Abstract
Homogentisate 1, 2 dioxygenase (HGD) is one of six enzymes required for the catabolism of the aromatic amino acids phenylalanine and tyrosine. Here we present the nucleotide sequence of transcripts of the bovine HGD gene. The full length cDNA of bovine HGD has been identified, encoding a deduced protein of 445 amino acids (Accession No. FJ515744). The bovine HGD gene comprises 14 exons and 13 introns. This is the first published cDNA bovine sequences that share high sequence similarity with other species. Semi-quantitative RT-PCR analysis demonstrated that the bovine HGD transcript was mainly expressed in liver and kidney tissues. Nine single nucleotide polymorphisms (SNPs) were identified, five in the coding region and four intronic. Four of the SNPs change an amino acid in the HGD protein sequence. Genotype and allelic frequencies were determined in Chinese red cattle breeds. Ten haplotypes were determined based upon the genotype of 9 SNPs. Moreover, for the first time an association was reported between HGD gene polymorphism and meat quality traits in Chinese red cattle (n = 224). Marker-trait association analyses showed that the HGD/PvuII genotypes showed a significant effect on meat cooking rate, muscle fiber diameter, and shear force (P < 0.05). The HGD DraIII genotypes showed a significant effect on muscle fiber diameter, shear force, and drip loss (P < 0.05). The HGD/AluI genotypes showed a significant effect on meat cooking rate, shear force, and drip loss (P < 0.05). The HGD/DraI genotypes showed a significant effect on meat cooking rate and shear force (P < 0.05). The HGD/EcoRV genotypes showed a significant effect on meat cooking rate, muscle fiber diameter, and shear force (P < 0.05). In all loci, no statistically significant differences were observed for pHu (P > 0.05). This is the first incidence were polymorphisms of a bovine HGD gene have demonstrated a correlation with meat traits in Chinese red cattle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the field of meat-producing animals, the influence of genetics on meat quality is now well recognized but still poorly understood. Domestic animals have been subjected to intensive selection since the beginning of domestication, particularly concerning character traits related to growth, development, body composition, reproduction, behavior, and resistance to diseases [1]. In cattle, tenderness is the primary quality attribute for consumer acceptance of meat, followed by juiciness and flavor. Efforts are being made to control and improve these qualities but are still impaired by many genetic and environmental factors and the extensive studies required to elucidate the mechanisms involved. Breeding selection has certainly driven the accumulation of natural mutations responsible for phenotypic differences. Finding these mutations and discovering how they control a character trait is the major objective in farm animal genome research and in the understanding of complex phenotypic traits.
Homogentisate oxidase, called homogentisate 1,2 dioxygenase (HGD), is one of six enzymes required for the catabolism of the aromatic amino acids phenylalanine and tyrosine. Phenylalanine and tyrosine play a major role in the synthesis of some proteins, hormones, pigments, and neurotransmitters. Homogentisate is an intermediate in the degradation pathway of phenylalanine. HGD degrades homogentisate into fumarate and acetoacetate. The fumarate and acetoacetate participate in catabolism of acetone and sugar and may be degraded into smaller molecules needed for the organism [2].
Homogentisate 1,2 dioxygenase is encoded by the HGD gene. The human HGD gene maps to chromosome 3 (3q21–23) [3]. The pig HGD gene maps to chromosome 13 (13q46–49). Several previous studies have shown that quantitative trait loci (QTL) for fatness and meat quality were located on chromosome 13 of pig [4, 5]. Therefore, HGD may be considered a candidate gene associated with carcass and meat quality traits. In this study we investigated the sequence structure, bioinformatics, expression profile, and single nucleotide polymorphisms (SNPs) of the HGD gene. We also sought to determine the relationship between these polymorphisms and meat quality and carcass traits of Chinese red cattle to provide useful data for population genetic studies and molecular breeding of meat quality traits.
Materials and methods
Genomic DNA and total RNA isolation
Blood or muscle samples were collected from 224 Chinese red cattle (steers, 24–26-months-old) randomly selected from three sires that are a cattle breed native to China in the Branch of Animal Husbandry, Jilin Academy of Agricultural Sciences, People’s Republic of China between 2007 and 2009. All phenotypic data of meat quality traits were determined using the legal grading standard parameters endorsed by professional meat quality graders of the Animal Products Grading Service in China and referred to methods of related literatures.
Genomic DNA was extracted from blood samples as previously described [6] and dissolved in TE solution. The DNA samples were stored at –20°C and/or at 4°C. For the RNA extraction, samples were taken from liver (LI), lung (LU), spleen (S), kidney (K), duodenum (D), rumen (R), muscle longissimus dorsi (LD) and muscle triceps brachii (TB) from Chinese red cattle. Tissues were obtained within 20 min of dissection and immediately submerged in liquid nitrogen before storage at –70°C. A total of 100 mg of each sample tissue was homogenized in Trizol Reagent (Invitrogen) using a high-speed mechanical homogenizer. RNA integrity was monitored by denaturing 1% agarose gel electrophoresis. Concentrations and purities of RNA were measured by spectrophotometry (Amersham Pharmacia Biotech, Buckinghamshire, UK).
All procedures involving animals were approved by the animal care and use committee at the respective institution where the experiment was conducted. All procedures involving animals were approved and authorized by the Chinese Ministry of Agriculture.
Middle fragment cloning of the HGD cDNA
Reverse transcription was performed for preparation of cDNA using an RT-PCR kit (Toyobo, Tokyo, Japan) according to the manufacturer’s manual. The human HGD mRNA sequence (NM_000187) was used as a query sequence to screen by Blastn, the EST GenBank database. Seven bovine HGD ESTs were identified (DV785971, DT887175, DV784033, DV786692, DV788366, EE335411 and DV782809) and SeqMan program of DNASTAR 6.0 was used to assemble them into a contig. In order to complete the overall coding sequence of the bovine HGD gene, a set of primers (HGD-MF and HGD-MR) was designed according to the bovine contig (all primer sequences are located in the supplemental file). Bovine liver cDNA was used as a template and PCR was performed using Advantage® 2 PCR Kit (Clontech) according to the manufacturer’s manual. The PCR products were purified using the QIAquick PCR purification kit (Qiagen) and sequenced bidirectionally using Big Dye Terminator chemistry on an ABI Prism Cycle sequencing 3730 (Applied Biosystems) by Shanghai Sangon Biological Engineering Technology and Services Co., Ltd.
Rapid amplification of cDNA ends
For preparation of 5′- and 3′-cDNA, liver cDNA was synthesized using a SMARTTM rapid amplification of cDNA ends (RACE) cDNA Amplification Kit according to the manufacturer’s instructions (BD Clontech Biosciences, Mountain View, CA, USA). For the 5′-RACE, first round polymerase chain reaction (PCR) was performed with a gene-specific primer 5HGDGSP1, followed by a second nested PCR with another gene-specific primer 5HGDGSP2. For 3′-RACE, a first round of PCR was performed with a gene-specific primer 3HGDGSP1, followed by a second nested PCR with another gene-specific primer 3HGDGSP2. RACE PCR reactions was performed using SMART™ RACE cDNA Amplification Kit and Advantage® 2 PCR Kit according to the manufacturer’s manual.
The PCR products were analysed on 1% agarose gels stained with ethidium bromide. The PCR products were then purified using NucleoTrap® gel extraction kit (Clontech), and cloned into the pGEM-T Easy Vector (Promega, Madison, WI, USA). Bidirectional DNA sequences of clone inserts were determined using Big Dye Terminator chemistry on an ABI Prism Cycle sequencing 3730 (Applied Biosystems) by Shanghai Sangon Biological Engineering Technology and Services Co., Ltd.
Analysis of bioinformatics
Nucleotide BLAST programs at NCBI (http://www.ncbi.nlm.nih.gov/BLAST/) were used for sequence homology searches in public databases [7]. A search for open reading frame (ORF) and translation of the nucleotide sequences into the amino acids was performed using the Open Reading Frame Finder at NCBI (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The putative amino acid sequence of the bovine HGD was aligned with those of the mammalian species using the CLUSTAL_X program [8]. A neighbor-joining phylogenetic tree, based on genetic distances calculated with Kimura’s two-parameter method, was constructed using the phydit program version 3.1 [9].
Expression profile of the HGD gene
Reverse transcription was performed for preparation of cDNA using the RT-PRC kit (Toyobo, Tokyo, Japan) according to the manufacturer’s manual. The cDNA was used as template in a duplex RT-PCR for amplifying a part of the HGD mRNA and a part of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA, which was used as an endogenous control, and the relative expression level of target gene was expressed as the target/GAPDH ratio. Primers HGD2F and HGD2R amplified HGD while primers GAPDHF and GAPDHR amplified GAPDH. RT-PCR program was an initial denaturation step at 95°C for 3 min, followed by 32 cycles of denaturation for 30 s at 95°C, annealing for 30 s at 60°C and extension for 30 at 72°C. A last extension step for 7 min at 72°C was added. The 50× Advantage 2 Polymerase Mix (Clontech) was used. The PCR products were analyzed by agarose gel electrophoresis and visualized by ethidium bromide staining and quantitated using GelScan software (Ver.5.1, BioSciTec Science Group, Germany).
Polymorphism discovery and PCR–RFLP analysis
Single strand conformation polymorphism (SSCP) analysis for all exons and its part flank regions (that is, small intronic regions) and sequencing of PCR products were performed to identify SNPs. Primers for SNP amplification were designed based on GeneBank No. NC_007299. Because some primers did not detect SNPs, they have not been indicated. The PCR reactions were performed in a volume of 12 μl of 1× PCR buffer, consisting of 50 ng of genomic DNA, 10 pmol of each primer, 100 μM of each dNTP, 1.5 mM MgCl2 and 1.0 Units 50× Advantage 2 Polymerase Mix (Clontech). PCR parameters were an initial denaturation at 95°C for 3 min, followed by 32 cycles of 94°C for 30 s, appropriate annealing temperature for 30 s, and 72°C for 30 s, with a final extension at 72°C for 7 min. PCR products were purified using Microcon TM-100 (Millipore) and sequenced using Big Dye Terminator chemistry on an ABI Prism Cycle sequencing 3730 (Applied Biosystems) by Shanghai Sangon Biological Engineering Technology and Services Co., Ltd.
All polymorphisms identified by PCR-SSCP and sequencing were confirmed by PCR restriction fragment length polymorphism (RFLP) using HaeIII and BstXI (HGD1F/HGD1R), BseNI and PvuII (HGD4F/HGD4R), DraIII and DdeI (HGD10F/HGD10R), AluI (HGD12F/HGD12R), and DraI and EcoRV (HGD14F/HGD14R) restriction enzymes (New England BioLabs). Genotyping of population researched was confirmed by PCR–RFLP using these restriction enzymes.
Statistical analyses
Genotype and allele frequencies were calculated for each polymorphism by direct counting. Gene homozygosity (Ho), gene heterozygosity (He), effective number of alleles (n e ), and polymorphism information content (PIC) of all loci were calculated [10–12]. Haplotype frequencies were analyzed by PHASE v2.1.1 [13]. The phenotypic data included the cooking loss, muscle fibre diameter, shear force, drip loss, and pHu. The effect of HGD genotypes on the meat quality traits of Chinese red cattle were analysed using GLM procedure of SAS [14]. Significance was considered at P < 0.05. The following model was used:
where Y ijkl is the observation of the trait; μ is the population mean; Genotype i is the fixed effect of ith genotype (i = 1, 2 and/or 3); Sire j is the fixed effect of jth sire (j = 1, 2 and 3); YS k is the fixed effect of kth seasons of slaughter (l = 1, 2, 3 and 4) and e ijkl is the random residue.
Results
Analysis of bovine HGD cDNA and gene structure
To study the structure of the bovine HGD gene, we analyzed the bovine HGD cDNA. We used the bovine HGD cDNA obtained (Accession No. FJ515744) to search for contigs containing the bovine HGD gene in the publicly available draft sequences from the bovine genome. A contig containing the whole HGD genomic DNA sequence was identified (Accession no. NC_007299) and gene structure was determined by comparing genomic and cDNA sequences. The bovine HGD gene is organized in 14 exons and 13 introns and extends over more than 42 kb of genomic DNA. The first exon constitutes 77 bp of 5′ UTR and the translation initiation codon ATG. Exon 14 contains the translation termination codon TGA and 198 bp (excluding the number of polyA) of 3′ UTR. The 3′ UTR contained a normal AATAAA polyadenylation signal sequence.
Comparisons of exon and intron sizes in bovine, human. and mouse HGD genes were performed (data not shown). The lengths of exons and introns were also investigated in comparison with other mammalian species. Exon sizes were almost identical, but variable intronic sizes were observed among the three mammalian species.
Analysis of the deduced amino acid sequence
The complete amino acid sequence was deduced from sequence analysis of cDNA and genomic DNA. HGD cDNA encoded for a protein consisting of 445 amino acids, with a predicted MW of 50 kDa and an isoelectric point of 7.12. In order to study the evolutionary relationship of six orthologous HGD proteins, multiple sequence alignment was carried out. The deduced amino acid sequence of bovine HGD gene shows 94, 92, 91, 85, and 70% similarity with its orthologous human, mouse, rat, chicken and zebrafish proteins, respectively.
A phylogenetic tree was built from the HGD protein sequences of bovine, human, mouse, rat, chicken, and zebrafish. Three clusters of proteins were grouped; the bovine and human HGD, the mouse and rat HGD, and the chicken and zebrafish HGD. Moreover, the phylogenetic tree shows that the human protein is nearest to the bovine, whereas the zebrafish and the chicken proteins are the most distant to it and also to the rest of the species (phylogenetic tree not shown).
Tissue distribution of the HGD transcript
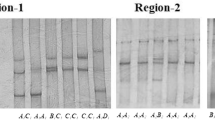
For the study of the tissue distribution of the bovine HGD cDNA, duplex reverse-transcription PCR was performed, using the GAPDH gene as an endogenous control. As shown in Fig. 1, HGD transcript was present in every tissue analyzed. In liver and kidney tissue, relativel mRNA levels were distinctly higher than the rest of the tissue, which was 0.601 and 0.319, respectively. Moreover, the relative mRNA levels of the other tissues (LD, TB, S, LU, R and D) analyzed were very low, which were 0.012, 0.01, 0.22, 0.10, 0.018 and 0.023, respectively.
Expression analysis of the bovine HGD gene in various tissues. Duplex RT–PCR was performed for the amplification of HGD and GAPDH, which was used as an endogenous control. Agarose gel electrophoresis of the RT–PCR products for several bovine tissues (a). Expression levels of the HGD gene in several bovine tissues (b). Here the means ± SEM of 3 experiments are presented
SNPs and haplotype analysis
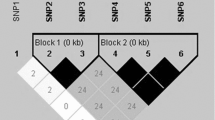
The HGD gene was screened for polymorphisms by using genomic DNA from a native bovine of China (Chinese red cattle). PCR fragments covering all exons and some introns of the gene were analyzed using the PCR-SSCP technique, and fragments harboring various electrophoresis profiles were then sequenced. A total of 9 SNPs were identified, among which five were in the coding region, and four were intronic (Fig. 2). Four of these polymorphisms induce a change in the amino acid sequence of the HGD protein and one of these polymorphisms occurs at the termination codon (TAA/TGA), which has not changed the status of the stop code (Fig. 2). All four mutations induce a change in the amino acid subclass (hydrophilic, hydrophobic, acidic, or basic). All SNPs were genotyped by PCR–RFLP. Allelic frequencies were determined in the population of Chinese red cattle. Genotype and allelic frequencies for nine SNPs that were genotyped on all individuals are listed in Table 1. Ho, He, ne, and PIC were calculated (Table 2). Nine SNPs, for which the genotypes were available for Chinese red cattle, were used for haplotype determination. Ten different haplotypes (Table 3) were identified among which were a very common one (frequency = 0.279), a second common one (frequency = 0.192), and a third common one (frequency = 0.158), with frequency of the other haplotypes lower (frequency <0.1).
Location of the nine possible SNPs along the bovine HGD gene. Black boxes indicate exons and lines connecting the boxes indicate introns. The number and length of exons (bp) are shown under the gene. SNPs are indicated according to their position in the sequence (NC_00729). SNPs create/disappear a relevant restriction site for these restriction enzymes. Gray boxes indicate amino acid change or silent mutation at relevant mutation site
Association of SNP genotypes with meat quality traits
We believe we are the first to have revealed that polymorphism in the HGD gene is associated with meat quality traits. Meat quality traits (meat cooking rate, muscle fiber diameter, shear force, drip loss, and pHu) were analyzed in the Chinese red cattle population. The HGD/PvuII (g.11205A > G) genotypes showed a significant effect on meat cooking rate, muscle fiber diameter, and shear force (P < 0.05). The HGD DraIII (g.29858G > A) genotypes showed a significant effect on muscle fiber diameter, shear force, and drip loss (P < 0.05). The HGD/AluI (g.34232C > G) genotypes showed a significant effect on meat cooking rate, shear force, and drip loss (P < 0.05). The HGD/DraI (g.42332C > T) genotypes showed a significant effect on meat cooking rate and shear force (P < 0.05). The HGD/EcoRV (g.42459A > G) genotypes showed a significant effect on meat cooking rate, muscle fiber diameter, and shear force (P < 0.05). In all loci, no statistically significant differences were observed for pHu (Table 4).
Discussion
The bovine genome map, although well developed, suffers from the imbalance of the number of genes versus random markers. We aimed to identify polymorphic sites in genes preferentially expressed in liver in order to add them to the genetic map of cattle and evaluate their potential role in traits related to growth, carcass or meat quality.
We analyzed the sequence of the bovine HGD cDNA. This structure is the same as the human HGD gene. The human HGD gene spans 54,363 bp and codes for a 1715 bp transcript, which is split into 14 exons ranging from 35 to 360 bp [15]. Splice donor and acceptor consensus sequences were identified at the exon and intron boundaries and conformed to the GU-AG rule [16]. The major non-canonical splice site pair is GC-AG [17]. The size of the exons and introns was also investigated in comparison with other mammalian species. Exon sizes were almost identical, but variable intronic sizes were observed among the three mammalian species. Bovine HGD protein shared 94, 92, and 91% amino acid sequences identity to those of human, mouse, and rat, respectively. This indicated that the HGD gene was highly evolutionarily conserved in mammals. The conservation is also demonstrated by multiple sequence alignment among different species. On the other hand, HGD mRNA expression analysis in eight tissues displayed variations. The expression in liver and kidney tissues was higher than other tissues, with relatively lower expression in muscle tissues. These results were consistent with the fact that HGD has a physiological function in liver and kidney tissue [18].
SNP analysis is a well-established tool for the identification of genes associated with traits of economic interest in livestock populations [19–21]. More recently, haplotype analysis has become an area of intense research for complex genetic phenotypes [22, 23]. A haplotype can be defined as a set of SNPs on a single chromosome that are closely linked and inherited as a unit. The knowledge of haplotype for several SNPs in one gene could provide more information about genotype-phenotype associations than individual underlying SNPs.
The present study is the first report on polymorphisms of the bovine HGD gene. We have identified nine novel SNPs of HGD in exons and introns. The first striking observation is that some mutant alleles have a high allelic frequency, suggesting that mutations occurred independently and were then more or less spread (Table 1). The other frequency of mutation alleles is lower. The fact that these mutant alleles are very rare suggests that they might be recent mutations that have not been spread so far.
We have calculated the parameter of genetic diversity at 9 SNP sites in Chinese red cattle. In the present study, loci HGD/DraIII and HGD/AluI appeared to be a little (PIC < 0.25) informative, whereas the other loci studied were moderately informative (0.25 < PIC < 0.5) [12]. The values of He, ne, and PIC in the HGD/BseNI loci (0.3854, 1.6677, and 0.3589, respectively) were higher than that of other loci, which implied that the polymorphism and genetic variation of HGD/BseNI were higher than that of other loci. This reflected that there was a higher genetic diversity within the HGD gene of Chinese red cattle in analyzed populations.
Four of these polymorphisms induced a change in the amino acid sequence of the HGD protein. Only one SNP (g.34232C > G) induced an essential amino acid (Thr) change to a nonessential amino acid (Ser). One SNP (g.42459A > G) resulted in a termination codon (TAA, “wild type”) change to another stop codon (TGA) with the frequency of the mutation allele (G = 0.594) higher than the wild type (A = 0.406). It is possible that there was a bias codon selection in the termination of the HGD gene that may affect the secondary structure of the mRNA, protein structure and/or HGD protein function during the progress of evolution. Indeed, codon use in mammals is biased and correlates with GC content; the preferred codons being the ones ending with a G or C [24, 25].
In human, twelve novel mutations have been identified: eight missense mutations that result in amino acid substitutions at positions conserved in HGD in different species; one frameshift mutation, two intronic mutations, and one splice-site mutation. They report characterization of five polymorphic sites in HGD gene and describe the haplotypic associations of alleles at these sites in normal and AKU chromosomes. In exon 4, a relatively frequent nucleotide substitution (c.407T/A) was identified, resulting in an amino acid change (H80Q) [26].
The result of least square analysis confirmed the significant association with meat quality in Chinese red cattle. The Chinese red cattle with the GG genotype have significantly higher meat cooking rate than that with AA/AG genotypes and have significantly lower muscle fiber diameter and shear force than that with AA genotype at the HGD/PvuII locus (P < 0.05). The Chinese red cattle with the GG genotype also have significantly lower muscle fiber diameter and drip loss than that with the GA genotype and significantly higher shear force than that with the GA genotype at the HGD/DraIII locus (P < 0.05). The Chinese red cattle with the CC genotype have significantly higher meat cooking rates, significantly lower shear force and drip loss than that of the CG genotype at the HGD/AluI locus (P < 0.05). The Chinese red cattle with CT genotype have significantly higher meat cooking rate than that of the CC and TT genotypes and have significantly lower shear force than that of the CC genotype at the HGD/DraI locus (P < 0.05). The Chinese red cattle with the AG genotype have significantly higher meat cooking rate than that of the AA/GG genotypes and have significantly lower muscle fiber diameter, and shear force than that of the AA genotype at the HGD/EcoRV locus (P < 0.05). Therefore, we suggest that allele G at the HGD/PvuIIlocus, allele G at the HGD/DraIII locus, allele C at the HGD/AluI locus, allele C at the HGD/DraI locus, and allele G at the HGD/EcoRV locus are the beneficial alleles for meat quality traits for breeding programs in Chinese red cattle. Despite all the available information concerning the HGD gene in human and mouse, there is no information known in bovine. Considering the economic importance of the meat quality traits to the livestock industry, it appears clearly essential to further research on the bovine HGD gene. Indeed, HGD has been shown to strongly influence meat quality and carcass traits [27]. In conclusion, we found evidence of a large novel variability in the HGD gene in Chinese red cattle. We found the variability caused by polymorphism to be associated with phenotypic traits related to meat quality. This study will be practical for the improvement of breeding native Chinese cattle and for purposes of meat consumption.
References
Andersson L, Georges M (2004) Domestic-animal genomics: deciphering the genetics of complex traits. Nat Rev Genet 5:202–212
Rodriguez JM, Timm DE, Titus GP, Beltran-Vale de Bernabe D, Criado O, Mueller HA, Rodriguez de Cordoba S, Penalva MA (2000) Structural and functional analysis of mutations in alkaptonuria. Hum Mol Genet 9:2341–2350
Fernandez-Canon JM, Granadino B, Beltran-Valero de Bernabe D, Renedo M, Fernandez-Ruiz E, Pen alva MA, Rodrıguez de Cordoba S (1996) The molecular basis of alkaptonuria. Nat Genet 14:19–24
Yu TP, Wang L, Tuggle CK, Rothschild MF (1999) Mapping genes for fatness and growth on pig chromosome 13: a search in the region close to the PIT1 gene. J Anim Breed Genet 116:269–280
Yue G, Russo V, Davoli R, Sternstein I, Brunsch C, Schroffelova D, Stratil A, Moser G, Bartenschlager H, Reiner G, Geldermann H (2003) Linkage and QTL mapping for Sus scrofa chromosome 13. J Anim Breed Genet 120(Suppl 1):103–110
Rouzaud F, Martin J, Gallet PF, Delourme D (2000) A first genotyping assay of French cattle breeds based on a new allele of the extension gene encoding the melanocortin-1 receptor (Mc1r). Genet Sel Evol 32:511–520
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Chun J (2001) PHYDIT version 3.1. http://plaza.snu.ac.kr/~jchun/phydit/)
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Hines HC, Zikakis JP, Haenlein GF (1981) Linkage relationships among loci of polymorphisms in blood and milk of cattle. J Dairy Sci 64:71–76
Botstein D, White RL, Skolnick M, Davis RW (1980) Construction of a genetic linkage map in human using restriction fragment length polymorphisms. Am J Hum Gene 32:314–331
Stephens JC, Schneider JA, Tanguay DA, Choi J, Acharya T, Stanley SE, Jiang R (2001) Haplotype variation and linkage disequilibrium in 313 human genes. Science 293:489–493
AS S (1999) Statistical analysis system institute. Incorporation, Cary, NC, USA
Granadino B, Beltran-Valero de Bernabe D, Fernandez-Canon JM, Penalva MA, Rodrıguez de Cordoba S (1997) The human homogentisate 1, 2-dioxygenase (HGO) gene. Genomics 43:115–122
Breathnach R, Chambon P (1981) Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem 50:349–383
Burset M, Seledtsov IA, Solovyev VV (2000) Analysis of canonical and non-canonical splice sites in mammalian genomes. Nucleic Acids Res 28:4364–4375
Edwin JA, Veldhuizen FredericH (2005) Steady-state kinetics and inhibition of anaerobically purified human homogentisate 1, 2-dioxygenase. Biochem J 386:305–314
He XP, Xu XW, Liu B (2009) Molecular characterization, chromosomal localization and association analysis with back-fat thickness of porcine LPIN2 and LPIN3. Mol Biol Rep 36:1819–1824
Zhang CL, Wang YH, Chen H, Lan XY, Lei CZ, Fang XT (2009) Association between variants in the 50-untranslated region of the bovine MC4R gene and two growth traits in Nanyang cattle. Mol Biol Rep 36:1839–1843
Lai XS, Lan XY, Chen H, Wang XL, Wang KY, Wang M, Yu H, Zhao M (2009) A novel SNP of the Hesx1 gene in bovine and its associations with average daily gain. Mol Biol Rep 36:1677–1681
Ciobanu DC, Bastiaansen JWM, Lonergan SM, Thomsen H, Dekkers JCM, Plastow GS, Rothschild MF (2004) New alleles in calpastatin gene are associated with meat quality traits in pigs. J Anim Sci 82:2829–2839
Stone RT, Casas E, Smith TPL, Keele JW, Harhay G, Bennett GL, Koohmaraie M, Wheeler TLS, Shackelford D, Snelling WM (2005) Identification of genetic markers for fat deposition and meat tenderness on bovine chromosome 5: development of a low-density single nucleotide polymorphism map. J Anim Sci 83:2280–2288
Marin A, Bertranpetit J, Oliver JL, Medina JR (1989) Variation in G + C-content and codon choice: differences among synonymous codon groups in vertebrate genes. Nucleic Acids Res 17:6181–6189
Porter TD (1995) Correlation between codon usage, regional genomic nucleotide composition, and amino acid composition in the cytochrome P-450 gene superfamily. Biochim Biophys Acta 1261:394–400
Beltran-Valero de Bernabe D, Granadino B, Chiarelli I, Porfirio B, Mayatepek E, Aquaron R, Moore MM, Festen JJM, Sanmarti R, Penalva MA, Rodriguez de Cordoba S (1998) Mutation and polymorphism analysis of the human homogentisate 1, 2-dioxygenase gene in alkaptonuria patients. Am J Hum Genet 62:776–784
Li JH, Yang MS, Xu NY (2008) Single nucleotide polymorphism of porcine HGD gene exon 14 and its association with carcass and meat quality traits. J Northwest AF Univ 36:22–26
Acknowledgements
This work was supported by the National High Technology Research and Development Project of China (No.2008aa101010), the National Fundamental Resources Platform of Science and Technology of P. R. China (2005DKA21101) and Natural Science fund of Shandong province of P. R. China (Y2007D33).
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Dudgeon and M. Li are contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, G., Dudgeon, C., Li, M. et al. Molecular cloning of the HGD gene and association of SNPs with meat quality traits in Chinese red cattle. Mol Biol Rep 37, 603–611 (2010). https://doi.org/10.1007/s11033-009-9860-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9860-4