Abstract
Salinity and alkalinity are the two main environmental factors that limit rice production. Better understanding of the mechanisms responsible for salinity and alkaline stress tolerance would allow researchers to modify rice to increase its resistance to salinity and alkaline stress. MicroRNAs (miRNAs) are ~21-nucleotide RNAs that are ubiquitous regulators of gene expression in eukaryotic organisms. Some miRNAs acts as an important endogenous regulator in plant responses to abiotic stressors. miR393 is a conservative miRNA family that occurs in a variety of different plants. The two members of the miR393 family found in rice are named osa-MIR393 and osa-MIR393b. We found that the osa-MIR393 expression level changed under salinity and alkaline stress, whereas that of osa-MIR393b did not. Target genes of osa-MIR393 were predicted, and some of these putative targets are abiotic related genes. Furthermore, we generated transgenic rice and Arabidopsis thaliana that over-expressed osa-MIR393, and the phenotype analysis showed that these transgenic plants were more sensitive to salt and alkali treatment compared to wild-type plants. These results illustrate that over-expression of osa-MIR393 can negatively regulate rice salt-alkali stress tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Land plants face unpredictable stresses, such as salt, drought, cold, pests, and disease. For rice (Oryza sativa), salinity and alkaline stress are the main factors that affect rice yield, as they inhibit crop growth and development, reduce agricultural production, and, in severe conditions, result in plant death. Unlike animals, which can move to avoid environmental stresses, plants are immobile and thus have acquired particular mechanisms to cope with these stresses. Plants have evolved complex molecular and physiological regulatory systems to cope with adverse environmental changes. In order to increase rice yield, a better understanding of the mechanisms responsible for salinity and alkaline stress tolerance is needed. Such information would allow researchers to modify rice to increase its resistance to salinity and alkaline stress.
MicroRNAs (miRNAs) are ~21-nucleotide RNAs, and some of them have been shown to play important gene regulatory roles during plant development and in stress tolerance [1–3]. miRNAs repress expression of endogenous genes by guiding cleavage of complementary target mRNAs [4]. miR393 is a conservative miRNA family found in plants such as rice and Arabidopsis, and the expression level of its members has been shown to change under abiotic stress conditions in Arabidopsis [5–7].
In this study, we verified that the level of a miR393 member in rice (osa-MIR393) is regulated by salinity and alkalinity. Target genes of osa-MIR393 were predicted, and some of them were verified by an in vivo biological experiment. Target analysis showed that some of these target genes are stress-related genes. Furthermore, we generated transgenic Arabidopsis thaliana and rice that over-expressed osa-MIR393, and phenotypic analysis revealed that over-expression of osa-MIR393 decreased salinity and alkaline stress tolerance in these transgenic plants compared with wild type plants. These results confirm that osa-MIR393 is associated with salinity and alkaline stress in rice.
Materials and methods
Seedling cultivation and treatment
Dehulled seeds of rice (O. sativa L., cv. Kongyu 131, from the College of Agriculture, Northeast Agricultural University, Harbin, Heilongjiang Province, China) were sterilized with 5% sodium hypochlorite for 15 min and rinsed three times with sterilized water. The sterilized seeds were placed in glass tubes filled with 30 ml 1/8 Murashige and Skoog medium (MS medium) with 1% agar and then cultivated in a controlled cabinet under a 14/10 h-light, 30/24°C-temperature cycle for 10 days. Select seedlings showing consistent growth were and transferred to Yoshida nutrient solution according to the International Rice Research Institute (IRRI) [8] for 1 week. For salt and alkali treatment, seedlings were transferred to plastic containers containing Yoshida nutrient solutions with 150 mM NaCl or 75 mM NaHCO3 for different periods of time.
Seeds of Arabidopsis ecotype Columbia-0 were sterilized with 5% sodium hypochlorite for 10 min and rinsed two times with sterilized water. Sterilized seeds were sown on plates containing 1/2 MS medium with 0.9% agar. Plates were stratified in darkness at 4°C for 4 days and then transferred to controlled conditions (16/8 h-light, 23/20°C-temperature cycle) for 1 week. Seedlings showing consistent growth were selected and transferred to square-shaped plastic dishes (Cat. No. DS001-100, BBI, Markham, ON, Canada) filled with 1/2 MS medium (0.9% agar) for 3 weeks. For salt and alkali treatment, seedlings were transferred to dishes containing 1/2 MS-medium (0.9% agar) with 150 mM NaCl or 100 mM NaHCO3.
Reverse transcription PCR (RT-PCR)
Total RNA was extracted from each leaves tissue sample using plant RNA isolation reagent (Cat. No. 12322-012, Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. Reverse transcription reactions were performed in 20 μl using 5 μg of RNA by M-MuLV reverse transcriptase (Cat. No. #M0253 V, NEB, Ipswich, MA, USA). RT-PCR conditions for the elongation factor 1-α gene ef1-α of rice and for Arabidopsis (osa-ef1-α and ath-ef1-α) amplification were as follows: 94°C for 5 min; (94°C for 30 s, 55°C for 30 s, 72°C for 1 min) × 25 cycles; and 72°C for 5 min. For amplification of osa-MIR393 and osa-MIR393b, the same conditions were used, but the number of PCR cycles was increased to 33 and 30, respectively. The primer pairs used for RT-PCR and the predicted amplicon sizes were as follows:
-
osa-ef1-α: forward, 5′-GGAAGCCGCTGAGATGAACAA, and reverse, 5′-AAGAGCCTCAAGCAAGGTGGG (529 bp)
-
ath-ef1-α: forward, 5′-GTATGGTTGTTACCTTTGCTCCCACAG, and reverse, 5′-CGTCACTTCTTCCCACGGTTTACTAC (500 bp)
-
Precursor of osa-MIR393: forward, 5′-GCATCCAAAGGGATCGCAT, and reverse, 5′-TGATCCGTCAAGCTGCTTG (83 bp)
-
Precursor of osa-MIR393b: forward, 5′-GCATTGATCTGGCTAGCTATC, and reverse, 5′-AGGAAAATTCCAAAGGGATTG (91 bp)
Target gene analysis
The sequence of mature osa-MIR393 was input into psRNA Target (http://bioinfo3.noble.org/psRNATarget/), and putative targets with an expectation score of ≤3 were selected.
Vectors containing sequences of osa-MIR393 target genes were provided by the Rice Genome Resource Center (RGRC). The targets were ligated downstream of the 35S promoter, followed by the green fluorescent protein (GFP) gene and the Nos terminators in pCAMBIA1302. The HptII gene was inserted in T-DNA as a selection marker gene (see Supplementary Data). The transformation procedure of Nicotiana benthamiana was an adaptation of the methods described by Chakrabarty et al. [9]. Primary transformants were selected on MS agar medium supplemented with 10 mg/l hygromycin (Cat. No. K4378, Sigma, St. Louis, MO, USA).
Transgenic N. benthamiana plants expressing target-GFP fusion protein were selected by ultraviolet radiation using a 100 W hand-held long-wave ultraviolet lamp (Model B-100AP, UVP, Upland, CA, USA). Next, 3–5 week-old GFP-expressing seedlings were infiltrated with Agrobacterium tumefaciens (EH105 strain) containing the 35S promoter-driven osa-MIR393 precursor expression plasmid, and vectors used as controls. Infiltration of A. tumefaciens was based on a previously described method [10]. Seven days after infiltration, GFP-expressing conditions of transgenic N. benthamiana plants were observed by UV lamp. Infiltrated leaves were photographed using a camera (EOS 350D, Canon) with an orange filter (Gelatin filter No. 15, Kodak).
Phenotypic analysis of transgenic rice and Arabidopsis
miRNA precursor (pre-miRNA) of osa-MIR393 was synthesized by the GenScript Corporation (Piscataway, NJ, USA). The osa-MIR393 precursor was ligated downstream of the 35S promoter and followed by the 0.26 kb Nos terminators in the pCTx vector. The bar gene was inserted in T-DNA as a selection marker gene (see Supplementary Data). The expression plasmids were introduced into Arabidopsis and rice by A. tumefaciens (LBA4404 strain)-mediated transformation following the methods of Clough and Bent [11] and Toki [12]. Transgenic Arabidopsis seeds were identified by screening on medium containing 50 mg/l glufosinate (Cat. No. 45520, Sigma, St. Louis, MO, USA), and transgenic rice seedlings were selected using 0.5 mg/l glufosinate. The T1 plants of transgenic rice were also analyzed by Southern blotting for examination of transmission of transgenes.
The root length of wild and T3 transgenic Arabidopsis plants in square-shaped plastic dishes was measured (30 transgenic plants for every treatment, and the experiment was repeated three times) after stress treatment for 15 days. Data were analyzed and graphed using SPSS 13.0 for Windows, and data are expressed as means ± standard error (SE). Comparisons between transgenic and wild plant groups were made using the Student’s t-test. The non-treatment plant group was analyzed as the control.
For phenotypic analysis of transgenic rice, T1 transgenic rice plants were selected using 10 mg/l glufosinate. Ten days after germination, transgenic and wild rice seedlings showing consistent growth were transferred to 150 mM NaCl, 75 mM NaHCO3, or no stress Yoshida nutrient solution. Seven days after treatment, rice growth conditions were recorded using a camera.
Results
Salinity and alkaline stress regulate the osa-MIR393 level
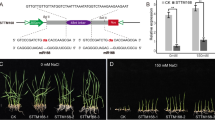
Two miRNA precursor sequences of miR393 family members in rice (osa-MIR393 and osa-MIR393b) were retrieved from miRBase. The time-series of expression level of these genes under salt and alkali treatment were detected by RT-PCR (Fig. 1). Because of obvious differential expression, osa-MIR393, a stress-regulated miRNA, was selected for further analysis.
Target gene analysis of osa-MIR393
MicroRNAs exert their biological function by regulating expression levels of target genes. We used psRNA Target to predict osa-MIR393 targets, and eight putative target sequences with a score of ≤3 were selected (see Supplementary Data). GO annotation revealed that these genes encode transport inhibitor response proteins, oxidoreductase, the phytosulfokine receptor precursor, GRF-interacting factor (GIF), and some unknown expressed protein.
To validate the interaction between miRNA and the predicted targets in vivo, we used a GFP-dependent method. Briefly, putative targets were fused to the 5′ end of GFP and introduced into N. benthamiana. Leaves from transgenic N. benthamiana were infiltrated with A. tumefaciens harboring the 35S promoter-driven osa-MIR393 precursor. If there is interaction between miRNA and its putative target, the area around the infiltrated point on the leaves will turn red under UV illumination due to the degradation of target-GFP fusion mRNA.
Three putative osa-MIR393 targets were selected to verify the interaction. Positive results were obtained in all the three tested genes: LOC_Os02g06260, LOC_Os05g41010, and LOC_Os05g05800 (Fig. 2). These target genes encode the phytosulfokine receptor precursor, a putative transport inhibitor response protein, and oxidoreductase, respectively. Base on previous studies [13, 14], these genes are involved in the salt and alkali stress response.
In vivo validation of interaction between osa-MIR393 and targets (LOC_Os02g06260, LOC_Os05g41010, and LOC_Os05g05800). N. benthamiana seedlings constitutively expressing target-GFP fusion proteins were selected under UV illumination. Leaves were infiltrated by A. tumefaciens harboring 35S promoter-driven osa-MIR393. Seven days after infiltration, the fluorescence was recorded by camera under a UV lamp. Arrows indicate the site of infiltration. The reddish area around the infiltration site indicates a positive interaction between osa-MIR393 and the target. Leaves infiltrated by an empty vector were used as controls
Phenotypic analysis of transgenic rice and Arabidopsis plants
To investigate the function of osa-MIR393 in plants, we over-expressed the osa-MIR393 precursor in transgenic Arabidopsis under the control of the CaMV 35S promoter. T3 generation seedlings of transgenic plants were then subjected to salt and alkali treatments followed by root length assay. When 150 or 100 mM NaHCO3 were applied, the growth of transgenic plants was dramatically inhibited (Fig. 3).
Effect of osa-MIR393 expression on salt and alkali tolerance in transgenic Arabidopsis plants. a Stress tolerance of plants over-expressing 35S-osa-MIR393. Top: wild-type and transgenic plants without stress treatment; middle: wild-type and transgenic seedlings treated with 150 mM NaCl for 15 days before photographs were taken; bottom: wild-type and transgenic seedlings treated with 100 mM NaHCO3 for 15 days before photographs were taken. b Root length of transgenic and wild-type seedlings under NaCl or NaHCO3 treatment. Error bars indicate SE. Analysis of significant differences and the graph were generated using SPSS, ** p ≤ 0.01. c Expression pattern of osa-MIR393 in wild type and transgenic lines by RT-PCR. cDNAs were normalized using the ath-ef1-α gene
We also generated transgenic rice (O. sativa L., cv. Kongyu 131) plants that over-expressed osa-MIR393. The T0 transgenic line was verified by Southern blotting analysis (Fig. 4a) and RT-PCR (Fig. 4b). T1 seeds from the transgenic rice line were germinated on 1/8 MS with 10 mg/l glufosinate for 10 days before being transferred to Yoshida nutrient solution with 150 mM NaCl or 75 mM NaHCO3. Similar to Arabidopsis, over-expression of osa-MIR393 decreased the salt and alkali stress tolerance of transgenic rice (Fig. 4c).
Effect of osa-MIR393 over-expression on salt and alkali tolerance in transgenic rice plants. a Southern blot analysis of transgenic rice. Rice DNA and vector DNA were digested by HindIII, run on a 0.8% agarose gel, transferred to a nylon membrane, and probed with a 32P-labeled fragment of the bar gene sequence. Positive vector and wild rice DNA were used as controls. b RT-PCR analysis of osa-MIR393 in transgenic rice. cDNAs were normalized using the osa-ef1-α gene. c Stress tolerance of osa-MIR393 transgenic rice and wild-type rice subjected to 150 mM NaCl or 75 mM NaHCO3 treatment. Top: wild-type and transgenic plants without stress treatment; middle: wild-type and transgenic seedlings treated with 150 mM NaCl for 15 days before photographs were taken; bottom: wild-type and transgenic seedlings treated with 75 mM NaHCO3 for 15 days before photographs were taken
Discussion
MicroRNA (miRNA)-guided post-transcriptional gene regulation is essential for normal growth and development and adaptation to stress conditions. Many computational predictions of potential plant miRNAs have been conducted [15], and characterization of miRNAs involved in plant stress responses is an active area of research [16, 17]. Previous studies suggested that members of the miR393 family likely are involved in plant stress response [18, 19]. Here we found that osa-MIR393 exhibits a more obvious expression change in response to salinity and alkaline stress compared with osa-MIR393b. This result prompted us to conduct further experiments to characterize the function of osa-MIR393 in plant salinity and alkaline stress responses.
Plant miRNAs generally execute their biological function via negative regulation of specific targets. Thus, it is necessary to study the targets of osa-MIR393. To do so, we conducted in silico target prediction for osa-MIR393 and then used an in vivo target validation method to detect the interaction between osa-MIR393 and its putative targets. In a previous study, Liu et al. generated a artificial microRNA (amiRNA) complemented of GUS-GFP sequence [20]. In the present study, we used a target-GFP fusion protein to detect the miRNA–target interaction. The results provided direct evidence that osa-MIR393 regulates salt and alkali stress responses in Arabidopsis and rice by targeting at least three genes: LOC_Os02g06260, LOC_Os05g41010, and LOC_Os05g05800. When we over-expressed osa-MIR393 in rice and Arabidopsis, transgenic plants showed enhanced sensitivity to salinity and alkaline stress. These results suggest that osa-MIR393 likely functions as a negative regulator of plant salt-alkali stresses responses.
Abbreviations
- miRNA:
-
MicroRNA
- GRF:
-
Growth-regulating factor
- GIF:
-
GRF-interacting factor
- MS medium:
-
Murashige and Skoog medium
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse transcription PCR
- pre-miRNA:
-
miRNA precursor
- GFP:
-
Green fluorescent protein
- RACE:
-
Rapid-amplification of cDNA ends
References
Bartel B, Bartel DP (2003) MicroRNAs: at the root of plant development? Plant Physiol 132:709–717
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Lai EC (2003) MicroRNAs: runts of the genome assert themselves. Curr Biol 13:R925–R936
Mallory AC, Vaucheret H (2006) Functions of microRNAs and related small RNAs in plants. Nat Genet Suppl 38:S31–S36
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16(8):2001–2019
Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 14(6):787–799
Zhao B, Liang R, Ge L, Li W, Xiao H, Lin H, Ruan K, Jin Y (2007) Identification of drought-induced microRNAs in rice. Biochem Biophys Res Commun 354(2):585–590
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice. IRRI, Los Banos
Chakrabarty R, Banerjee R, Chung SM, Farman M, Citovsky V, Hogenhout SA, Tzfira T, Goodin M (2007) pSITE vectors for stable integration or transient expression of autofluorescent protein fusions in plants: probing Nicotiana benthamiana-virus interactions. Mol Plant Microbe Interact 20:740–750
Voinnet O, Baulcombe DC (1997) Systemic signalling in gene silencing. Nature 389:553
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Toki S (1997) Rapid and efficient Agrobacterium-mediated transformation in rice. Plant Mol Biol Rep 15:16–21
Huang J, Zhang H, Wang J, Yang J (2003) Molecular cloning and characterization of rice 6-phosphogluconate dehydrogenase gene that is up-regulated by salt stress. Mol Biol Rep 30(4):223–227
Yang W, Liu X, Zhang J, Feng J, Li C, Chen J (2009) Prediction and validation of conservative microRNAs of Solanum tuberosum L. Mol Biol Rep. doi:10.1007/s11033-009-9881-z
Wang YC, Qu GZ, Li HY, Wu YJ, Wang C, Liu GF, Yang CP (2010) Enhanced salt tolerance of transgenic poplar plants expressing a manganese superoxide dismutase from Tamarix androssowii. Mol Biol Rep 37(2):1119–1124
Sunkar R, Chinnusamy V, Zhu J, Zhu JK (2007) Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci 12:301–309
Phillips JR, Dalmay T, Bartels D (2007) The role of small RNAs in abiotic stress. FEBS Lett 581:3592–3597
Sunkar R, Jagadeeswaran G (2008) In silico identification of conserved microRNAs in large number of diverse plant species. BMC Plant Biol 8:37
Liu HH, Tian X, Li YJ et al (2008) Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 14:836–843
Liu C, Zhang L, Sun J, Luo Y, Wang MB, Fan YL, Wang L (2010) A simple artificial microRNA vector based on ath-miR169d precursor from Arabidopsis. Mol Biol Rep 37:903–909
Acknowledgments
This project was supported by a grant from the Key Research Plan of Heilongjiang Province (GA06B103-3), the Innovation Research Group of NEAU (CXT004), the “863” project (2008AA10Z153), and the Basic Research Preliminary Study Foundation of the Ministry of Science and Technology of the PRC (2003CCA03500).
Author information
Authors and Affiliations
Corresponding author
Additional information
Peng Gao and Xi Bai contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, P., Bai, X., Yang, L. et al. osa-MIR393: a salinity- and alkaline stress-related microRNA gene. Mol Biol Rep 38, 237–242 (2011). https://doi.org/10.1007/s11033-010-0100-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0100-8