Abstract
As more and more re-sequencing genome data in crops were released, SNPs (single nucleotide polymorphisms) are easily achieved for genotyping and show the highest abundance among all kinds of molecular markers. However, high-throughput SNP genotyping methods, such as KASP (Kompetitive Allele Specific PCR), TaqMan, or ARMS (amplification refractory mutation system), are always labor on DNA extraction and depending on expensive equipment. Therefore, most breeders are undergoing the bottleneck of lacking an easy, cost-saving, and stable genotyping system. Here, we report a direct PCR–based medium-throughput SNP marker–assisted selection (D-MAS) system suits available major crops including rice, wheat, maize, and rapeseed. The D-MAS system, which reduces the time of manual operation and result analysis dramatically, contains (1) seedling breeding in greenhouse; (2) high-throughput DNA extraction by alkaline lysis; and (3) gel-free SNP marker detection with 384-well by PARMS (penta-primer amplification refractory mutation) or KASP genotyping system. The stability of alkaline lysis DNA was validated by flexible dilution fold and long storing time under low temperatures. The alkaline lysis DNA from four F2 populations of rice, wheat, maize, and rapeseed showed equal efficiency in SNP calling relative to the ones from cetyl trimethylammonium bromide (CTAB) method. With the alkaline lysis DNA, PAMRS showed denser genotype cluster than KASP. Furthermore, the D-MAS system was adaptive with rice old leaves. The throughput and efficiency of the D-MAS system were validated in the hybrid rice seed purity test with a Xian-Geng-specific SNP marker which is also recommended to select ipa1-2d gene in the rice molecular breeding. In conclusion, we proposed a direct PCR–based SNP calling pipeline, which could be a simple, cheap, and robust standard operation procedure (SOP) of molecular breeding for different crops and get extensive use in most laboratory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the first reference genome sequence of crops, the genome sequence of rice variety Nipponbare was released in 2005 (International Rice Genome Sequencing Project 2005). In the following decades, a number of crop genomes or draft genomes were released, including maize (Schnable et al. 2009), sorghum (Paterson et al. 2009), soybean (Schmutz et al. 2010), cotton (Wang et al. 2012), setaria (Bennetzen et al. 2012), and wheat (International Wheat Genome Seqencing Consortium, IWGSC 2014). The boosting of next-generation sequencing and crop functional genome researches like genome-wide association study and map-based cloning provided a great number of causative SNPs and information for functional genes. Until now, more than 3000 Asian cultivated rice were sequenced (Wang et al. 2018), about 3300 genes were cloned (https://funricegenes.github.io/), and 200 of 3300 genes were map-based cloned (Zhang et al. 2016). Huang et al. constructed a high-density haplotype map (Huang et al. 2010), and they further revealed the major heterosis contributing gene (Huang et al. 2015, Huang et al. 2016). The genome re-sequencing data are ongoing boosting in other crops as well, such as the genomic database HapMap3 containing 916 inbred lines in maize (Bukowski et al. 2018). All these works paved the way to molecular breeding and, further, the genomic breeding.
The crop breeding method was developed from phenotype selection to marker-assisted selection and now turned to the rational design or genomic breeding; this process is more and more depending on genotyping. In the general MAS breeding protocol, 50% individuals with target gene could be selected in a backcrossing population. The rational selection is an accurate and efficient breeding strategy in rice. In this breeding scheme, less than 5% individuals with multiple target genes were selected (Zeng et al. 2017). The genomic breeding strategy suggested a stricter molecular marker selection method by identifying the recombination between the target gene and linked 100 kb background of the recurrent parent. In order to introduce less than 200 kb chromosome segment of a target gene, only less than 1% individuals could be selected in the genomic breeding scheme (Wing et al. 2018). It is clear to see that the more accurate the breeding, the more genotyping was needed. However, previous genotyping methods either based on gel electrophoresis or automated fluorescence reading may have certain defects which limited the development of molecular breeding.
Gel electrophoresis–based molecular markers such as SCAR (sequence characterized amplified region), SSR (simple sequence repeat), and CAPS/dCAPS (cleaved amplified polymorphic sequence/deviated cleaved amplified polymorphic sequence) were widely used in molecular breeding. The throughput of these techniques, however, is limited because electrophoresis is laborious and time-consuming. The on gel result reading and data point recording were depending on human labor completely. This bottleneck was broken by employing integrated automation molecular system (Dayteg et al. 2007). The gel-free SNP marker system has enormous higher throughput potentiality than SSR markers and makes fully automated SNP marker system a more robust solution in molecular breeding. A convincing example is Monsanto who switched to SNP marker system in 2000, which promoted 40-fold increase of total marker data point production in the next 6 years (Eathington et al. 2007). However, the real high throughput might combine the SNP system with Fluidigm or the Douglas Scientific Array Tape system, which is too expensive to afford for most breeders (Thomson 2014, Batley 2015). Because of this, among all the molecular markers, the SSR/InDel marker system is still dominant.
Because of the high-throughput potential of SNP genotyping system, SNP markers have been extensively developed for further application in the rice, wheat, and maize (Ma et al. 2017, Pan et al. 2017, Rasheed et al. 2016, Yang et al. 2019, and Zhao et al. 2019). TaqMan (Thermo Fisher, USA) and KASP (LGC Genomics, UK) are currently two major SNP marker–detecting methods suitable for automated genotyping of a large number of individuals with a few markers simultaneously. However, the laborious DNA extraction limited these medium-throughput SNP genotyping. Conventional CTAB and SDS plant DNA extraction procedures need many tedious steps like pipetting, centrifuge, precipitation, salt washing, and elution, which are slow and exhausting. Reported high-throughput DNA extraction method for SNP genotyping was conducted with magnetic bead kit (Thomson 2014) which is expensive. Besides that, the TaqMan probe synthesis is much more expensive than normal primers.
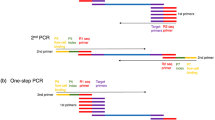
So, the current states of genotyping methods could be concluded as below: (1) traditional gel–based methods are time- and labor-consuming and have low throughput but are cheap. (2) Medium-throughput automated genotyping method, such as TaqMan and KASP, needs high-quality DNA and has expensive primer/probe design and PCR mix. (3) High-throughput automated genotyping needs much more expensive equipment. So, molecular breeders of different crops are facing the same defects in different genotyping platforms. It is still lacking a cheap, medium-throughput, easy, and robust genotyping system which could be conducted in most of laboratories. Here, we reported a D-MAS system which combined modified rapid alkaline lysis DNA extraction with a medium-throughput SNP genotyping by PARMS (Gentides, China) or KASP (LGC Genomics, UK). PARMS is a KASP-like SNP genotyping technique combining the ARMS which is also named as allele-specific PCR (Heim and Meyer, 1990; Newton et al. 1989) and universal energy transfer–labeled primers (Nuovo et al. 1999; Myakishev et al. 2001). The two universal primers of PARMS share the same two common 21 base 3′ oligonucleotide ends with the SNP genotyping technique of KASP (Rosas et al. 2014), which makes PARMS and KASP theoretically interchangeable using the same SNP primer set. This D-MAS system is adaptable to rice, wheat, maize, and rapeseed. What is more, it was adaptable to different competitive PCR platforms (KASP and PARMS) and old leaves. Only 1 day was needed from sampling to genotyping of 1500 samples. The medium-throughput genotyping and robust genotyping of the D-MAS system are both beneficial for molecular breeding or gene fine-mapping among different crops in most laboratories.
Materials and methods
Plant material for genotyping test
A total of four F2 segregating populations, each from one of four crops, were used in this study. The rice, wheat, and maize populations were derived from crosses between Zhenshan97 (ZS97) and Minghui63 (MH63), Shannong22 and Emai398, and R62 and S909, respectively. The seeds of rapeseed F2 population 16H134 were kindly provided by Dr. Haitao Li. All seeds from four populations were germinated and planted in 48- or 96-well plastic cave dish containing nutrition soil and vermiculite (ratio of 3:1) in a greenhouse under natural light condition (Online Resource 1). The old leaves of 40 doubled haploid lines, which were derived from Xian-Geng hybrid cultivar YongYou 2640, were harvested in the field, and another five hybrid rice cultivars were sampled to test the D-MAS system.
Sampling and DNA isolation
For rice, wheat, and maize at trefoil stage and for rapeseed at four-leaf stage, about 2-cm-long or 1-cm-diameter leaves from seedlings were collected into 2-ml centrifuge tubes or 96-deep-well plates for DNA isolation. The DNA was extracted in a 96-deep-well plate by alkaline lysis approach using the following procedures: (1) adding 67 μl of 0.3 M NaOH solution and two 4 mm diameter stainless balls into each sample well. After grinding at 50 Hz for 60 s using TissueLyser (JINGXIN, China), the deep-well plate was placed into a water bath at 96 °C for 1 min (the heating step was not performed in the non-boil treatment). (2) Adding 200 μl of 0.75 M Tris-HCl (pH 7.5–7.8). (3) A centrifuge (TD5A-WS, CENCE, China) at 3000g for 1 min separates the supernatants, which were further transferred to a new 200-μl 96-well PCR plate. (4) Gradient dilution to 5, 10, 20, and 30 folds with ultrapure water. Such alkaline lysis DNA solutions can be used as PCR templates. All the experimental pictures are shown in Online Resource 2. As a control, the CATB DNA was extracted using CTAB protocol (Murray and Thompson 1980), and the concentration was measured by NanoDrop 2000 (Thermo Scientific, USA) and adjusted to 15 ng/μl. Except for the dilution test, which conducted four diluted alkaline lysis DNA and one CTAB DNA as templates, the other tests were all conducted only with 10 times dilution of alkaline lysis DNA and CTAB DNA. CATB DNA and alkaline lysis DNA were stored at 4 °C and − 20 C for storing time test. Twelve individuals were used in the dilution test, and 48 individuals were used for test of different primers and crops. DNA extraction for old leaves of rice is identical to young leaves.
Selection and design of SNP marker primer sets
The primer sequence information of SNP markers for wheat and maize was obtained from publications which were used as KASP markers before (Ma et al. 2017, Pan et al. 2017, and Zhao et al. 2019). The SNP primer sets for rapeseed and rice were kindly provided by Dr. Haitao Li and Dr. Yanyu Yang, respectively. The rice finger print SNP primers were designed on line. Firstly, we collected SNPs between ZhenShan97 and MingHui63 from a rice database RiceVarMap (http://ricevarmap.ncpgr.cn/v2/two_cultivars_compare/), and then, the franking sequencing of each SNP was downloaded from Nipponbare V6 genome through rice Gbrowse programme in RiceVarMap (http://ricevarmap.ncpgr.cn/cgi-bin/gb2/gbrowse/ricevarmap/). The primers were both designed by an online software snpway (www.snpway.com). After removing non-specific matching primers by blast, a total of 24 primers located on the 12 chromosomes of rice were selected to test the success rates of primer design.
In order to design a tightly linked SNP marker of ipa1-2d gene, an ipa1-2d functional dominant marker was obtained from Zhang et al. (2017) to detect this gene in 48 hybrid or inbred rice cultivars. And then, the whole-genome SNPs of ipa1-2d carried cultivars were detected by GSR44K whole-genomic breeding chip (Greenfafa, China), and the 100 kb franking SNP of the ipa1-2d gene was selected and compared with Nipponbare and 9311 reference genome to choose the specific SNPs. The primer design method was the same as the previous description. All primer sequences are listed in Online Resource 3.
Genotyping and SNP calling
Genotyping tests were carried out with PARMS (Gentides, China) and KASP (LGC Genomics, UK). The PCR reactions are set in 384-well PCR plates for both PARMS and KASP genotyping. The 5 μl PCR reaction system contains 2× PARMS or KASP PCR reaction mix, 150 nM each allele-specific primer, 400 nM locus-specific primer, and 1.4 μl alkaline lysis or CTAB DNA template. Five-microliter mineral oil is added into each well of 384 PCR plate to prevent evaporation of PCR mix. The thermal cycler program of PARMS is denaturing at 95 °C for 15 min and then 10 cycles of denaturation at 95 °C for 20 s and annealing started at 65 °C for 1 min, then decreasing 0.8 °C per cycle to the annealing temperature at 57 °C. This was followed by 32 cycles of denaturation at 95 °C for 20 s and annealing at 57 °C for 1 min. For KASP, the program is denaturing at 95 °C for 15 min and then 10 cycles of denaturation at 95 °C for 20 s and annealing started at 61 °C for 1 min, then decreasing 0.6 °C per cycle to the annealing temperature at 55 °C. This was followed by 32 cycles of denaturation at 95 °C for 20 s and annealing at 55 °C for 1 min. Endpoint scanning of fluorescent intensity was carried out in BMG Omega F (LGC Genomics, UK); SNP calling and plots were carried out on the KlusterCaller software (LGC Genomics, UK) combining manual modification. The bad date point is defined by the following rules: (1) The individual genotyping result from alkaline lysis DNA could not be repeated using CTAB DNA. (2) The data point in the plot did not cluster into any of three main groups. (3) The data points cannot be distinguished with NTC (non-template control).
Result
The SOP of the D-MAS system
We proposed a pipeline aiming at constructing a SOP of direct PCR–based medium-throughput SNP marker–assisted molecular breeding, which is easy to be conducted in most laboratories. Firstly, seeds were sowed in 96 plugs and bred in the greenhouse (Online Resource 1), followed by sampling young leaves into the 96-deep-well plates. Four steps were needed for DNA extraction by alkaline lysis, including adding NaOH, grinding, neutralization, and dilution (Online Resource 2). The alkaline lysis DNA was taken as the template for SNP calling by PARMS or KASP system. In order to increase the throughput of SNP genotyping, a 384 PCR system is suggested (Fig. 1).
Determination of alkaline lysis DNA concentration and genotyping platform
For PARMS genotyping platform, a promising concentration range for genotyping was determined by 0- to 30-fold gradient diluted alkaline lysis DNA, using CATB DNA as the control. As shown in Fig. 2 and Online Resource 4, the alkaline lysis DNA of all four crops without dilution could cause failure of PARMS PCR amplification. All 5- to 30-fold dilution alkaline lysis DNA achieved successful genotyping. Over 20-fold dilution could cause a slight decline of genotyping fluorescence intensity, although it did not have impact on the genotype determination of each group (Fig. 2 and Online Resource 4).
Alkaline lysis DNA dilution series compared with CTAB DNA. a–d Rice, wheat, maize, and rapeseed alkaline lysis DNA dilution test, respectively. Black dot: NTC; pink dot: non-dilution alkaline lysis DNA; blue dot: CTAB DNA; red dot: 10 times dilution alkaline lysis DNA; green dot: 30 times dilution alkaline lysis DNA. The dots which are clustered in three dotted circles represent two different homozygous (Y:Y and X:X) genotypes and one heterozygous (X:Y) genotype
We also compared PARMS and KASP systems using alkaline lysis DNA. Two identical PCR systems were used for evaluation, and the thermal cycler programs were set according to the manufacturers’ protocol. The results proved that the alkaline lysis method was compatible both in the PARMS and KASP system. And PARMS, which showed a denser genotype cluster than that of KASP, was less sensitive to the PCR inhibitor in the alkaline lysis DNA Online Resource (5).
The D-MAS system is stable under the PARMS platform
We tested the stability of alkaline lysis DNA with PARMS platform. Genotyping was conducted with three primer sets in four crops, and the results were completely consistent between alkaline lysis DNA and CATB DNA in four F2 populations of rice, wheat, maize, and rapeseed (Fig. 3, Table 1). The fluorescence intensity was identical using two types of DNA samples in all 3 rice primer sets. In maize and rapeseed, two of the three primers showed no significant difference in fluorescence intensity between these two types of DNA samples, while one primer set showed lower fluorescence using alkaline lysis DNA. The fluorescence intensity of alkaline lysis DNA was lower than that of reaction using CATB DNA in all primer sets of wheat, but it had no impact on the genotyping results. Besides the universal test between different crops that was conducted by the available primers, we also tested the success rates of primer design through 24 rice fingerprint primers. Finally, 87.5% (21/24) software batch designed primers worked in the fingerprint experiment Online Resource (6).
Genotyping with alkaline lysis DNA and CTAB DNA of rice, wheat, maize, and rapeseed using three primers under the PARMS platform. The red and blue dots represent different homozygous genotypes, and the green dots represent heterozygous genotype; the black dots are NTC. The selected dots with light red, green, and blue color are the genotyping results of alkaline lysis DNA, and the dots with dark red, green, and blue are corresponding CTAB DNA. The 384 plate below the dot picture showed the genotyping results of individual sample comparing alkaline lysis and CTAB methods. The upper selected 48 samples were alkaline lysis DNA and lower unselected 48 samples were CTAB DNA. a–c Genotyping with rice primers RMoc1S3, TESTR3, and REHD1S1, respectively, under the PARMS platform. d–f Genotyping with wheat primers Sdr-B1, Zds-A1, and Rht-D1, respectively, under the PARMS platform. g–i Genotyping with maize primers Ks6, MkqPH1B, and MkqTBN3A, respectively, under the PARMS platform. j–l Genotyping with rapeseed primers KB4, KB6, and KB14, respectively, under the PARMS platform
The optimized DNA extraction steps were robust in the storing and genotyping
We compared the alkaline lysis DNA extracted with or without the heating step. All four crops genotyping results indicated that the heating step is vital for a better result and longer storing time to the alkaline lysis DNA sample (Table 1). Surprisingly, rice alkaline lysis DNA could be stored at 4 °C for 2 weeks when both boiled or non-boiled alkaline lysis DNA showed excellent coincident genotyping results. Up to 91.1% rice non-boiled alkaline lysis DNA could be genotyping successfully after 4-week storing at 4 °C. Unfortunately, wheat non-boiled alkaline lysis DNA may degenerate after 14-day storing at 4 °C, although the boiled alkaline lysis DNA showed stable amplification reaction as the CATB extracted DNA did. The genotyping signals from maize and rapeseed alkaline lysis DNA were more disperse than those of CATB DNA, and the boiled ones were slightly better than un-boiled ones Online Resource (7). The alkaline lysis DNA could be stored in − 20 °C for 8 weeks at least (Table 1; Online Resource 8). Next, the same method was used to genotyping old leaves of rice in flowering stage, which showed identical result with CTAB DNA Online Resource (9).
A case of rapid detection of rice hybrid seed purity with ipa1-2d special SNP marker
In order to test the throughput and efficiency of the D-MAS system, one ipa1-2d-linked SNP marker RK0825A was designed. The genotyping result of 48 hybrid and inbred rice cultivars by a functional dominant marker Repspecific F/R (Zhang et al. 2017) showed that ipa1-2d gene was very rare in the Xian-Xian hybrid rice (1/33) and Geng (0/8) rice cultivars but predominant in the Xian-Geng (7/7) rice cultivars (Online Resource 10). Thus, two Xian-Geng hybrid rice cultivars were genotyped by whole-genomic SNP genechip, and the gene franking SNP was compared with the reference genome. Finally, a SNP marker RK0825A which derived from SNP locus R0825195113AG, was proved to co-separate with the functional dominant marker locus (Online Resources 3 and 10). Four commercial Xian-Geng hybrid rice cultivars were used to screen the purity of hybrid seeds by RK0825A. The whole pipeline is shown in Fig. 1. Nearly 1536 leaf samples were harvested in 3 h, and the alkaline lysis DNA extraction was finished in 2 h, followed by 1 h for preparing PCR mix and 2 h for PCR reaction. The four 384 PCR plates were scanned by BMG Omega F (LGC Genomics, UK) in 10 min. The purity of 4 hybrid rice was 98.15%, 98.42%, 99.21%, and 98.36%, respectively. The purity test results are shown in Fig. 4. It is easy to identity the homozygous ones which may come from restorer or sterile line.
Discussion
The D-MAS system is a simple, cost-saving, and robust SNP genotyping SOP for different crops
Any SOP should own the characters of easy and stable. In the D-MAS SOP, firstly, only four simple steps were needed in the alkaline lysis DNA preparation: (1) adding NaOH, (2) grinding, (3) neutralization, and (4) dilution. Secondly, it is easy to balance the DNA concentration and PCR inhibitor in the alkaline lysis DNA. From 5-fold to 30-fold dilution, alkaline lysis DNA could output correct genotyping result. Thirdly, both PARMS and KASP SNP genotyping platform could use alkaline lysis DNA as template. Fourthly, the alkaline lysis DNA and CTAB DNA from the same leaf samples of four crops have the identical genotyping results, and the alkaline lysis DNA could be stored for 2 months at − 20 °C at least. It was also suited for rice old leaf (Online Resource 9) and cotton leaf (personal communication with Dr. Yanyu Yang). Furthermore, the success rates of PARMS primer design were quite high (87.5%) with the free online software snpway. It coincides with the search data which showed 94.8% SNPs could be transferred into the KASP primers (Yang et al. 2019). To reduce the cost of genotyping, we used cheap disposable 384-well PCR plates and mineral oil to seal the plate instead of plastic membrane which is more expensive and recommended to use in all the fluorescence reading. The endpoint reading in the BMG Omega F (LGC Genomics, UK) is adaptable to normal clear PCR plates plus mineral oil sealing. However, white plate is preferred if using other microplate readers. The cost of DNA extraction, PCR mix, and the operation steps was dramatically reduced in the MAS of four crops, and the results were stable. The three characteristics of easy operation steps, cheap reagent, and stable genotyping in four crops make D-MAS the potential genotyping SOP for most crop breeders which will also reduce the cost of industry.
Updating the way of breeding
The traditional breeding methods prefer to select individuals by phenotypic information which is highly depending on the breeding experience. However, experience-based breeding ability may obtain from more than a decade’s working practice because most of the yield-related, abiotic, and biotic resistance traits are difficult to observe. Moreover, the traditional method is not time and labor saving at all. Functional genome research in crops has been developed for more than 30 years; as RICE2020 project described (Zhang et al. 2008), scientists made great effort to determine the function of every gene along with the haplotypes in rice and other crop genomes and further try to improve crops with the findings of functional genome researches. In the genomic breeding scheme, elite genes/alleles integrate into a certain background to form a series of individual near isogeneic lines (NILs) with who will easily pyramid all of these genes/alleles together without genetic drag. It has a great potential to pyramiding more superior alleles together because the rice inbred lines only carry less than half of superior alleles, and the hybrid varieties have several (Huang et al. 2015, Huang et al. 2016). As above suggestions presented, no more than 1% individuals have the chance to be the candidate during the construction of NIL lines. If more than 99% individuals were discarded, 99% field can be saved as well as the corresponding manpower input. Molecular breeding is more accurate in selecting QTL traits which are difficult to be observed in the field. In the period of genomic breeding, every breeder may reduce more than 99% field working by experimental genotyping, and every inbred line may clearly be labeled with genotype; thus, the further gene pyramiding event could be known directly in the next genetic modification. The simple, cost-saving, and robust D-MAS SOP could help most breeders to achieve this goal.
References
Batley J (2015) Plant genotyping: methods and protocols. In: Methods in Molecular Biology, vol 1245. Springer, New York
Bennetzen JL, Schmutz J, Wang H, Percifield R, Hawkins J, Pontaroli AC, Estep M, Feng L, Vaughn JN, Grimwood J, Jenkins J, Barry K, Lindquist E, Hellsten U, Deshpande S, Wang X, Wu X, Mitros T, Triplett J, Yang X, Ye CY, Mauro-Herrera M, Wang L, Li P, Sharma M, Sharma R, Ronald PC, Panaud O, Kellogg EA, Brutnell TP, Doust AN, Tuskan GA, Rokhsar D, Devos KM (2012) Reference genome sequence of the model plant Setaria. Nat Biotechnol 30(6):555–561. https://doi.org/10.1038/nbt.2196
Bukowski R, Guo X, Lu Y, Zou C, He B, Rong Z, Wang B, Xu D, Yang B, Xie C, Fan L, Gao S, Xu X, Zhang G, Li Y, Jiao Y, Doebley JF, Ross-Ibarra J, Lorant A, Buffalo V, Romay MC, Buckler ES, Ware D, Lai J, Sun Q, Xu Y (2018) Construction of the third-generation Zea mays haplotype map. GigaScience 7(4):1–12. https://doi.org/10.1093/gigascience/gix134
Dayteg C, Tuvesson S, Merker A, Jahoor A, Brantestam KA (2007) Automation of DNA marker analysis for molecular breeding in crops: practical experience of a plant breeding company. Plant Breed 126(4):410–415. https://doi.org/10.1111/j.1439-0523.2007.01306.x
Eathington SR, Crosbie TM, Edwards MD, Reiter RS, Bull JK (2007) Molecular markers in a commercial breeding program. Crop Sci 47(Supplement_3):S-154
Heim M, Meyer UA (1990) Genotyping of poor metabolisers of debrisoquine by allele-specific PCR amplification. Lancet 336(8714):529–532. https://doi.org/10.1016/0140-6736(90)92086-W
Huang X, Wei X, Sang T, Zhao Q, Feng Q, Zhao Y, Li C, Zhu C, Lu T, Zhang Z, Li M, Fan D, Guo Y, Wang A, Wang L, Deng L, Li W, Lu Y, Weng Q, Liu K, Huang T, Zhou T, Jing Y, Li W, Lin Z, Buckler ES, Qian Q, Zhang QF, Li J, Han B (2010) Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet 42(11):961–967. https://doi.org/10.1038/ng.695
Huang X, Yang S, Gong J, Zhao Y, Feng Q, Gong H, Li W, Zhan Q, Cheng B, Xia J, Chen N, Hao Z, Liu K, Zhu C, Huang T, Zhao Q, Zhang L, Fan D, Zhou C, Lu Y, Weng Q, Wang ZX, Li J, Han B (2015) Genomic analysis of hybrid rice varieties reveals numerous superior alleles that contribute to heterosis. Nat Commun 6:6258. https://doi.org/10.1038/ncomms7258
Huang X, Yang S, J-Y G, Zhao Q, Feng Q, Zhan Q, Zhao Y, Li W, Cheng B, Xia J, Chen N, Huang T, Zhang L, Fan D, Chen J, Zhou C, Lu Y, Weng Q, Han B (2016) Genomic architecture of heterosis for yield traits in rice. Nature 537. https://doi.org/10.1038/nature19760
International Rice Genome Sequencing Project (2005) The map-based sequence of the rice genome. Nature 436(7052):793–800. https://doi.org/10.1038/nature03895
International Wheat Genome Seqencing Consortium(IWGSC) (2014) A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345(6194):1251788. https://doi.org/10.1126/science.1251788
Ma C, Ma X, Yao L, Liu Y, Du F, Yang X, Xu M (2017) qRfg3, a novel quantitative resistance locus against Gibberella stalk rot in maize. Theor Appl Genet 130(8):1723–1734. https://doi.org/10.1007/s00122-017-2921-5
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8(19):4321–4325
Myakishev MV, Khripin Y, Hu S, Hamer DH (2001) High-throughput SNP genotyping by allele-specific PCR with universal energy-transfer-labeled primers. Genome Res 11(1):163–169
Newton CR, Graham A, Heptinstall LE, Powell SJ, Summers C, Kalsheker N, Smith JC, Markham AF (1989) Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res 17(7):2503–2516. https://doi.org/10.1093/nar/17.7.2503
Nuovo GJ, Hohman RJ, Nardone GA, Nazarenko IA (1999) In situ amplification using universal energy transfer-labeled primers. J Histochem Cytochem 47(3):273–279. https://doi.org/10.1177/002215549904700301
Pan Q, Xu Y, Li K, Peng Y, Zhan W, Li W, Li LAND, Yan J (2017) The genetic basis of plant architecture in 10 maize recombinant inbred line populations. Plant Physiol 175:858–873
Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, Schmutz J, Spannagl M, Tang H, Wang X, Wicker T, Bharti AK, Chapman J, Feltus FA, Gowik U, Grigoriev IV, Lyons E, Maher CA, Martis M, Narechania A, Otillar RP, Penning BW, Salamov AA, Wang Y, Zhang L, Carpita NC, Freeling M, Gingle AR, Hash CT, Keller B, Klein P, Kresovich S, McCann M, Ming R, Peterson DG, Mehboob-ur-Rahman, Ware D, Westhoff P, Mayer KF, Messing J, Rokhsar DS (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457(7229):551–556. https://doi.org/10.1038/nature07723
Rasheed A, Wen W, Gao F, Zhai S, Jin H, Liu J, Guo Q, Zhang Y, Dreisigacker S, Xia X, He Z (2016) Development and validation of KASP assays for genes underpinning key economic traits in bread wheat. Theor Appl Genet 129(10):1843–1860. https://doi.org/10.1007/s00122-016-2743-x
Rosas JE, Bonnecarrère V, Pérez de Vida F (2014) One-step, codominant detection of imidazolinone resistance mutations in weedy rice (Oryza sativa L.). Electron J Biotechnol 17:95–101
Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, Xu D, Hellsten U, May GD, Yu Y, Sakurai T, Umezawa T, Bhattacharyya MK, Sandhu D, Valliyodan B, Lindquist E, Peto M, Grant D, Shu S, Goodstein D, Barry K, Futrell-Griggs M, Abernathy B, du J, Tian Z, Zhu L, Gill N, Joshi T, Libault M, Sethuraman A, Zhang XC, Shinozaki K, Nguyen HT, Wing RA, Cregan P, Specht J, Grimwood J, Rokhsar D, Stacey G, Shoemaker RC, Jackson SA (2010) Genome sequence of the palaeopolyploid soybean. Nature 463(7278):178–183. https://doi.org/10.1038/nature08670
Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, Liang C, Zhang J, Fulton L, Graves TA, Minx P, Reily AD, Courtney L, Kruchowski SS, Tomlinson C, Strong C, Delehaunty K, Fronick C, Courtney B, Rock SM, Belter E, du F, Kim K, Abbott RM, Cotton M, Levy A, Marchetto P, Ochoa K, Jackson SM, Gillam B, Chen W, Yan L, Higginbotham J, Cardenas M, Waligorski J, Applebaum E, Phelps L, Falcone J, Kanchi K, Thane T, Scimone A, Thane N, Henke J, Wang T, Ruppert J, Shah N, Rotter K, Hodges J, Ingenthron E, Cordes M, Kohlberg S, Sgro J, Delgado B, Mead K, Chinwalla A, Leonard S, Crouse K, Collura K, Kudrna D, Currie J, He R, Angelova A, Rajasekar S, Mueller T, Lomeli R, Scara G, Ko A, Delaney K, Wissotski M, Lopez G, Campos D, Braidotti M, Ashley E, Golser W, Kim H, Lee S, Lin J, Dujmic Z, Kim W, Talag J, Zuccolo A, Fan C, Sebastian A, Kramer M, Spiegel L, Nascimento L, Zutavern T, Miller B, Ambroise C, Muller S, Spooner W, Narechania A, Ren L, Wei S, Kumari S, Faga B, Levy MJ, McMahan L, van Buren P, Vaughn MW, Ying K, Yeh CT, Emrich SJ, Jia Y, Kalyanaraman A, Hsia AP, Barbazuk WB, Baucom RS, Brutnell TP, Carpita NC, Chaparro C, Chia JM, Deragon JM, Estill JC, Fu Y, Jeddeloh JA, Han Y, Lee H, Li P, Lisch DR, Liu S, Liu Z, Nagel DH, McCann M, SanMiguel P, Myers AM, Nettleton D, Nguyen J, Penning BW, Ponnala L, Schneider KL, Schwartz DC, Sharma A, Soderlund C, Springer NM, Sun Q, Wang H, Waterman M, Westerman R, Wolfgruber TK, Yang L, Yu Y, Zhang L, Zhou S, Zhu Q, Bennetzen JL, Dawe RK, Jiang J, Jiang N, Presting GG, Wessler SR, Aluru S, Martienssen RA, Clifton SW, McCombie W, Wing RA, Wilson RK (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326(5956):1112–1115. https://doi.org/10.1126/science.1178534
Thomson JM (2014) High-throughput SNP genotyping to accelerate crop improvement. Plant Breed Biotechnol 2(3):195–212
Wang K, Wang Z, Li F, Ye W, Wang J, Song G, Yue Z, Cong L, Shang H, Zhu S, Zou C, Li Q, Yuan Y, Lu C, Wei H, Gou C, Zheng Z, Yin Y, Zhang X, Liu K, Wang B, Song C, Shi N, Kohel RJ, Percy RG, Yu JZ, Zhu YX, Yu S (2012) The draft genome of a diploid cotton Gossypium raimondii. Nat Genet 44(10):1098–1103. https://doi.org/10.1038/ng.2371
Wang W, Mauleon R, Hu Z, Chebotarov D, Tai S, Wu Z, Li M, Zheng T, Fuentes RR, Zhang F, Mansueto L, Copetti D, Sanciangco M, Palis KC, Xu J, Sun C, Fu B, Zhang H, Gao Y, Zhao X, Shen F, Cui X, Yu H, Li Z, Chen M, Detras J, Zhou Y, Zhang X, Zhao Y, Kudrna D, Wang C, Li R, Jia B, Lu J, He X, Dong Z, Xu J, Li Y, Wang M, Shi J, Li J, Zhang D, Lee S, Hu W, Poliakov A, Dubchak I, Ulat VJ, Borja FN, Mendoza JR, Ali J, Li J, Gao Q, Niu Y, Yue Z, Naredo MEB, Talag J, Wang X, Li J, Fang X, Yin Y, Glaszmann JC, Zhang J, Li J, Hamilton RS, Wing RA, Ruan J, Zhang G, Wei C, Alexandrov N, McNally K, Li Z, Leung H (2018) Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557(7703):43–49. https://doi.org/10.1038/s41586-018-0063-9
Wing RA, Purugganan MD, Zhang Q (2018) The rice genome revolution: from an ancient grain to Green Super Rice. Nat Rev Genet 19(8):505–517. https://doi.org/10.1038/s41576-018-0024-z
Yang G, Chen S, Chen L, Sun K, Huang C, Zhou D, Huang Y, Wang J, Liu Y, Wang H (2019) Development of a core SNP arrays based on the KASP method for molecular breeding of rice. Rice 12:21. https://doi.org/10.1186/s12284-019-0272-3
Zeng D, Tian Z, Rao Y, Dong G, Yang Y, Huang L, Leng Y, Xu J, Sun C, Zhang G, Hu J, Zhu L, Gao Z, Hu X, Guo L, Xiong G, Wang Y, Li J, Qian Q (2017) Rational design of high-yield and superior-quality rice. Nat Plants 3:17031. https://doi.org/10.1038/nplants.2017.31
Zhang J, Chen L-L, Xing F, Kudrna DA, Yao W, Copetti D, Mu T, Li W, Song J-M, Xie W, Lee S, Talag J, Shao L, An Y, Zhang C-L, Ouyang Y, Sun S, Jiao W-B, Lv F, Du B, Luo M, Maldonado CE, Goicoechea JL, Xiong L, Wu C, Xing Y, Zhou D-X, Yu S, Zhao Y, Wang G, Yu Y, Luo Y, Zhou Z-W, Hurtado BEP, Danowitz A, Wing RA, Zhang Q (2016) Extensive sequence divergence between the reference genomes of two elite indica rice varieties Zhenshan 97 and Minghui 63. Proc Natl Acad Sci U S A 113(35):E5163–E5171. https://doi.org/10.1073/pnas.1611012113
Zhang L, Yu H, Ma B, Liu G, Wang J, Gao R, Li J, Liu J, Xu J, Zhang Y, Li Q, Huang X, Qian Q, Han B, He Z (2017) A natural tandem array alleviates epigenetic repression of IPA1 and leads to superior yielding rice. Nat Commun 8:14789. https://doi.org/10.1038/ncomms14789
Zhang Q, Li J, Xue Y, Han B, Deng XW (2008) Rice 2020: a call for an international coordinated effort in rice functional genomics. Mol Plant 1(5):715–719. https://doi.org/10.1093/mp/ssn043
Zhao J, Wang Z, Liu H, Li T, Hou J, Zhang X, Hao C (2019) Global status of 47 major wheat loci controlling yield, quality, adaptation and stress resistance selected over the last century. BMC Plant Biol 19(1):5. https://doi.org/10.1186/s12870-018-1612-y
Acknowledgments
We thank Dr. Haitao Li, Dr. YiKe Liu, and Mr. Duo Wang for their kindly providing rapeseed, wheat, and maize F2 population. We gratefully acknowledge Dr. Huihui Yu for the advice and revision of this manuscript.
Funding information
This research was supported by the Fundamental Research Funds for the Central Universities of China (Grant No. 2662017QD033), Hubei Provincial Natural Science Foundation of China (Grant No. 2019CFA061), Special Major Projects for Technological Innovation in Hubei Province (2019ABA104), National Natural Science Foundation of China (Grant No.31600983), and the Earmarked Fund for the China Agriculture Research System of China (Grant No. CARS-01-05).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Lu, J., Hou, J., Ouyang, Y. et al. A direct PCR–based SNP marker–assisted selection system (D-MAS) for different crops. Mol Breeding 40, 9 (2020). https://doi.org/10.1007/s11032-019-1091-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-019-1091-3