Abstract
Salsola kali pollen is a common cause of pollinosis during summer and early fall in desert and semi-desert regions. The aim of this study was the identification and characterization of Sal k 3, a new allergen from S. kali pollen. S. kali pollen extract was fractionated by SDS-PAGE and the allergenic profile was determined by IgE-immunoblotting using twelve S. kali allergic patients. Protein identification was carried out by the means of mass spectrometry. Using degenerated primers, two DNA fragments encoding N- and C-terminal domain of Sal k 3 were amplified by PCR, then cloned into the PTZ57R/T vector and sequenced. The open reading frame of Sal k 3 fragments were subcloned in the pET-32b(+) vector, expressed in E. coli, and purified by Ni2+ affinity chromatography. The IgE-binding capacity of rSal k 3 fragments was then studied by IgE-immunoblotting, inhibition assays, and skin prick tests. A 45-kDa allergen was identified as a fragment of the cobalamin-independent methionine synthase (MetE) by mass spectrometry and was detected in the sera of 8/12 (66.6%) of S. kali allergic patients. Moreover, inhibition assays demonstrated that the purified rSal k 3 fragments were similar to their counterparts in the crude extract. Sal k 3 represents a new allergen of S. kali pollen and seems to be an important allergenic compound in S. kali pollen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salsola kali (Russian thistle), a well-known member of the Amaranthaceae family, is common throughout most arid and semiarid regions of the world, including the central and western regions of Canada, the United States and the coasts of Europe, North Africa, Asia and Australia [1–6]. Moreover, the inhalation of Salsola kali (S. kali) pollen is one of the main causes of respiratory allergic diseases in semiarid countries such as Iran, Saudi Arabia, and Kuwait, where the frequency of sensitization ranges from 53 to 76.7% [1, 2, 5, 6].
In 1981, Shafiee et al. reported two allergenic proteins with molecular weights of 39.0 and 42.0 kDa in S. kali pollen [7]. Furthermore, in two separate studies, a S. kali pollen-allergen with an apparent molecular weight (MW) of 40 to 43 kDa was identified, which also showed pectin methylesterase (PME) properties [8, 9]. This allergen was named Sal k 1 and is considered a major allergen of the S. kali pollen. More recently, using sera from Iranian S. kali allergic patients, the IgE-binding profile of the S. kali pollen extract was explored and showed that it was mainly composed of five allergenic components including 25, 39, 45, 66, and 85 kDa [2].
In this study, a new allergen of the S. kali pollen was identified and characterized as a cobalamin-independent methionine synthase. This was designated as Sal k 3 according to the WHO/IUIS system of allergen nomenclature.
Materials and methods
Patients, pollen extract preparation and skin prick test (SPT)
Twelve patients, seven women and five men, presenting to the Immunology Research Center of Avicenna Research Institute were included in this study on the basis of a case history of seasonal allergy without asthma, and a positive SPT to S. kali pollen extract (Supplementary Table 1). Four subjects who showed negative SPT responses and no specific IgE to S. kali pollen extract (numbers 13–16) were considered as negative control (Supplementary Table 1).
Two grams of the S. kali defatted pollen (purchased from Greer Laboratories, USA) with a purity of more than 95% were extracted as described previously [2]. The protein content of the extract was measured by Bradford’s method [10].
SPT with S. kali pollen extract was performed as described previously [2]. The Human Ethics Committee of Mashhad University of Medical Sciences (Mashhad, Iran) approved the study protocol with informed written consent from each patient.
Measurement of total and specific IgE
Total serum levels of IgE were measured in duplicate by a commercial ELISA kit (RADIM, Italy) according to the manufacturer’s instructions. To determine specific IgE reactivity of S. kali pollen extract, all 12 sera were evaluated by ELISA as previously described [11]. The four control subjects who showed negative SPTs to S. kali pollen extract (Supplementary Table 1, sample numbers 13–16) were also used as negative controls. Optical density (OD450) greater than four times the median values of negatives control was considered to be positive.
SDS-PAGE and IgE-immunoblotting
The S. kali extract and recombinant proteins were fractionated by means of SDS-PAGE in accordance with the method of Laemmli [12] on Bio-Rad Miniprotean II System gels (12.5% polyacrylamide) and then electrotransferred onto polyvinylidene difluoride (PVDF) membranes, as described elsewhere [2].
MALDI-TOF MS analysis
The IgE-reactive protein band with the MW of 45 kDa from the S. kali pollen extract which had been identified by immunoblotting was excised from the Coomassie brilliant blue-stained SDS-PAGE gel and sent for mass spectrometry. Mass spectrometry was performed at the Genomic Research Center of the University of Hong Kong, using ABI 4800 MALDI-TOF/TOF Analyzer (Applied Biosystems, USA).
The proteins were reduced, alkylated, and digested with trypsin. Peptide mass fingerprinting data was also employed to search in the NCBI nonredundant protein databases using Mascot software from Matrix Science (http://www.matrix-science.com) of Viridiplantae and other green plants as the taxonomy. One missed cleavage of trypsin per peptide, an accuracy level of 75 ppm and a mass tolerance of 1 Da were allowed.
Amplification of Sal k 3 cDNA by PCR and determination of nucleotide sequence
According to the results, immunoblotting and mass spectrometry, we assumed that the protein band with the apparent MW of 45 kDa, which identified as cobalamin-independent methionine synthase (MetE with the approximate MW of 85 kDa) was a breakdown fragment of the MetE. This assumption was in line with several previous observations that some proteins in pollen extracts may be proteolytically degraded during extract preparation and purification [13–16]. Moreover, Utley et al. showed that the reduction of Met synthase molecule during SDS-PAGE resulted in the appearance of three bands with MWs of almost 90, 45, and 35 kDa. Crystallographic structure of MetE of Arabidopsis thaliana (AtMetE) also revealed that inside the members of the zinc metalloenzymes family, there were two conserved domains including the N-terminal and C-terminal which linked together with a loop [17]. We thus designed two pairs of degenerate oligodeoxynucleotide primers for cDNA amplification of N- and C-terminal domains of S. kali MetE (SkMetE) based on the amino acid sequence comparisons of different plant MetEs. The primers used were the sense primer: 5′- ATGGCDTCHCACRTTGTTGG-3′ and the antisense primer: 5′-AACAGCRTWATTHMTBACRCMSGG-3′ for N-terminal domain and the sense primer: 5′- AGAGTVACDAARGAAGCTGTTCA-3′ and the antisense primer: 5′-GATGRKCTTRGCHGCAKMMACCAT-3′ for C-terminal domain of MetE with a seven nucleotides overlap. Two fragments of SkMetE coding region were amplified with Pfu DNA polymerase (Fermentas, Lithuania) and were cloned into the pTZ57R/T vector included in Ins TAcloneTM PCR cloning kit (Fermentas, Lithuania). The resulting plasmids were used to transform TOP10 E. coli cells. The recombinant plasmids were then subjected to sequencing (SeqLab, Germany).
Construction of prokaryotic expression plasmid carrying Sal k 3 fragments
Based on the obtained sequences, specific primers for both Sal k 3 fragments were designed. These primers contained overhangs with NotI and XhoI restriction sites for direct cloning into the expression plasmid pET-32b(+) (Novagen, USA) as follows: the sense primer (5′-TCCGCGGCCGCAATGGCGTCTCACGTTGTTG-3′) and the anti-sense primer (5′-TCTCTCGAGAACAGCTTCGTTGTTAACTC-3′) for the N-terminal domain, and the sense primer (5′-TCCGCGGCCGCAAGAGTTACCACCGAAGCTG-3′) and the anti-sense primer (5′-TCTCTCGAGGATGGGCTTGGCCGCAT-3′) for the C-terminal domain of SkMetE (the NotI and the XhoI restriction sites are underlined).
The resulting amplification products were digested by the NotI and the XhoI restriction enzymes as the manufacturer’s protocol (Fermentas, Lithuania). The purified digested PCR products were then ligated into the digested pET-32b(+) plasmid with the same enzymes. The recombinant expression vectors provided a sequence coding for a thioredoxin (TrxA) partner, to increase the solubility, and two hexahistidyl (6×His) affinity tags at the 5′ and 3′ ends of the N- and the C-terminal of SkMetE (Sal k 3) coding sequence.
Expression and purification of recombinant Sal k 3 fragments
The E. coli expression strain BL21 (DE3)pLysS (Invitrogen, USA) was transformed by recombinants plasmids harboring the genes of the N-terminal and C-terminal domains of the SkMetE, cultured in LB medium containing 50 μg/ml ampicillin, and incubated at 37°C. Subsequent to reaching an OD600 of 0.4, protein expression was induced by the addition of isopropyl-β-D-thiogalactosidase (IPTG) to a final concentration of 0.2 mM [18]. After induction, in order to improve the solubility of rSal k 3, the cultures were incubated at 18°C and the cells were allowed to grow for a period of 12 h. The cells were then disrupted by freezing in liquid nitrogen following by thawing at 37°C.
The purification of rSal k 3 was performed by a Ni-NTA agarose (Invitrogen, USA) from the soluble phase of lysate, according to the manufacturer’s instructions. Protein concentration was determined according to the Bradford’s method [10].
Sequence analysis and homology modeling of SkMetE
The deduced amino acid sequence of SkMetE was subjected to a BLAST similarity search. Conserved residues between SkMetE and the other MetEs of Beta vulgaris (BAE07181), Solanum tuberosum (AAF74983) and Arabidopsis thaliana (NP-197294) were mapped with multiple sequence alignment using the ClustalW software (http://www.ebi.ac.uk/clustalw/).
The homology model of Sal k 3 was generated using the coordinates from the cobalamin-independent methionine synthase of Arabidopsis thaliana (AtMetE) (PDB Number: 1U1 J) by the Internet server Swiss Model (http://swissmodel.expasy.org/SWISS-MODEL.html), as previously described [11]. The AtMetE has 83% sequence identity with the SkMetE.
ELISA inhibition
ELISA inhibition assays were performed as described above, except that a pooled serum (1:2 v/v) from S. kali allergic patients (Nos. 1, 2, 3, 6, and 9) was preincubated for 1 h at room temperature with either 1000, 100, 1, 1, 0.1 or 0.01 μg of the purified specific recombinant proteins as inhibitors or with BSA as a negative control. Percentage of inhibition was calculated using the following formula: (OD of sample without inhibitor − OD of sample with inhibitor/OD of sample without inhibitor) × 100.
Immunoblotting inhibition
The IgE-immunoblot inhibition experiments were performed as described previously [2], except that the pooled sera (1:5 v/v) from S. kali allergic patients (Nos. 1, 2, 3, 6, and 9) was preincubated for 3 h at room temperature with an equal volume of each purified inhibitor or with BSA as negative control.
Results
Immunoreactivity of S. kali pollen extract
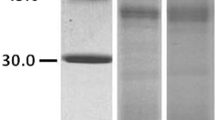
SDS-PAGE of S. kali pollen extract indicated several proteins with MWs approximately from 15 to 97 kDa (Fig. 1a). The results also revealed that the overall pattern of migration of the Sal k 3 and its expected N- and C-terminal fragments was not considerably changed under both non-reducing and reducing conditions (data not shown). However, on the reduced gel, the content of the two bands with the apparent MWs of 45- and 39-kDa were greater than those on the unreduced gel.
SDS-PAGE and immunoreactivity of S. kali pollen extract and recombinant Sal k 3 fragments. a Coomassie Brilliant Blue (CBB) stained SDS-PAGE of crude extract of the S. kali pollen, recombinant C- and N-terminal fragments of Sal k 3 (rSal k 3-CF and rSal k 3-NF, respectively) on a 12.5% polyacrylamide gel. Lane M, molecular weight (Amersham, UK); b IgE-immunoblot of S. kali pollen extract using allergic patients’ sera; lanes 1–12, probed with individual allergic patients’ sera; lane C−, negative control; c and d IgE-immunoblotting analysis of N-terminal and C-terminal fragments of the rSal k 3 using 12 S. kali allergic patients, respectively
The levels of specific IgE to S. kali pollen extract were determined in 12 individual patient’s sera (Supplementary Table 1). All patients had significantly elevated specific IgE to S. kali pollen extract. Serum samples from S. kali allergic patients were further tested for IgE reactivity to S. kali pollen extract by immunoblotting assay. The IgE-binding profile of S. kali pollen extract was mainly composed of several allergenic components of approximately 25, 39, 45, 66, and 85 kDa (Fig. 1b).
MALDI-TOF MS
The protein band corresponding to the identified allergen and with the apparent MW of 45-kDa from the S. kali pollen (Fig. 1a) was excised from the polyacrylamide gel and analyzed by MALDI-TOF MS. The MS profile of the protein band is shown in Fig. 2. By comparison with the established databases, a protein with a score of >85 and more than two matched peptides was identified as significant. According to the peptide mass fingerprint analysis, the masses obtained from the excised protein band showed the highest correlation with the methionine synthase (MetE) from Solanum tuberosum, Hordeum vulgare, Helianthus annuus, Glycine max and Arabidopsis thaliana (Table 1). As shown in Fig. 2, seven out of the nine matched peaks belonged to prominent mass peaks. Although, significant expectation values were achieved for the protein with the apparent MW of 45-kDa, there was a difference between its theoretical molecular weight and that observed by the MALDI-TOF MS. An explanation of this difference may be that the protein with the MW of about 45-kDa is essentially a fragment of the MetE with the apparent MW of 85 kDa. This assumption is in line with several studies which showed an enzymatic degradation of pollen extract proteins during extract preparation and purification and also following the during SDS-PAGE [13–16, 19]. Moreover, a prominent band with the approximate MW of 85 kDa from the S. kali pollen extract was also observed.
Cloning of Sal k 3 cDNA, sequence analysis and homology modeling
The size of the PCR products of two N- and C-terminal fragments of Sal k 3 were 1194–1098 bp, respectively. Sequence analysis of Sal k 3 showed an open-reading frame of 2,271 bp coding for 757 amino acid residues with a predicted molecular mass of 83.57 kDa, a calculated pI of 6.17, and three potential N-glycosylation sites at Asn-101, -339, and -566. The obtained sequence was deposited in the NCBI GenBank (Accession Number: FJ821454). Comparison of the deduced amino acid sequence of Sal k 3 with other plant MetE in a protein database was performed (Supplementary Fig. 3). The results showed that SkMetE shared the highest amino acid sequence identity with the MetE of Beta vulgaris (91%) (BAE07181) belonging to the Amaranthaceae family. Moreover, SkMetE shared 89% identity with the MetE of Solanum tuberosum (AAF74983) and 88% with MetE of Arabidopsis thaliana (NP-197294).
Three-dimensional structure of the SkMetE is shown in supplementary Fig. 4. The models were evaluated in terms of stereochemical and geometric parameters such as bond lengths, bond angles, torsion angles, G-factor, and packing environment, and it was found to satisfy all stereochemical and geometric criteria. No residue was located in the disallowed regions of the Ramachandran map. After energy minimization of the models, the overall conformational energy of comparative models of the SkMetE was 180 kcal/mol. Main-chain Cα atoms of 1LULJ and the SkMetE superimpose with an RMS deviation of 0.74 Å.
Based on previous crystallographic analysis of the MetE of A. thaliana (AtMetE) [17], MetE has a monomeric structure and is built by two domains, including the N-terminal domain extending from residues 2 to 391 and the C-terminal domain from residues 392 to 765. Moreover, there is a linker between the two domains consists of a loop which extends from residues 390 to 395 (Supplementary Figs 3 and 4).
Expression and purification of rSal k 3 fragments
The recombinant N- and C- terminal fragments of Sal k 3, with the apparent MWs of 64 and 59 kDa, respectively (Fig. 1a), were present as soluble form in the supernatant. These two fragments were further purified by Ni2+-chelate affinity chromatography. The purified rSal k 3 fragments were quantified using the Bradford’s protein assay which showed that approximately 1.2 and 1.5 mg of the N- and C-terminal fragments of the recombinant SkMetE had been purified from 1 l of bacterial expression medium, respectively.
Immunoreactivity of rSal k 3 fragments
The levels of specific IgE to each fragment of purified rSal k 3 were determined using 12 individuals patients’ sera (Supplementary Table 1). Eight patients (Nos. 1, 2, 3, 5, 6, 9, 10, and 12) had significant specific IgE levels to both purified rSal k 3 fragments.
Serum samples from the patients allergic to S. kali pollen were further tested for IgE reactivity to the N- and C-terminal fragments of the rSal k 3 by immunoblotting assays. Analysis of purified recombinant proteins revealed that the recombinant forms of both fragments of the Sal k 3 were reactive with eight individual sera (Fig. 1c and d, lane 1, 2, 3, 5, 6, 9, 10, and 12). These results were consistent with those obtained from specific IgE ELISA.
IgE-binding reactivity of the purified TrxA protein was evaluated by immunoblotting and ELISA assays and showed no reaction with the pooled sera from S. kali allergic patients (data not shown).
IgE-binding capacity of rSal k 3 fragments
In order to evaluate the inhibition of IgE-binding to immobilized rSal k 3 fragments by natural S. kali pollen extract and recombinant Sal k 3 fragments, ELISA inhibition assays were performed using a pooled serum from the allergic patients (Nos. 1, 2, 3, 6, and 9). An almost complete inhibition was obtained when 1 mg/ml of each fragment of the rSal k 3 used was as a positive control (Fig. 3).
Immunoblot inhibition assays were performed to investigate the IgE-binding capacity of the purified rSal k 3 compared to its natural counterpart in S. kali pollen extract. Pre-incubation of serum samples with each fragment of the rSal k 3 completely inhibited the IgE binding to all three protein bands with apparent MWs of 85-, 45- and 39 kDa (Fig. 4, lane 3). These observations may be another indication of proteolytically degradation of SkMetE in S. kali pollen extract during extract preparation or the reduction during SDS-PAGE. Taken together, inhibition experiments demonstrated the similar IgE reactivity for both purified rSal k 3 fragments and its natural counterpart (Sal k 3) in S. kali pollen extract. In addition, the results showed that pre-incubation of serum samples with native crude extract of S. kali pollen completely inhibited the IgE binding to natural Sal k 3 counterparts in S. kali pollen extract and other reactive proteins (Fig. 4, lane 2). However, pre-incubation of the pooled serum with PBS-BSA (Fig. 4, lane 1) or the purified TrxA protein (data not shown) did not affect on its IgE-reactivity to both fragments of the natural Sal k 3.
IgE-immunoblotting inhibition analysis. Pollen extract of S. kali were separated by SDS-PAGE and blotted onto PVDF. IgE-binding of a pooled serum, including five S. kali-allergic patients, Nos. 1, 2, 3, 6, and 9 were studied after pre-incubation with PBS (lane 1), S. kali extract (50 μg/ml) (lane 2) and purified N- and C-terminal fragments of rSal k 3 (50 μg/ml) (lane 3); lane M, molecular weight (Amersham, UK)
Capacity of rSal k 3 fragments to elicit immediate cutaneous reactions
The capacity of rSal k 3 fragments to induce immediate skin reactions were investigated in 12 S. kali allergic patients by in vivo SPTs (Supplementary Table 1). Following application of purified rSal k 3 fragments on inner side of the forearms of allergic patients, eight of them showed positive reaction while four patients (Nos. 4, 7, 8, and 11) were negative for both rSal k 3 fragments. These in vivo results were confirmed in vitro immunoreactivity assays. In control patients no skin reactions elicited with application purified rSal k 3 fragments.
Discussion
This study identified the cobalamin-independent methionine synthase (MetE) as a new allergen of S. kali pollen. This allergen was designated Sal k 3 by the WHO/IUIS Allergen Nomenclature Subcommittee.
Methionine synthases catalyze the transfer of a methyl group from N5-methyl-5,6,7,8-tetrahydrofolate (CH3–H4folate) to L-homocysteine (Hcy), the terminal step in the biosynthesis of methionine. Two unrelated families of proteins catalyze this reaction: cobalamin-dependent methionine synthase (MetH) and MetE. The cobalamin-independent Met synthase shares no sequence similarity with MetH. Both MetE and MetH are found in bacteria, whereas fungi and plants possess only the cobalamin-independent Met synthase. It is believed that MetE belongs to a distinct family of zinc metalloenzymes [17, 20, 21]. The SkMetE shares a high degree of amino acid sequence homology with the MetE from other plants including Beta vulgaris (91%) (belonging to the family of Amaranthaceae), Solanum tuberosum (89%), and Arabidopsis thaliana (88%). This high degree of homology may assume the same tertiary fold. Moreover, site-directed mutagenesis and crystallographic studies of MetE revealed that nearly all of conserved residues involved in biological functions are located in the C-terminal domain. In accordance with the previous studies [17, 21], the comparison of amino acid sequences showed that biological residues and potential N-glycosylation sites of the MetE from selected plants were highly conserved and that the C-terminal domain of these proteins were significantly more conserved than the N-terminal domain. Noteworthy, as shown by in vivo and in vitro immunoassays, no significant difference was observed in the IgE-binding capacity of the N- and C-terminal fragments of SkMetE. According to these results, it may be assumed that the IgE-binding regions of Sal k 3 are distributed throughout its entire structure while the potential conformational epitopes of the Sal k 3 remain undetermined.
To our knowledge, this study is the second report on allergenic MetEs among plants. In the first study, Chardin et al. [22] showed that the amino acid sequence of a high molecular weight allergenic protein (approximately 80 kDa) from the oilseed rape (Brassica napus) pollen was exceedingly similar to that of the cobalamin-independent methionine synthase of A. thaliana (AtMetE). The authors demonstrated that this 80-kDa protein represented an allergen from the oilseed rape pollen.
In the present study, two protein fragments from SkMetE (Sal k 3) were cloned by the PCR strategy based on the codons of conserved amino acid sequences of MetEs from different plants. The open reading frame of Sal k 3 contains 2,271 bases and encodes an approximately 85 kDa-protein, which correlates with the molecular weight characteristics of the known plant MetEs. During the expression process of the rSal k 3 fragments, the temperature of the culture medium was lowered to 18°C following IPTG induction. A significant increase in the amount of both soluble fragments of rSal k 3 was detected, thus confirming that aggregation was reduced at a lower temperature [23, 24]. This can be explained by the chaperone-assisted folding pathways in E. coli.
In order to confirm that the two purified fragments of rSal k 3 were correctly folded and bound to IgE as their natural counterpart in S. kali pollen extract, immunoblotting assays were carried out. The results showed that the recombinant and the natural proteins had the same IgE reactivity to patients’ sera. Furthermore, a complete inhibition of IgE-binding to natural Sal k 3 fragments in S. kali pollen extract was obtained after pre-incubation of pooled serum with the purified recombinant Sal k 3 fragments. The results of the following ELISA inhibition experiments were also entirely consistent with those obtained from immunoblotting assays. Concurrent with the results of SPTs with the S. kali pollen extract, four patients (Nos. 4, 7, 8, and 11) were not positive by SPTs with both fragments of rSal k 3. Taken together, it seems that rSal k 3 fragments are comprised of IgE-epitopes similar to those of their natural counterparts in S. kali pollen extract.
The results of immunochemical assays strongly suggested that SkMetE underwent partial proteolysis which led to its breakdown into two fragments with the MWs of approximately 45 and 39 kDa. Previous studies hypothesized that this breakdown occurs during pollen extract preparation and storage [13–16, 19]. Concurrent with this hypothesis, the present study showed that pre-incubation of sera from S. kali allergic patients with purified rSal k 3 fragments leads to complete inhibition of the IgE-binding capacity of the three protein bands with MWs of approximately 39, 45, and 85 kDa.
SDS-PAGE results indicated that the pattern of migration of Sal k 3 and its fragments did not change in both non-reducing and reducing conditions (data not shown). It was thus suggested that five cysteine residues of Sal k 3 may not be associated with interchain disulfide bonds. However, in a previous study, the authors stated that the methionine synthase of the human placenta was partially degraded into two fragments with approximate MWs of 45 and 35 Da [19]. Furthermore, Dixon et al. determined that due to limited proteolysis, the methionine synthase of A. thaliana was degraded into several polypeptides with different molecular masses [25]. Taken together, these observations suggested that methionine synthase is susceptible to degradation as a result of proteolysis or exposure to a reducing condition. Moreover, it is possible that the number or size of the products of methionine synthase degradation depends on the conditions of pollen extract preparation and storage. Nevertheless, additional studies are required to elucidate the patterns of degradation and the number and the size of cleavage products.
So far, increasing scientific evidence has accumulated indicating that in heavily air polluted zones, pollens express a larger amount of proteins that previously reported as allergenic [26–30]. In a recent study, the authors suggested that the cobalamin-independent methionine synthase was up-regulated in high CO2 condition, even though the specific role of CO2 concentration has not been determined [31]. Further studies are needed to determine the role of air pollution in the up-regulation of the MetE from S. kali pollen.
In conclusion, this study described a new allergen from S. kali pollen, Sal k 3, with a detectably specific IgE in approximately 67% of S. kali allergic patients. Sal k 3 is a cobalamin-independent methionine synthase (MetE) that may be partially degraded under reducing condition or during the extract preparation and purification procedures. However, it seems that the partial fragmentation of Sal k 3 has no effect on the IgE-binding capacity of its broken-down products. Future studies may be focused on the characterization of potential allergenic epitopes recognized by T and B cells and also on exploring possible diagnostic and specific therapeutic tools.
References
Al-Dowaisan A, Fakim N, Khan MR, Arifhodzic N, Panicker R, Hanoon A, Khan I (2004) Salsola pollen as a predominant cause of respiratory allergies in Kuwait. Ann Allergy Asthma Immunol 92:262–267
Assarehzadegan MA, Sankian M, Jabberi Azad F, Noorbakhsh R, Varasteh A (2009) Allergy to Salsola kali in a Salsola Incanescens-rich area: role of extensive cross allergenicity. Allergol Int 58:261–266
Colas C, Monzon S, Venturini M, Lezaun A (2006) Double-blind, placebo-controlled study with a modified therapeutic vaccine of Salsola kali (Russian thistle) administered through use of a cluster schedule. J Allergy Clin Immunol 117:810–816
Colas C, Monzon S, Venturini M, Lezaun A, Laclaustra M, Lara S, Fernandez-Caldas E (2005) Correlation between Chenopodiacea/Amaranthacea pollen counts and allergic symptoms in Salsola kali monosensitized patients. J Investig Allergol Clin Immunol 15:254–258
Ezeamuzie CI, Thomson MS, Al-Ali S, Dowaisan A, Khan M, Hijazi Z (2000) Asthma in the desert: spectrum of the sensitizing aeroallergens. Allergy 55:157–162
Suliaman FA, Holmes WF, Kwick S, Khouri F, Ratard R (1997) Pattern of immediate type hypersensitivity reactions in the Eastern Province, Saudi Arabia. Ann Allergy Asthma Immunol 78:415–418
Shafiee A, Yunginger JW, Gleich GJ (1981) Isolation and characterization of Russian thistle (Salsola pestifer) pollen allergens. J Allergy Clin Immunol 67:472–481
Carnes J, Fernandez-Caldas E, Marina A, Alonso C, Lahoz C, Colas C, Lezaun A (2003) Immunochemical characterization of Russian thistle (Salsola kali) pollen extracts. Purification of the allergen Sal k 1. Allergy 58:1152–1156
Barderas R, Garcia-Selles J, Salamanca G, Colas C, Barber D, Rodriguez R, Villalba M (2007) A pectin methylesterase as an allergenic marker for the sensitization to Russian thistle (Salsola kali) pollen. Clin Exp Allergy 37:1111–1119
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Sankian M, Varasteh A, Pazouki N, Mahmoudi M (2005) Sequence homology: a poor predictive value for profilins cross-reactivity. Clin Mol Allergy 3:13
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Plunkett G (2008) Stability of allergen extracts used in skin testing and immunotherapy. Curr Opin Otolaryngol Head Neck Surg 16:285–291
Petersen A, Suck R, Hagen S, Cromwell O, Fiebig H, Becker WM (2001) Group 13 grass allergens: structural variability between different grass species and analysis of proteolytic stability. J Allergy Clin Immunol 107:856–862
Nelson HS, Ikle D, Buchmeier A (1996) Studies of allergen extract stability: the effects of dilution and mixing. J Allergy Clin Immunol 98:382–388
Nelson HS (1979) The effect of preservatives and dilution on the deterioration of Russian thistle (Salsola pestifer), a pollen extract. J Allergy Clin Immunol 63:417–425
Ferrer JL, Ravanel S, Robert M, Dumas R (2004) Crystal structures of cobalamin-independent methionine synthase complexed with zinc, homocysteine, and methyltetrahydrofolate. J Biol Chem 279:44235–44238
Song J, Zhang H, Liu Z, Ran P (2008) Mango profilin: cloning, expression and cross-reactivity with birch pollen profilin Bet v 2. Mol Biol Rep 35:231–237
Utley CS, Marcell PD, Allen RH, Antony AC, Kolhouse JF (1985) Isolation and characterization of methionine synthetase from human placenta. J Biol Chem 260:13656–13665
Zeh M, Leggewie G, Hoefgen R, Hesse H (2002) Cloning and characterization of a cDNA encoding a cobalamin-independent methionine synthase from potato (Solanum tuberosum L.). Plant Mol Biol 48:255–265
Pejchal R, Ludwig ML (2005) Cobalamin-independent methionine synthase (MetE): a face-to-face double barrel that evolved by gene duplication. PLoS Biol 3:e31
Chardin H, Mayer C, Senechal H, Tepfer M, Desvaux FX, Peltre G (2001) Characterization of high-molecular-mass allergens in oilseed rape pollen. Int Arch Allergy Immunol 125:128–134
Chen Y, Wang H, Wang X, Cao A, Chen P (2006) Cloning and expression of peroxisomal ascorbate peroxidase gene from wheat. Mol Biol Rep 33:207–213
Fan Z, Yue C, Tang Y, Zhang Y (2009) Cloning, sequence analysis and expression of bacterial lipase-coding DNA fragments from environment in Escherichia coli. Mol Biol Rep 36:1515–1519
Dixon DP, Skipsey M, Grundy NM, Edwards R (2005) Stress-induced protein S-glutathionylation in Arabidopsis. Plant Physiol 138:2233–2244
Bartra J, Mullol J, del Cuvillo A, Davila I, Ferrer M, Jauregui I, Montoro J, Sastre J, Valero A (2007) Air pollution and allergens. J Investig Allergol Clin Immunol 17(Suppl 2):3–8
D’Amato G (2002) Environmental urban factors (air pollution and allergens) and the rising trends in allergic respiratory diseases. Allergy 57(Suppl 72):30–33
Cortegano I, Civantos E, Aceituno E, del Moral A, Lopez E, Lombardero M, del Pozo V, Lahoz C (2004) Cloning and expression of a major allergen from Cupressus arizonica pollen, Cup a 3, a PR-5 protein expressed under polluted environment. Allergy 59:485–490
Armentia A, Lombardero M, Callejo A, Barber D, Martin Gil FJ, Martin-Santos JM, Vega JM, Arranz ML (2002) Is Lolium pollen from an urban environment more allergenic than rural pollen? Allergol Immunopathol (Madr) 30:218–224
Suarez-Cervera M, Castells T, Vega-Maray A, Civantos E, del Pozo V, Fernandez-Gonzalez D, Moreno-Grau S, Moral A, Lopez-Iglesias C, Lahoz C, Seoane-Camba JA (2008) Effects of air pollution on cup a 3 allergen in Cupressus arizonica pollen grains. Ann Allergy Asthma Immunol 101:57–66
Pedreschi R, Hertog M, Robben J, Noben J, Nicolai B (2008) Physiological implications of controlled atmosphere storage of ‘Conference’ pears (Pyrus communis L.): a proteomic approach. Posth Biol Tech 50:110–116
Acknowledgments
This study was supported by Research administration of Mashhad University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Assarehzadegan, M.A., Sankian, M., Jabbari, F. et al. Identification of methionine synthase (Sal k 3), as a novel allergen of Salsola kali pollen. Mol Biol Rep 38, 65–73 (2011). https://doi.org/10.1007/s11033-010-0078-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0078-2