatments with Lorenzo’s oil and docosahexaenoic acid for neurological development in a case with Zellweger syndrome. Brain Dev 29:586–589. doi:10.1016/j.braindev.2007.02.005
Innis SM (2007) Dietary (n-3) fatty acids and brain development. J Nutr 137:855–859
Peyron-Caso E, Taverna M, Guerre-Millo M, Veronese A, Pacher N, Slama G, Rizkalla SW (2002) Dietary (n-3) polyunsaturated fatty acids up-regulate plasma leptin in insulin-resistant rats. J Nutr 132(8):2235–2240
Khan S, Minihane AM, Talmud PJ, Wright JW, Murphy MC, Williams CM, Griffin BA (2002) Dietary long-chain n-3 PUF as increase LPL gene expression in adipose tissue of subjects with an atherogenic lipoprotein phenotype. J Lipid Res 43:979–985
Peyron-Caso E, Quignard-Boulange A, Laromiguiere M, Feing-Kwon-Chan S, Veronese A, Ardouin B, Slama G, Rizkalla SW (2003) Dietary fish oil increases lipid mobilization but does not decrease lipid storage-related enzyme activities in adipose tissue of insulin-resistant, sucrose-fed rats. J Nutr 133(7):2239–2243
Hsu JM, Wang PH, Liu BH, Ding ST (2004) The effect of dietary docosahexaenoic acid on the expression of porcine lipid metabolism-related genes. J Anim Sci 82:683–689
Jump DB (2002) The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem 277:8755–8758. doi:10.1074/jbc.R100062200
Gregoire FM, Smas CM, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78:783–809
Yen CF, Jiang YN, Shen TF, Wong IM, Chen CC, Chen KC, Chang WC, Tsao YK, Ding ST (2005) Cloning and expression of the genes associated with lipid metabolism in Tsaiya ducks. Poult Sci 84(1):67–74
Deng X, Elam MB, Wilcox HG, Cagen LM, Park EA, Raghow R, Patel D, Kumar P, Sheybani A, Russell JC (2004) Dietary olive oil and menhaden oil mitigate induction of lipogenesis. Endocrinology 145(12):5847–5861. doi:10.1210/en.2004-0371
Mckenney JM, Sica D (2007) Prescription omega-3 fatty acids for the treatment of hypertriglyceridemia. Am J Health Syst Pharm 64:595–605. doi:10.2146/ajhp060164
Siddiqui RA, Harvey KA, Zaloga GP (2008) Modulation of enzymatic activities by n-3 polyunsaturated fatty acids to support cardiovascular health. J Nutr Biochem 19(7):417–437. doi:10.1016/j.jnutbio.2007.07.001
Abeywardena MY, Head RJ (2001) Longchain n-3 polyunsaturated fatty acids and blood vessel function. Cardiovasc Res 52:361–371. doi:10.1016/S0008-6363(01)00406-0
Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405:421–424. doi:10.1038/35013000
Chambrier C, Bastard JP, Rieusset J, Chevillotte E, Bonnefont-Rousselot D, Therond P, Hainque B, Riou JP, Laville M, Vidal H (2002) Eicosapentaenoic acid induces mRNA expression of peroxisome proliferator-activated receptor γ. Obes Res 10:518–525. doi:10.1038/oby.2002.70
Spurlock ME, Houseknecht KL, Portocarrero CP, Cornelius SG, Willis GM, Bidwell CA (2000) Regulation of PPARγ but not obese gene expression by dietary fat supplementation. J Nutr Biochem 11:260–266. doi:10.1016/S0955-2863(00)00076-0
Trombetta A, Maggiora M, Martinasso G, Cotogni P, Canuto RA, Muzio G (2007) Arachidonic and docosahexaenoic acids reduce the growth of A549 human lung-tumor cells increasing lipid peroxidation and PPARs. Chem Biol Interact 165:239–250. doi:10.1016/j.cbi.2006.12.014
Madsen L, Petersen RK, Kristiansen K (2005) Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim Biophys Acta 1740:266–286
Nakatani T, Kim HJ, Kaburagi Y, Yasuda K, Ezaki O (2003) A low fish oil inhibits SREBP-1 proteolytic cascade, while a high-fish-oil feeding decreases SREBP-1 mRNA in mice liver: relationship to anti-obesity. J Lipid Res 44:369–379. doi:10.1194/jlr.M200289-JLR200
Yoshikawa T, Shimano H, Yahagi N, Ide T, Amemiya-Kudo M, Matsuzaka T et al (2002) Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J Biol Chem 277(3):1705–1711. doi:10.1074/jbc.M105711200
Kim HJ, Takahashi M, Ezaki O (1999) Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. A possible mechanism for down-regulation of lipogenic enzyme mRNAs. J Biol Chem 274(36):25892–25898. doi:10.1074/jbc.274.36.25892
Ou J, Tu H, Shan B, Luk A, DeBose-Boyd RA, Bashmakov Y et al (2001) Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc Natl Acad Sci USA 98(11):6027–6032. doi:10.1073/pnas.111138698
Xu J, Teran-Garcia M, Park JHY, Nakamura MT, Clarke SD (2001) Polyunsaturated fatty acids suppress hepatic sterol regulatory element-binding protein-1 expression by accelerating transcript decay. J Biol Chem 276:9800–9807. doi:10.1074/jbc.M008973200
Ou J, Luong A, Goldstein JL, Brown MS (2001) Unsaturated fatty acids down-regulate srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells. J Biol Chem 276(6):4365–4372. doi:10.1074/jbc.M007273200
Mater MK, Thelen AP, Pan DA, Jump DB (1999) Sterol response element-binding protein 1c (SREBP-1c) is involved in the polyunsaturated fatty acid suppression of hepatic S14 gene transcription. J Biol Chem 274(46):32725–32732. doi:10.1074/jbc.274.46.32725
Xu J, Cho H, Malley SO, Park JHY, Clarke SD (2002) Dietary polyunsaturated fats regulate rat liver sterol regulatory element binding proteins-1 and -2 in three distinct stages and by different mechanisms. J Nutr 132:3333–3339
Sekiya M, Yahagi N, Matsuzaka T, Najinma Y, Nakakuki M, Nagai R, Ishibashi S, Osuga J, Yamada N, Shimano H (2003) Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology 38:1529–1539
Peterson DG, Matitashvili EA, Bauman DE (2004) The inhibitory effect of trans-10, cis-12 CLA on lipid synthesis in bovine mammary epithelial cells involves reduced proteolytic activation of the transcription factor SREBP-1. J Nutr 134:2523–2527
Kraemer FB, Shen WJ (2002) Hormone-sensitive lipase: control of intracellular tri-(di-) acylglycerol and cholesteryl ester hydrolysis. J Lipid Res 43:1585–1594. doi:10.1194/jlr.R200009-JLR200
Osuga JI, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O (2000) Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci USA 97:787–792. doi:10.1073/pnas.97.2.787
Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386. doi:10.1126/science.1100747
Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW (2004) Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem 279(47):48968–48975. doi:10.1074/jbc.M407841200
Gilham D, Ho S, Rasouli M, Martres P, Vance DE, Lehner R (2003) Inhibitors of hepatic microsomal triacylglycerol hydrolase decrease very low density lipoprotein secretion. FASEB J 17:1685–1687
Shen WJ, Patel S, Yu Z, Jue D, Kraemer FB (2007) Effects of rosiglitazone and high fat diet on lipase/esterase expression in adipose tissue. Biochim Biophys Acta 1771:177–184
Dolinsky VW, Gilham D, Hatch GM, Agellon LB, Lehner R, Vance DE (2003) Regulation of triacylglycerol hydrolase expression by dietary fatty acids and peroxisomal proliferator-activated receptors. Biochim Biophys Acta 1635:20–28
Acknowledgments
This work was supported by a grant from The National Nature Science Foundation of China (30871785), Program for New Century Excellent Talents in University of the Chinese Ministry of Education (NCET-06-0865) and the key Project of Chinese Ministry of Education (105167).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, C., Wei, Zw. & Li, Y. DHA regulates lipogenesis and lipolysis genes in mice adipose and liver. Mol Biol Rep 38, 731–737 (2011). https://doi.org/10.1007/s11033-010-0160-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0160-9