Abstract
Glutathione S-transferases (GSTs) M1 and T1 are known to be polymorphic in humans. Both polymorphisms are due to gene deletions which are responsible for the existence of null genotypes. Previous studies have suggested that GST genotypes may play a role in determining susceptibility to a number of unrelated cancers, including lung cancer. The GSTM1 and GSTT1 polymorphisms were determined by PCR-based analysis in 75 lung cancer patients and 55 controls. The unconditional logistic regression analysis was used to calculate ORs and 95% CI. The frequencies of GSTM1 and GSTT1 null genotypes were 37.3 and 22.7% in lung cancer patients and 27.3 and 16.4% in controls, respectively. When analyzed by histology the GSTM1 null genotype was more prevalent in squamous-cell carcinoma and adenocarcinoma patients. Whereas, GSTT1 null genotype frequency was lower in small-cell lung cancer patients than controls. But these differences were not statistically significant. According to smoking status, null genotype for both gene are associated with an increase in risk for lung cancer. Our results suggest that GSTM1 and GSTT1 polymorphisms may play a role in the development of lung cancer for some histological subtypes and modifies the risk of smoking-related lung cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of human cancer is a highly complex process with both environmental and genetic determinants. Substantial evidence indicate that there is interindividual variation in the susceptibility to lung cancer [1]. This susceptibility is modulated, at least in part, by polymorphisms in genes encoding DNA repair proteins, cell-cycle control proteins and metabolic enzymes. One sub-class of metabolizing enzymes comprises xenobiotic metabolizing enzymes which are classically divided into two categories: the “phase I” and “phase II” enzymes. The phase I enzymes are Cytochrome p-450 system and phase II enzymes contain the glucoronyl-, glutathione- (GSH) and sulpho-transferases. Cytochrome p-450 enzymes catalyze the oxidation of polyaromatic hydrocarbons (PAHs) to electrophilic intermediates capable of initiating the carcinogenic process. These electrophilic intermediates may undergo conjugation or hydration reactions catalyzed by glutathione S-transferases (GSTs) and epoxide hydrolase producing hydrophilic compounds that are excreted from the body. Polyaromatic hydrocarbons (PAH+) found in cigarette smoke are the precursors of many cancer-causing chemicals [2]. These exert their effect only after being metabolically activated to intermediates capable of binding to DNA and causing mutations. There is convincing evidence that cancer risk from PAH is mediated in part by cytochrome p4501A1 (CYP1A1) aryl hydrocarbon hydroxylase (AHA), which can transform these xenobiotics into reactive DNA-binding intermediates.
Another genetic susceptibility factor is the inherited absence of the glutathione S-transferases (GSTs) which play an important role in the defense of the body against reactive compounds [3]. Therefore, it has been hypothesized that individuals with a high activity of oxidative enzymes or a low activity of detoxifying enzymes may be at increased risk for cancer caused by tobacco products [4]. Based primarily on protein sequence similarity, the soluble glutathione S-transferases have been divided into four (classes Alpha, Mu, Pi and Theta) protein families. Two of the GST isoenzymes, the μ class enzyme GSTM1 and the θ class enzyme GSTT1 have been shown to be polymorphic in humans [5, 6]. Both polymorphisms result from deletions of genes which are responsible for the existence of null alleles. Homozygosity of null alleles results in the null phenotype with absence of enzyme activity. Approximately 50% of the European and Asian [7] populations are homozygously deleted for this allele. GSTM1 is known to detoxify mutagenically active forms of several carcinogens in the tobacco smoke, including electrophilic benzo(a)pyrenes by conjugating them with cellular glutathione [8]. Lack of GSTM1 alleles has been reported to be associated with an increased susceptibility for tobacco smoke-induced lung [5] or bladder cancer [9] and possibly also with colorectal [10, 11] hepatocellular, gastric, esophageal, head and neck as well as cutaneous cancers [12].
In humans, the theta class GSTs are represented by two izoenzymes; GSTT1 [6, 13, 14] and GSTT2 [15, 16]. GSTT1 is implicated in the activation of specific halogenated alkanes to carcinogenic metabolites [17]. A similar deletion polymorphism has been observed for the GSTT1 gene which leads to a functional deficiency of the enzyme activity. Large ethnic differences in the prevalence of the homozygously deleted GSTT1 genotype have been observed. The prevalence of the GSTT1 null genotype is highest among Chinese (64%), followed by Koreans (60%), African-Americans (22%), Caucasians (29%) and Asian-Indians (16%), whereas it is lowest among Mexican-Americans (10%) [18, 19]. The GSTT1 gene deletion may therefore modify the individual risk associated with exposure to toxic and carcinogenic chemicals. Specifically, the gene defect of GSTT1 has been associated with an increased risk of myelodysplastic syndromes [20], astrocytoma and menengiomas [21].
The aim of this study was to investigate the role of GSTM1 and GSTT1 polymorphisms in the risk of developing lung cancer. Specifically we sought to determine whether smokers who lack genes for either the GSTM1 or GSTT1 enzymes are at increased risk for lung cancer.
Materials and methods
This study included 75 cases with lung cancer and 55 controls. All individuals were interviewed by a questionnaire to obtain information on lifestyle (including a lifetime history of tobacco use). The control group consisted of unrelated individuals who were free of any benign or malignant tumor. The histological classification of the lung tumors were; squamous cell carcinoma (n = 32), adenocarcinoma (n = 21), small cell carcinoma (n = 14) and mixed and non-classifiable type carcinoma (n = 8).
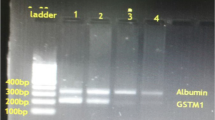
Genomic DNA for molecular analysis was isolated from peripheral blood samples by proteinase K digestion and a salting-out with sodium chloride. Multivariate logistic regression analysis was used to calculate the risk of lung cancer in association with smoking status, histologic grade and polymorphisms of the GSTM1 and GSTT1 genes. For detection of polymorphic deletions of GSTM1 and GSTT1 genes, an internal-standard-controlled PCR was used.100–500 ng of genomic DNA was amplified in a final volume of 25 μl containing 10 mM Tris–HCl (pH 8.8), 50 mM KCl, 0.08% Nonidet p40, 0.2 mM dNTP, 2 mM MgCl2, 1U Taq polymerase and 10 pmol of each primer. For the GSTM1 polymorphism, we used the primer pair 5′-GAACTCCCTGAAAAGCTAAAGC-3′ and 5′-GTGGGGCTCAAATATACGGTGG-3′ and 35 amplification cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 1 min. Final extension was allowed to proceed at 72°C for 5 min using the oligonucleotide primers 5′-TTCCTTACTGGTCCTCACATCTC-3′ and 5′-TCACCGGATCATGGCCAGCA-3′, amplification was performed for the GSTT1 with an annealing temperature of 64°C for 30 cycles. Co-amplification of the β-globin gene fragment served as an internal positive control for a successful amplification reaction. The PCR products were separated on 2% agarose gels for 1 h at 100 V. The gels were evaluated using the video gel documentation system (Vilber-Lourmat, Cedex, France) and the BIOPROFIL 1D software. The internal standard fragment amplified from β-globin was 268 bp of length, whereas presence of the GSTM1 and GSTT1 genes were identified by 219 and 480 bp fragments, respectively. The presence or absence of the GSTM1 and GSTT1-specific amplification products indicated the respective positive and null genotypes of the genes.
All data was analyzed by SPSS for Windows software (version 13.0). A value of <0.05 was regarded as statistically significant. The logistic regression results appear as odds ratios (OR) and %95 confidence interval (CI).
Results
The characteristics of the study population are shown in Table 1. The age distribution was different between cases and controls, the mean age being 41.6 ± 27.5 and 61 ± 26.5 years for the controls and cases, respectively. Distributions of GSTM1 and GSTT1 genotypes are shown in Table 2.
The frequency of the GSTM1 null type was higher in the cases (37.3%) as compared to controls (27.3%), but the difference was not significant (P = 0.261). The GSTT1 null genotype was observed in 16.4% of the control subjects and in 22.7% of the patients with lung cancer. The difference between patients and controls was not significant (P = 0.506).
The frequency of the null GST genotype varied according to the histology of the tumor for both genes. Although no statistically significant associations were found between the GSTM1 null genotype and lung cancer patients in general, the frequency was higher in squamous cell (40.6%) than that observed in the controls (27.3%). The odds ratio, after adjustment for age and cigarette smoke was 1.356 (95% CI: 0.273–6.746) for squamous cell carcinoma. The adjusted ORs of small cell and adenocarcinomas for the GSTM1 null genotype were 0.952 (95% CI: 0.180–5.027) and 0.421(95% CI: 0.056–3.141), respectively. Thus, the GSTM1 null genotype was associated with a lower risk of small cell lung cancer.
According to the histology of the tumor, the frequency of the null GSTT1 genotype appears to be lower in small cell (14.3%) cancer compared to control group (16.4%). Therefore, the GSTT1 null genotype was significantly associated with a lower risk of specific histologies.
These analyses were repeated for the smoking groups. Evaluation of the interactions between GSTM1 and GSTT1 polymorphisms and smoking status in cases are summarized in Tables 3 and 4, respectively. We observed that the lung cancer risk was 2.5–4.5-fold higher in all smoking groups carrying the GSTM1 null genotype. The GSTT1 null genotype was associated with an increased risk only in heavy smokers.
Discussion
A great interest is focused on the identification of genes and their modifying effect on cancer risk by environmental pollutants or exposure to occupational carcinogens. Many reports indicate that in the detoxification of carcinogens, genetic differences may affect individual predisposition to lung cancer. A number of studies have attempted to establish links between polymorphic expression of different GSTs and lung cancer risk in different ethnic populations [22–24] but the results have been conflicting [25]. This study was conducted to investigate the association of the GSTM1 and GSTT1 polymorphisms in relation to cigarette smoking and histological subtypes in lung cancer patients.
Some reports have revealed an increased risk of lung cancer in patients carrying the GSTM1 null genotype [22, 26–28]. On the other hand, an association between the GSTM1 null allele and lung cancer has been refuted in other reports [29–31]. We observed a modest increase in the risk associated with the GSTM1 null genotype, consistent with the results of Nazar et al. [28] and Belobugova et al. [26] Our study showed that the GSTM1 null genotype slightly increased lung cancer risk (OR: 1.57, 95% CI: 0.328–7.543). However, this difference was not statistically significant (P = 0.26). In consistence with our results, a recent meta-analysis involving 43 published case–control studies including over 18,000 individuals showed only a slight excess of lung cancer risk (OR: 1.17, 95% CI: 1.07–1.27) in those with the GSTM1 null genotype [32]. A significant association between lung cancer and GSTM1 polymorphism was observed in analyze based on histological subgroups. Wang et al. [24] showed that the GSTM1 null genotype increased the lung cancer risk 1.6 times in squamous cell carcinoma in contrast to Lewis et al. [33] who indicated that the GSTM1 null genotype has a protective role in squamous cell carcinoma. Our results, suggest that GSTM1 null genotype does not have any affect in development of squamous cell carcinoma while it has a protective effect against small cell carcinoma. These different results may be due to small sizes of the study groups, or ethnic differences.
In our study the increase in the GSTM1 null genotype frequency was found in the smoker group. A similar observation has already been reported by Ford et al. [34] and can be explained by the enzymatic properties of GSTM1. Since GSTM1 detoxifies activated form of PAHs which are found in tobacco smoke, in the absence of GSTM1 enzyme, PAHs may contribute to the development of lung cancer.
Consistent with several published reports [24, 26, 28, 33] we could not find a relationship between GSTT1 null genotype and lung cancer. Previous studies have also indicated that the risk associated with different genotypes varies with different histological subtypes [27]. Our results indicate that the GSTT1 null genotype decreased lung cancer risk particularly in patients with small cell carcinoma. On the other hand, Chan et al. [29] reported that GSTT1 null genotype is associated with higher risk in patients with adenocarcinoma. But their study group was composed of non-smoking lung cancer patients.
In our study multivariate logistic regression analysis showed that heavy smokers with the GSTT1 genotype had an approximately 4.5-fold higher relative risk when compared with non-smoking patients. These results indicate that several carcinogenic compounds in the tobacco smoke may also contribute to the development of lung cancer.
References
Sqita MR, Wei Q, Li G, Wu X (1999) Genetic susceptibility to tobacco carcinogenesis. Cancer Invest 17:661–662
Pelkonen O, Nebert DW (1982) Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev 34:189–222
Mannervik B, Danielson UH (1988) Glutathione transferases structure and catalytic activity. CRC Crit Rev Biochem 23:283–337
Harris CC (1986) Tobacco smoke and lung disease: who is susceptible? Ann Intern Med 5:607–609
Seidegard J, Pero RW (1985) The hereditary transmission of high glutathione transferase activity toward transtilbene oxide in human mononuclear leukocytes. Hum Genet 69:66–68
Pemble S, Schröder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB (1994) Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J 300:271–276
Kliencke JK, Kelsey KT, Lamela RA, Toscano WA (1990) Human glutathione S-transferase deficiency as a marker of susceptibility to epoxide-induced cytogenetic damage. Cancer Res 50:1585–1590
Seidegard J, Pero RW, Miller DG, Beattie EJ (1986) A glutathione transferase in human leukocytes as a marker fort he susceptibility to lung cancer. Carcinogenesis 7:751–753. doi:10.1093/carcin/7.5.751
Bell DA, Taylor JA, Paulson DF, Robertson CN, Mohler JL, Lucier GW (1993) Genetic risk and carcinogen exposure: a common inherited defect of the carcinogen metabolism gene glutathione-transferase M1 (GSTM1) that increases susceptibility to bladder cancer. J Natl Cancer Inst 85:1159–1164. doi:10.1093/jnci/85.14.1159
Chenevix-Trench G, Young J, Coggan M, Board P (1995) Glutathione S-transferase M1 and T1 polymorphisms: susceptibility to colon cancer and age of onset. Carcinogenesis 16:1655–1657. doi:10.1093/carcin/16.7.1655
Zhong S, Wyllre AH, Barnes D, Wolf CR, Spurr NK (1993) Relationship between the GSTM1 genetic polymorphism and susceptibility to bladder, breast and colon cancer. Carcinogenesis 14:1821–1824. doi:10.1093/carcin/14.9.1821
Hengstler JG, Arand M, Herrero ME, Oesch F (1998) Recent results cancer research: polymorphisms of N-acetyltransferases, glutathione S-transferases, microsomal epoxide hydrolase and sulfotransferases: influence on cancer susceptibility. In: Schwab M (ed) Genes and environment. Springer, New York, pp 47–85
Meyer DJ, Coles B, Pemble SE, Gilmore KS, Fraser GM, Ketterer B (1991) Theta, a new class of glutathione-transferases purified from rat and man. Biochem J 274:409–414
Schroeder KR, Hallier E, Peter H, Bolt HM (1992) Dissociation of a new glutathione S-transferase in human erythrocytes. Biochem Pharmacol 43:1671–1674
Hussey AJ, Hayes JD (1992) Characterization of a human class-theta glutathione S-transferase with activity towards 1-menaphthyl sulphate. Biochem J 286:926–935
Tan KL, Webb GC, Baker RT, Board PG (1995) Molecular cloning of a cDNA and chromosomal localisation of a human theta-class glutathione S-transferase gene (GSTT2–2) to chromosome 22. Genomics 25:381–387
Hallier E, Schröder KR, Asmuth K, Dommermuth A, Aust B, Goergens HW (1994) Metabolism of dichloromethanemethylchloride to formaldehydein human erythrocytes: influence of polymorphism of glutathione transferase theta (GSTT1-1). Arch Toxicol 68:423–427
Lee EJ, Wong JY, Yeoh PN, Gong NH (1995) Glutathione S-transferase theta (GST1) genetic polymorphism among Chinese, Malays and Indians in Singapore. Pharmacogenetics 5:332–334
Nelson HH, Wiencke JK, Christiani DC, Cheng TJ, Zuo ZF, Schwartz BS, Lee BK, Spitz MR, Wang M, Xu X (1995) Ethnic differences in the prevalence of the homozygously deleted genotype of glutathione S-transferase theta. Carcinogenesis 16:1243–1245
Chen H, Sandler DP, Taylor JA, Shore DL, Liu E, Bloomfield CD, Bell DA (1996) Increased risk for myelodysplastic syndromes in individuals with glutathione transferase θ 1 (GSTT1) gene defect. Lancet 347:295–297
Elexpuru-Camiruaga J, Buxton N, Kandula V, Dias PS, Campbell D, McIntosh J, Broome J, Jones P, Inskip A, Alldersea J (1995) Susceptibility to astrocytoma and meningioma: influence of allelism at glutathione S-transferase (GSTT1 and GSTM1) and cytochrome p-450 (CYP2D6) loci. Cancer Res 55:4237–4239
Jourenkova-Mironova N, Wikman H, Bouchardy C, Voho A, Dayer P, Benhamou S, Hirvonen A (1998) Role of glutathione S-transferase GSTM1, GSTM3, GSTP1 and GSTT1 genotypes in modulating susceptibility to smoking-related lung cancer. Pharmacogenetics 8:495–502
Kihara M, Noda K (1999) Lung cancer risk of the GSTM1 null genotype is enhanced in the presence of the GSTP1 mutated genotype in male Japanese smokers. Cancer Lett 137:53–60
Wang J, Deng Y, Cheng J, Ding J, Tokudome S (2003) GST genetic polymorphisms and lung adenocarcinoma susceptibility in a Chinese population. Cancer Lett 201:185–193. doi:10.1016/S0304-3835(03)00480-4
Hayes JD, Strange RC (2000) Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology 61:154–166. doi:10.1159/000028396
Belogubova EV, Togo AV, Karpova MB, Kuligina E, Buslov KG, Ulibina JM, Lemehov VG, Romanenko SM, Shutkin VA, Hanson KP, Hirvonen A, Imyanitov EN (2004) A novel approach for assessment of cancer predisposing roles of GSTM1 and GSTT1 genes: use of putatively cancer resistant elderly tumor-free smokers as the referents. Lung Cancer 43:259–266. doi:10.1016/j.lungcan.2003.08.019
Houlston RS (1999) Glutathione S-transferase M1 status and lung cancer risk: a meta analysis. Cancer Epidemiol Biomarkers Prev 8:675–682
Nazar-Stewart V, Vaughan TL, Stapleton P, VanLoo J, Nicol-Blades B, Eaton DL (2003) A population-based study of glutathione S-transferase M1, T1 and P1 genotypes and risk for lung cancer. Lung Cancer 40:247–258. doi:10.1016/S0169-5002(03)00076-X
Chan-Yeung M, Tan-Un KC, Ip MSM, Tsang KWT, Ho SP, Ho JCM, Chan H, Lam WK (2004) Lung cancer susceptibility and polymorphisms of glutathione S-transferase genes in Hong Kong. Lung Cancer 45:155–160. doi:10.1016/j.lungcan.2004.01.016
Raimondi S (2005) Metabolic gene polymorphisms and lung cancer risk in non-smakers. An update of the GSEC study. Mutat Res 592:45–57. doi:10.1016/j.mrfmmm.2005.06.002
Schneider J, Bernges U, Philipp M, Woitowitz HJ (2004) GSTM1, GSTT1 and GSTP1 polymorphism and lung cancer risk in relation to tobacco smoking. Cancer Lett 208:65–74. doi:10.1016/j.canlet.2004.01.002
Benhamou S, Lee WJ, Alexandrie AK, Boffetta P, Bouchardy C, Butkiewicz D, Brockmöller J, Clapper ML, Daly A, Dolzan V, Ford J, Gaspari L, Haugen A, Hirvonen A, Husgafvel-Pursiainen K, Ingelman-Sundberg M, Kalina I, Kihara M, Kremers P, Le Marchand L, London SJ, Nazar-Stewart V, Ono-Kihara M, Rannug A, Romkes M, Ryberg D, Seidegard J, Shields P, Strange RC, Stücker I, To-Figueras J, Brennan P, Taioli E (2002) Meta- and pooled analysis of the effects of glutathione S-transferase M1 polymorphisms and smoking on lung cancer risk. Carcinogenesis 23:1343–1350. doi:10.1093/carcin/23.8.1343
Lewis SJ, Cherry NM, Niven RML, Barber PV, Povey AC (2002) GSTM1, GSTT1 and GSTP1 polymorphisms and lung cancer risk. Cancer Lett 180:165–171. doi:S0304-3835(02)00028-9
Ford JG, Li Y, O’Sullivan MM, Demopoulos R, Garte S, Taioli E, Brandt-Rauf PW (2000) Glutathione S-transferase M1 polymorphism and lung cancer risk in African-Americans. Carcinogenesis 21:1971–1975. doi:10.1093/carcin/21.11.1971
Acknowledgments
This work was supported by the Research Fund of the University of Istanbul. Project number: 251/23082004
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altinisik, J., Balta, Z.B., Aydin, G. et al. Investigation of glutathione S-transferase M1 and T1 deletions in lung cancer. Mol Biol Rep 37, 263–267 (2010). https://doi.org/10.1007/s11033-009-9673-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9673-5