Abstract
Acylsugars are important insect defense compounds produced at high levels by glandular trichomes of the wild tomato, Solanum pennellii. Marker-assisted selection was used to select for plants containing the three AGQTL named AG3QTL, AG4QTL, and AG11QTL from self-pollinated populations derived from an interspecific backcross population of CU071026 x (CU071026 x S. pennellii LA716). High acylglucose-accumulating lines were selected from these populations that possess these three AGQTL and the fewest number of extraneous S. pennellii LA716 introgressions. Incorporation of these three acylglucose QTL in the presence of the five standard S. pennellii introgressions of CU071026 altered acylsugar level and sugar moiety, demonstrating epistatic interactions between the acylglucose QTL on both of these traits. Comparison of the lines generated from the two breeding techniques indicated the three acylglucose QTL are essential but not necessarily sufficient for the production of elevated levels of acylglucose acylsugars. Fine-mapping of AG3QTL, AG4QTL, and AG11QTL resulted in less than 1 Mbp intervals for the locations of AG4QTL and AG11QTL; proposals of the causal genes underlying these acylglucose QTL are discussed. Characterization of the fatty acid profile of lines selected out of the interspecific backcross populations revealed an increase in the proportion of acylsugar n-C10 fatty acid acyl chains, possibly governed by one or more of the three acylglucose QTL. Characterization of the acylsugar profile of acylglucose lines selected from the interspecific backcross populations also demonstrated interactions among the acylglucose QTL to further modulate the diversity of acylsugars accumulated. Evaluation of an acylglucose line and controls against the tomato insect pest Frankliniella occidentalis demonstrated that levels of resistance differed among these lines and that the acylsugars accumulated by the acylglucose line were effective at reducing both F. occidentalis oviposition and incidence of Tomato spotted wilt orthotospovirus. However, of some of the acylglucose lines and hybrids tested against Spodoptera exigua did not indicate differences for larval weight gain and survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Durable resistance to insect herbivores and the pathogens they vector has long been a goal of plant breeders. Plants accumulate an extensive diversity of specialized metabolites, many of which function in resistance to herbivores. This has resulted in a growing interest in utilizing these metabolites for pest control over traditional practices, such as pesticides. Acylsugars are a promising group of specialized metabolites that are receiving considerable attention for their potential as a source of insect resistance. Numerous species in the nightshade family (Solanaceae) accumulate acylsugars, including Solanum pennellii, S. galapagense, S. habrochaites, S. berthaultii, and Nicotiana tabacum (Fobes et al. 1985; Severson et al. 1985; Burke et al. 1987; King et al. 1986, 1988; Shapiro et al. 1994; Kim et al. 2012; Schilmiller et al. 2015). Acylsugars are secreted as naked droplets from glandular trichomes and act as direct feeding and/or oviposition deterrents against a variety of insect herbivores (Severson et al. 1985; Goffreda and Mutschler 1989; Hawthorne et al. 1992; Rodriguez et al. 1993; Juvik et al. 1994; Liedl et al. 1995; Fancelli et al. 2005; Leckie et al. 2016; Weinhold et al. 2017) and also in indirect defense (Weinhold and Baldwin 2011). The acylsugars accumulated by tomato species (at moderate to high levels by wild relatives and trace levels by cultivated tomatoes) are composed of a sugar backbone, either sucrose or glucose, to which are esterified several aliphatic acids, ranging from 4 to 14 carbons in length. These fatty acid acyl chains can be straight-chained or branched (iso or anteiso) (Fobes et al. 1985; Burke et al. 1987; Shapiro et al. 1994; Schilmiller et al. 2010, 2012, 2016; Fan et al. 2016). A two-carbon extension method has been proposed and demonstrated in tomato to explain the production of medium length branched fatty acid acyl chains (Slocombe et al. 2008; Walters and Steffens 1990).
Breeding efforts to transfer increased levels of the acylsugars associated with insect resistance from S. pennellii LA716 into tomato (Goffreda and Mutschler 1989; Hawthorne et al. 1992; Rodriguez et al. 1993; Juvik et al. 1994; Shapiro et al. 1994; Liedl et al. 1995) created the Cornell benchmark line, CU071026, which accumulates moderate levels of acylsugars. CU071026 contains five S. pennellii LA716 introgressions on chromosomes 2, 3, 7, and 10 that are called AS2, AS3, AS7, AS10.1, and AS10.2, respectively (Leckie et al. 2012; Smeda et al. 2016).
Different S. pennellii accessions accumulate types of acylsugars, which vary with geographical locations where the accessions were collected (Shapiro et al. 1994; Ning et al. 2015), suggesting the possibility of co-evolution of specific metabolic profiles with local herbivore populations. The term “acylsugar chemotype” is used to summarize the aspects of the acylsugars such as acylsugar level, sugar moiety, location of acyl groups, and orientation of acyl groups that vary across species or accessions and which could play a role in resistance. S. pennellii LA716 predominantly accumulates acylglucoses with a characteristic array of fatty acids (Burke et al. 1987; Shapiro et al. 1994; Blauth et al. 1999; Schilmiller et al. 2012; Ning et al. 2015). In contrast, the acylsugar profile of the tomato line CU071026, which was bred from S. pennellii LA716, is exclusively acylsucroses with a dissimilar fatty acid profile (Leckie et al. 2014; Smeda et al. 2016). A recent study utilizing purified acylsugars from CU071026 and several S. pennellii accessions indicated that the purified acylsugars from CU071026 are less effective at equimolar levels than purified acylsugars of some S. pennellii accessions at controlling silverleaf whitefly (Bemisia tabaci) and western flower thrips (Frankliniella occidentalis) oviposition in laboratory assays (Leckie et al. 2016). Furthermore, the Leckie et al. (2016) study revealed synergistic interaction between acylsugar fractions, which led to improved insect resistance. While the mechanism(s) underlying the differential acylsugar efficacy and synergy observed in that study were not elucidated, those results suggest that the insect control of CU071026, or derived lines, could be improved by altering their acylsugar chemotype to increase diversity, which might lead to greater synergistic interactions among components of their acylsugar chemotypes. The potential benefits of a more diverse metabolic profile, including decreased insect feeding, improved adaptation and increased survival, has also been recognized in other studies evaluating plant-herbivore interactions (Duffey and Stout 1996; Castellanos and Espinosa-García 1997; Akhtar and Isman 2003; Whitehead and Bowers 2014). However, Whitehead and Bowers (2014) also demonstrate that interactions between compounds in a mixture can lead to either synergy or antagonism, and that the outcome is dependent on the consumer.
QTL from S. pennellii LA716 that impact acylsugar chemistry include three QTL (FA2QTL, FA7QTL, FA8QTL) that affect the acylsugar fatty acid composition (Blauth et al. 1999; Schilmiller et al. 2010, Leckie et al. 2014). These QTL were introgressed into CU071026 to generate mono-introgression lines that produced high levels of acylsucrose acylsugars with distinct fatty acid profiles (Leckie et al. 2014; Smeda et al. 2016). Further combinations of these fatty acid QTL generated additional acylsugar lines, revealing additive and epistatic interactions between these QTL that further modulated the constituent acylsugar fatty acids and the richness, evenness, and diversity of the acylsugars accumulated (Smeda et al. 2017). A series of recent studies identified four acylsugar acyltransferases (ASAT1-4) that are key acylsugar biosynthetic genes and also generate much of the acylsucrose diversity observed in cultivated tomato and several wild relatives by controlling the location of acyl chain attachment (Schilmiller et al. 2012, 2015; Fan et al. 2016). Since none of these ASAT genes map to the regions of S. pennellii LA716 that are present in CU071026 and necessary for increased acylsugar level, other genes within the introgressions in CU071026 must be responsible for the marked increase in acylsugar level compared to cultivated tomato.
QTL that affect acylsugar moiety (acylglucose vs acylsucrose) have also been identified in both inter and intra-specific mapping populations (Mutschler et al. 1996; Blauth et al. 1998; Leckie et al. 2013). Leckie et al. (2013) identified three acylglucose QTL (AG3, AG4, and AG11) that largely controlled acylglucose accumulation in a BC1F1 population of CU071026 x (CU071026 x S. pennellii LA716) and a subsequent BC1F2 population. One objective of our study was to create tomato lines that produce moderately high levels of acylglucoses by selecting high acylglucose-accumulating lines out of self-pollinated populations derived from a backcross population of CU071026 x (CU071026 x S. pennellii LA716) (Leckie et al. 2013) with selection to reduce the number of extraneous S. pennellii introgressions. The second objective was to extensively characterize these acylglucose lines for acylsugar chemotype, as well as to test a subset of the generated germplasm for resistance against Spodoptera exigua (weight gain and survival) and F. occidentalis (oviposition and vectored virus infection). Two acylglucose QTL: acylglucose 3 (AG3QTL) and acylglucose 11 (AG11QTL), previously shown to alter acylsugar level and moiety (Leckie et al. 2013), were introgressed into CU071026 to test the effect of these QTL in an acylsugar-producing tomato line. Simultaneously, selection of high acylglucose-accumulating acylsugar lines was conducted out of BC1F3, BC1F4, and BC1F5 populations derived from a BC1F2 plant generated by Leckie et al. (2013) and identification of plants with recombinations within the acylglucose QTL introgressions during selection allowed fine mapping of the QTL within them. The acylsugars produced by acylglucose-accumulating tomato lines were characterized by a spectrophotometric acylsugar assay to measure acylsugar level, by gas chromatography mass spectrometry (GC-MS) to determine relative proportions of the acylsugar fatty acids present and by liquid chromatography mass spectrometry (LC-MS), which determines the relative proportions of accumulated acylsugar molecules with information on the number and length of fatty acids esterified to the sugar backbone. The implications of these data are discussed, including how addition of the acylglucose QTL affected aspects of acylsugar chemotype and control of insects and vectored virus.
Materials and methods
Plant materials
CU071026 is the acylsugar-producing tomato benchmark line bred by the Cornell University tomato breeding program using S. pennellii LA716. The interspecific populations at the BC1F3, BC1F4, and BC1F5 generations used to select for high acylglucose-accumulating plants were derived from a single BC1F2 plant which was selected from the BC1F1 population CU071026 x (CU071026 x LA716) used in a prior study to map acylsugar QTL (Leckie et al. 2013, 2014).
A series of tomato lines with individual introgressions of S. pennellii LA716 DNA in the processing tomato M82 (a sub-selection of UC82-B) were produced by Eshed and Zamir (1994, 1995). Based on prior QTL analysis, the introgression lines (Leckie et al. 2013), IL3-5 and IL11-3, were used by the Cornell University tomato breeding program as a source of AG3QTL and AG11QTL, respectively, for the introgression line breeding strategy for transfer of QTL. The seed of IL3-5 and IL11-3 were produced at Cornell University, derived from seed obtained from D. Zamir (Hebrew University of Jerusalem, Rehovot, Israel).
Plant growth conditions
Conditions for germination, growth, and maintenance of plants were the same as those detailed in Smeda et al. (2016). Plants grown in CA for the thrips and virus work were grown in similar conditions except that seeds were germinated on paper towels under constant light for one week prior to transplanting and plants were grown in 6″ Round Thermoformed plastic pots (McConkey, WA).
Introgression line breeding strategy for transfer of QTL and selection of acylglucose lines
Selection of plants from the BC1F1 population was based on markers within the five S. pennellii LA716 introgressions possessed by CU071026 and markers within the additional introgressions being introduced. The CU071026 introgressions were selected for homozygosity and markers within the new IL introgressions were utilized to select for presence of the introgressions, as well as for recombinations. Markers within the IL introgressions were used in the BC1F2 population to select for homozygosity and recombinations.
To provide contrasting materials, two introgression lines (IL lines), each of which putatively contained either AG3QTL or AG11QTL, were selected for transfer of these two AGQTL. The selected IL lines were intermated and self-pollinated to generate a line homozygous for both AG3QTL and AG11QTL. The double introgression IL line was then crossed as the female parent to CU071026, and a resulting F1 plant backcrossed to CU071026 to create the BC1F1 population (CU071026 x (IL line x CU071026)).
Genotypic screening
Identity and location of all molecular markers utilized to select for CU071026 regions can be found in Smeda et al. (2016) in Supplementary Table S1. The identity and location of the markers used to introgress the AG3QTL and AG11QTL from the IL 3-5 and 11-3 lines to create lines with them and also the five CU071026 introgressions are provided in Supplementary Table S1. The identity and location of markers used to select for and fine-map AG3QTL, AG4QTL, and AG11QTL out of the BC1F3, BC1F4, and BC1F5 populations are provided in Supplementary Table S2. Genotyping by sequencing (GBS) was used on selections from the BC1F4 population to fine-map AG11QTL and to define the introgressions contained in the final acylglucose line selections. Genomic DNA was isolated with a DNeasy® Plant Mini Kit (Qiagen). GBS was performed as described by Elshire et al. (2011) and submitted to the Weill Cornell Medical College Genomics Resources Core Facility for 101-cycle single-end sequencing on one lane of a 16-lane flow cell of an Illumina HiSeq 2000 instrument (Illumina Inc., San Diego, CA, USA). The sequencing reads were processed with the GBS Discovery Pipeline for species with a reference genome implemented in TASSEL version 3.0 (Bradbury et al. 2007) and following the pipeline documentation (Glaubitz et al. 2014). The sequence tags for our GBS library were aligned to the EXPEN SL2.50 ITAG2.4 release of the Solanum lycopersicum genome (Fernandez-Pozo et al. 2014), and then positions were updated according to the EXPEN SL3.0 ITAG3.1 release.
Phenotypic screening
Acylsugar level and acylglucose concentration
Levels of total acylsugars and relative proportions of acylsucroses and acylglucoses for control plants and populations in the development of the acylglucose-accumulating acylsugar lines were ascertained using the methods detailed in Leckie et al. (2012) and Smeda et al. (2016). Acylsugar level data were analyzed using ANOVA in JMP Pro 12 (SAS Institute Inc. 2015), and means were separated by Tukey-Kramer HSD (p < 0.05). Prior to analysis, acylsugar level data were often Log10(x) or Cube-root(x) transformed to improve normality. Concentration of acylglucoses and percent acylglucose data were often Log10(x) or Cube-root(x) transformed prior to analysis to improve normality.
Fatty acid characterization
Proportions of the fatty acids cleaved from the acylsugars in each sample were ascertained using the methodology described in Leckie et al. (2014) and Smeda et al. (2016). Percent fatty acid GC data for the replicated evaluation of lines/hybrids was an average from two samples per plant from eight plants and was analyzed using ANOVA in JMP Pro 12 (SAS Institute Inc. 2015), and means separated by Tukey-Kramer HSD (p < 0.05). Prior to analysis, data for many fatty acids were log10(x + 1) or cube-root transformed to improve normality.
Acylsugar composition characterization
LC-MS was utilized to analyze the composition of acylsugars accumulated by each acylglucose-accumulating selection and control plants in the BC1F5 population. LC-MS sample preparation and analysis was done per the methodology described in Schilmiller et al. (2015) and Smeda et al. (2016). LC-MS data were analyzed by hierarchical clustering with a Pearson correlation using pairwise average-linkage clustering for both genotypes and acylsugars using the tools provided by GenePattern (Reich et al. 2006).
Insect assays
S. exigua experiment
Egg clutches of S. exigua were obtained from Benzon Research Inc. (Carlisle, PA) a few days before hatching and eggs were maintained at 4°C until hatching. Eggs were placed on pre-made general purpose lepidopteran diet (Frontier Agricultural Sciences, Newark, DE) 3 days before larvae were needed and kept at room temperature to facilitate uniform hatching. Primary lateral leaflets were taken from among the eight plants of each genotype and attached to a 9.5-mm rubber bumper (The Vibration Solution LLC, Burlington, NC) in a 4-oz food cup (Reditainer: Clear Lake Enterprises, Clear Lake, MN) (1 leaflet per cup) with a 1.6-mm map tack (Geomart-retail LLC, Fort Collins, CO). Twenty-one cups were set up for each genotype. Cups with leaflets were kept at approximately 21°C, and three 1st instar S. exigua larvae were placed on each leaflet with a paintbrush on a Monday. Twenty 1st instar S. exigua larvae were weighed on a UMX2 microbalance (Mettler-Toledo, Columbus, OH) to establish a starting weight. Fifteen to twenty 1st instar larvae were also placed on artificial diet to track the weight gain of the larvae on media over the course of the experiment as a reference. Replacement leaflets were taken from among the eight plants of a genotype on Wednesday and used to replace the initial leaflets. Larvae were transferred from the old leaflets to new leaflets with a fine paintbrush. On Friday afternoon, all living larvae were weighed on a microbalance, and larvae grown on media were weighed as a reference for attainable weight.
Western flower thrips virus-inoculation and oviposition experiments
A virus-free colony of the western flower thrips (WFT) (F. occidentalis, Pergande) and a separate viruliferous cohort were maintained at the University of California, Davis, under conditions described previously (Ullman et al. 1992). Approximately, 12 h 1st instar larvae were routinely removed to feed on virus-infected Datura stramonium plant for 24 h, to enable efficient transmission of Tomato spotted wilt orthotospovirus (TSWV) in virus-inoculation experiments, after which they were fed on fresh supplies of green beans, Phaseolus vulgaris L. Leaf disks (8 mm radius) were cut from leaflets adjacent to the terminal leaflet on the first fully expanded leaf. Two leaf disks of an acylsugar line, washed (under a stream water for 10 s to remove acylsugars) and unwashed, were placed abaxial face up on 1% agar in a polystyrene petri dish (height: 9.0 mm, diameter: 50.0 mm, Pall Corporation, NY) and were offered to four 24–72 h viruliferous adult females to possibly inoculate TSWV when feeding under constant light, at room temperature. Evaluation of this washing technique under the microscope showed that it removes trichome exudate droplets while leaving trichome structures intact, and the epidermal leaf layers undamaged. Removal of acylsugar droplets from leaflets with water was also used effectively by Weinhold et al. (2017). Five replicates of each treatment, including controls (two leaf disks: either washed or unwashed per petri dish), were included in each experiment and repeated five times. The thrips were removed after 24 h, and leaf disks were incubated another 48 h for virus to replicate. Infection with virus in each leaf disk was tested with a TSWV ELISA reagent set (Agdia, Elkhart, IN) following the manufacturer’s suggestions. Absorbance was read at 405 nm to determine infected leaf disks. The experimental design to test oviposition behavior of the WFT was set up similarly, but with 10 gravid females from the non-infected colony, no more than 72 h post adult eclosion, which were added to each petri dish and allowed to oviposit for 24 h. Eggs laid onto either leaf disk were counted using an adapted staining protocol from Backus et al. (1988). The staining protocol enables thrips eggs to be distinguishable from the leaves under light microscope (Leica MZ125). Each oviposition experiment included five replicates of each treatment as before, repeated a total of six times.
Results and discussion
Selection of high-accumulating acylglucose lines out of progenies of an interspecific backcross population
This strategy was based on selecting a plant out the BC1F2 population generated by Leckie et al. (2013) that produced seed, had exhibited high acylglucose production, and whose genome had relatively low number and size of additional S. pennellii LA716 introgressions. Because CU071026 and S. pennellii LA716 were used as parents in the breeding scheme, all plants in all populations were homozygous for the CU071026 introgressions that govern moderate/high accumulation of total acylsugar levels. The pedigree and breeding scheme outlining the populations and selection of high acylglucose-accumulating plants is depicted in Supplementary Fig. S1. Depiction of the size and location of introgressions in selections from this breeding strategy are depicted in Supplementary Fig. S2. The specific introgressions contained within the selections from this breeding strategy are outlined in Supplementary Table S3, and were used to make selections and breeding decisions.
The acylsugar levels and acylglucose accumulation of selections are shown in Table 1. While the data from our study largely support previous studies concluding acylglucose accumulation is governed by AG3QTL, AG4QTL, AG11QTL, and their epistatic interaction, there is some evidence from the BC1F3 selections that supports the hypothesis of an additional region(s) involved in acylglucose accumulation. Specifically, the selections in the BC1F3 that do not contain AG3QTL/AG4QTL or AG11QTL have detectable accumulation of acylglucoses compared to CU071026, which also lacks these QTL. The effect of the AG4QTL and AG11QTL on acylglucose accumulation and percent acylglucose was comparable to that seen in Leckie et al. (2013), but the effect on total acylsugar was different. Specifically, the class of plants in Leckie et al. (2013) homozygous for the AG3QTL, AG4QTL, and AG11QTL was comparable in total acylsugar level to the plants homozygous for the AG3QTL, but heterozygous for the AG4QTL and AG11QTL. In our study, comparison of those classes of plants demonstrated that the plants homozygous for AG4QTL/AG11QTL accumulated about half the level of acylsugars compared to plants heterozygous for AG4QTL/AG11QTL (BC1F4 Table 1). This incongruity could be explained by selection in our study against additional S. pennellii LA716 introgressions in the BC1F3 and BC1F4 populations, some of which could contain unidentified acylsugar QTL that have an interactive effect with AG3QTL, AG4QTL, and AG11QTL to control total acylsugar levels or specifically acylsucrose accumulation. There is considerable evidence for epistatic interactions in acylsugar biosynthesis, both for total acylsugar and acylglucose accumulation (Blauth et al. 1998; Leckie et al. 2012, 2013). The general trend observed in Leckie et al. (2013) and our study could suggest that homozygosity for the acylglucose QTL leads to greater relative proportions of acylglucoses through increased acylglucose accumulation and lower total acylsugar levels. However, S. pennellii LA716 accumulates mostly acylglucoses, and accumulates levels of acylsugars at least six times that of CU071026 (Leckie et al. 2013), demonstrating that homozygosity for the acylglucose QTL does not necessitate low acylsugar levels, and suggesting that additional yet unidentified QTL are necessary to combine with the acylglucose QTL to achieve even higher acylsugar levels.
Based on the acylglucose phenotype and the loss of several extraneous introgressions, one of the selections (141425-188) that was homozygous for the CU071026 introgressions and the AG11QTL introgression, and that set seed well, was designated as the initial plant for the new line, AG3/AG4/AG11/AS. Identity of the boundaries of the introgressions in 141425-188, outside of those from CU071026, and defined by SNPs obtained by GBS from the BC1F4 selection, 141195-353, are displayed in Supplementary Table S4. The selection (141425-042), lacking the AG11QTL introgression was selected as the initial plant for the new line, AG11/AG4/-AG11/AS, which provides a closely related high acylsucrose-accumulating line to compare with the AG3/AG4/AG11/AS line.
Use of S. pennellii introgression lines to transfer AG3QTL and AG11QTL into CU071026
The other breeding attempt to generate high acylglucose-accumulating acylsugar lines was through introgression of the S. pennellii regions containing acylglucose QTL into the acylsugar benchmark line CU071026 utilizing the mono-introgression lines created by Eshed and Zamir (1994, 1995) as sources of the additional regions. Based on the markers used by Leckie et al. (2013), the mono-introgression lines IL3-5 and IL11-3 were selected as those likely to contain the acylglucose QTL AG3QTL and AG11QTL, respectively. Line IL3-5 contained an introgression of ca. 3.4 Mbp from S. pennellii LA716 and IL11-3 contained an introgression of ca. 44.4 Mbp from S. pennellii LA716 (Long et al. 2013). BC1F1 and BC1F2 selections combining the putative AG3QTL/AG11QTL regions heterozygously and homozygously (Supplementary Table S5) accumulated trace or undetectable levels of acylglucoses. This approach was ultimately unsuccessful and suggested that AG4QTL was necessary for detectable accumulation of acylglucoses, but also that an additional region or regions might be necessary for high accumulation of acylglucoses. Evidence for this conclusion was that selections in the BC1F2 (Supplementary Table S5) homozygous for AG3QTL/AG11QTL accumulated only trace levels of acylsugars whereas selections in the BC1F3 (Table 1) homozygous for AG3QTL/AG11QTL accumulated lower but detectable levels of acylsugars including acylglucoses. A selection from the BC1F2 that set seed well and was homozygous for AG3QTL and AG11QTL was selected to establish the new line, designated AG3/AG11/AS. A depiction of the AG3/AG11/AS S. pennellii LA716 introgressions are displayed in Supplementary Fig. S2.
Characterization of acylsugar levels, fatty acid profile, and acylsugar profile of acylglucose lines and hybrids
Acylsugar biosynthesis in tomato is a complicated system that relies on interaction among a number of genes scattered across the genome. Previous studies have shown acylsugar level and the fatty acid profile of the acylsugars often display high heritability, but can experience significant environmental interaction (Shapiro et al. 1994; Smeda et al. 2016). Due to evaluation of single plants in the BC1F1-BC1F5, a replicated experiment growing a series of acylglucose lines, hybrids, and controls was important to properly characterize the effect of the acylglucose QTL on acylsugar level and acylglucose accumulation, and to evaluate the fatty acid profile of tomatoes containing the acylglucose QTL, which has not been previously evaluated. The entries in a replicated evaluation experiment included the CU071026 control, the AG3/AG11/AS line, the AG3/AG4/-AG11/AS line, the AG3/AG4/AG11/AS line, and a CU071026 x AG3/AG4/AG11/AS hybrid.
Total acylsugar level
Analysis of the replicated entries clearly demonstrated that the AG3QTL, AG4QTL, and AG11QTL and their interaction significantly influenced acylsugar level. The AG3/AG11/AS line accumulated trace levels of acylsugars (Table 2), which agreed with initial evaluation of selections from our IL BC1F2 population. Similarly, the AG3/AG4/AG11/AS line accumulated lower levels of acylsugars compared to CU071026, similar to initial evaluation of plants from the BC1F5 population. The CU071026 x AG3/AG4/AG11/AS F1 hybrid accumulated levels of acylsugars intermediate to the levels of CU071026 and the AG3/AG4/AG11/AS line, demonstrating the incomplete dominance of these three acylglucose QTL on acylsugar level. The AG3/AG4/-AG11/AS line accumulated higher levels of acylsugars, which is consistent with initial evaluation in the BC1F5 selections lacking the AG11QTL. The level of acylsugars accumulated in the AG3/AG4/-AG11/AS line were approximately twice the level observed in the AG3/AG4/AG11/AS line, which demonstrates the pronounced impact of the AG11QTL, when homozygous, to decrease total acylsugar levels.
Acylglucose accumulation
Analysis of the replicated entries also demonstrated that AG3QTL, AG4QTL, and AG11QTL profoundly impact acylglucose accumulation and the relative proportion of acylglucoses. CU071026 did not accumulate appreciable levels of acylglucoses, consistent with prior evaluation, whereas the AG3/AG4/AG11/AS line accumulated high levels of acylglucoses (Table 2). The CU071026 x AG3/AG4/AG11/AS F1 hybrid accumulated moderate levels of acylglucoses, which were slightly lower than that observed in AG3/AG4/AG11/AS; however, the percent of acylsugars that were acylglucoses in the CU071026 x AG3/AG4/AG11/AS hybrid was only about half that of AG3/AG4/AG11/AS. Conversely, the AG3/AG4/-AG11/AS and AG3/AG11/AS lines accumulated trace and no acylglucoses, respectively. The lack of acylglucose accumulation in the AG3/AG11/AS line and the trace acylglucose accumulation in the AG3/AG4/-AG11/AS line demonstrate clearly that both AG4QTL and AG11QTL are necessary for accumulation of high levels of acylglucoses. The high levels of acylglucoses in the CU071026 x AG3/AG4/AG11/AS F1 hybrid were only slightly less than that of the AG3/AG4/AG11/AS line, indicating that heterozygosity for all three acylglucose QTL is sufficient to generate significant acylglucose accumulation. The much lower relative percent of acylglucose acylsugars in the CU071026 x AG3/AG4/AG11/AS F1 hybrid compared to that of the AG3/AG4/AG11/AS line, though, matches well with the BC1F4 and BC1F5 selections (Table 1) which illustrated that homozygosity for either AG4QTL or AG11QTL or both is necessary for a large proportion of the accumulated acylsugars to be acylglucoses.
Fatty acid profile
Analysis of the entries revealed an unexpected impact on the fatty acid profile of the acylsugars produced, potentially due to the acylglucose QTL. Generally, the entries had fatty acid profiles that were similar to that of CU071026, as expected, but two entries (AG3/AG4/AG11/AS and AG3/AG4/-AG11/AS) displayed a decrease in ai-C5 fatty acids, and an increase in n-C10 fatty acids compared to CU071026 (Table 2). In addition, the AG3/AG11/AS line displayed a much lower proportion of i-C5 fatty acids, and higher proportion of n-C12 fatty acids than CU071026. However, the AG3/AG11/AS line has very low acylsugar levels, which can result in the GC-MS derived fatty acid profile being unreliable, as discussed in Smeda et al. (2016). The FA7QTL has also been shown to generate an increase in n-C10 fatty acids (Leckie et al. 2014; Smeda et al. 2016, 2017), however, an introgression containing FA7QTL was not present in any of these entries suggesting that one of the additional introgressions in the AG3/AG4/AG11/AS and AG3/AG4/-AG11/AS lines is involved in this trait. Because the AG3/AG4/-AG11/AS entry accumulates an increased proportion of n-C10 fatty acids, the AG11QTL introgression is not likely contributing to the increased n-C10. Likewise, the lack of increased n-C10 in AG3/AG11/AS suggests that the AG3QTL is not involved in the fatty acid profile change. Because the CU071026 x AG3/AG4/AG11/AS hybrid does not display an increase in n-C10 fatty acids, the S. pennellii LA716 alleles likely involved in the increased proportion of n-C10 appear to be recessive. Based on the other introgressions contained within the AG3/AG4/AG11/AS line (Supplementary Table S4), the most likely remaining introgressions contributing to the increased n-C10 are the large introgression on chromosome 4, additional introgression 4a (Add-int-4a), the introgression on the end of chromosome 10 (Add-int-10), or the introgression on the top of chromosome 12 (Add-int-12). Additionally, the AG3/AG4/-AG11/AS line contains a small introgression (ca. 2.8 Mbp) on the end of chromosome 11 that is also contained in AG3/AG4/AG11/AS (within Add-int-11), and not in AG3/AG11/AS, and therefore could also be involved in the increased proportion of n-C10 fatty acids.
LC-MS characterization
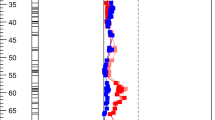
LC-MS was used for additional characterization of some BC1F5 selections to corroborate the spectrophotometric assay evaluation and also enable a detailed comparison of the specific acylsugars accumulated in the selections. Three BC1F5 plants homozygous for the AG11QTL introgression (141425-120, 141425-185, 141425-188), as well as a plant lacking the AG11QTL introgression (141425-042) and a plant heterozygous for the introgression (141425-112) were included in the LC-MS analysis with the CU071026 control. Representative LC-MS chromatograms for each plant are displayed in Supplementary Fig. S3. A total of 71 acylsugars were identified as being accumulated by at least one of the plant samples (Fig. 1). CU071026 was observed to accumulate exclusively acylsucroses, while the 141425-042 samples displayed a profile dominated by acylsucroses, but with accumulation of several acylglucoses, including G3:22 (acylglucose acylsugar with three fatty acid groups totaling 22 carbons) (ID 1). The 141425-120, 141425-185, and 141425-188 plants, homozygous for the AG11QTL, exhibited an acylsugar profile that was dominated by acylglucoses, but with significant accumulation of several acylsucroses. The peak areas of acylsucroses vs acylglucoses in 141425-120, 141425-185, and 141425-188 demonstrated that just over 50% of the total acylsugar peak area was comprised of acylglucoses (Supplementary Table S6 and Supplementary Fig. S3). Interestingly, however, the most abundant acylsugars in the 141425-120, 141425-185, 141425188 plants were two acylsucroses: S3:22 (ID 4) and S3:20 (ID 2). Acylsugar peak areas of the 141425-112 plants, heterozygous for the AG11QTL, revealed that only 36% was comprised of acylglucoses; like the plants homozygous for AG11QTL, the acylsugar profile was dominated most by the two acylsucroses with ID 2 and ID 4. The relative proportion of acylglucoses in these plants from LCMS peak areas indicated the plants homozygous for AG11QTL had a higher proportion of acylglucoses than plants heterozygous for AG11QTL; this result was consistent with the spectrophotometric invertase assay from the BC1F5 selections (Table 1).
Hierarchical cluster analysis with Pearson correlation using a pairwise average-linkage clustering method, indicating the predominant acylsucroses and acylglucoses accumulated by each plant. Three samples were analyzed per plant. Color across a row indicates relative levels (peak area/g leaf weight) of the respective acylsugar, with red indicating samples with the highest levels detected and blue/purple indicating low or no detection relative to the highest sample for the particular type of acylsugar. aThe proposed acylgroup number and length for an acylsugar whose identification was hampered by low abundance and peak overlap. bAn annotated acylglucose based on m/z range but whose acylgroup number and length was hampered by low abundance and peak overlap. cThe mass to charge ratio for each acylsugar followed by retention time in minutes. dAcylsugar nomenclature indicates S for sucrose backbone of the molecule and G for glucose, as well as the number of fatty acid acyl chains (2 to 4) with their cumulative length in carbons that are esterified to the sugar followed by the lengths in carbons of each acyl group in the respective acylsugar
Hierarchical cluster analysis (HCA) supports the impact of AG11QTL by illustrating a pattern of acylsugar composition largely defined by the presence or absence of AG11QTL (Fig. 1). Within the plants lacking the AG11QTL, the HCA clearly showed the 141425-042 samples clustered separately from the CU071026 samples, which is largely due to the moderate accumulation of several acylglucoses in the 141425-042 plant. Within the plants containing the AG11QTL, the three homozygous for AG11QTL (141425-120, 141425-185, and 141425-188) were virtually indistinguishable. The 141425-112 plant samples, on the other hand, clustered separately, likely due to three acylsugars only detected in this plant (ID 35, ID 36, and ID 37) and from decreased accumulation of acylglucoses and increased accumulation of acylsucroses. This distinct profile supports the incompletely dominant action of the AG11QTL or linked QTL.
For several acylsugars identified in the LC-MS analysis, there were clear chromatographic separation of compounds having identical mass and indistinguishable mass spectra, for example G3:22 (5,5,12) (ID 1) and G3:22 (5,5,12) (ID 3) (Fig. 1). These acylsugar isomers likely differ in either the position of acyl chain attachment or in the branching of the acyl chains. The abundance of these two acylglucoses differed between entries such that the acylglucose with ID 3 was highly abundant in the 141425-120, 141425-185, and 141425-188 plants, but also moderately accumulated in the 141425-112 and 141425-042 entries. Conversely, the acylglucose with ID 1 was abundant in the 141425-120, 141425-185, and 141425-188 plants, which are homozygous for AG11QTL, and moderately accumulated in the 141425-042 plant samples, but was not detectable in the 141425-112 plants. This observation suggests complex interactions between AG3QTL/AG4QTL and AG11QTL, where accumulation of the ID 1 acylglucose is possible when AG11QTL is homozygous S. pennellii or tomato, but not when heterozygous.
In addition to these acylglucose isomers, several acylsucrose isomers displayed presence/absence variation between entries. For example, a pair of acylsucrose isomers differing in retention time despite identical mass and indistinguishable mass spectra suggests a role for the AG4QTL in the HCA. Specifically, the S3:19 (4,5,10) ID 62 acylsugar is accumulated by both CU071026 and 141425-042, although the levels are 11× higher in 141425-042 (Supplementary Table S6), which is homozygous for AG4QTL. Conversely, the S3:19 (4,5,10) ID 69 acylsugar is only accumulated in 141425-042, although at a much lower level than the ID 62 acylsugar. The presence/absence of the ID 62 and 69 acylsugar in CU071026 and 141425-042 is consistent with data presented in Fan et al. (2016) showing the impact of Sp-ASAT2 to allow accumulation of a few acylsucroses with all acyl chains on the pyranose ring. Additionally, Fan et al. (2016) observed that the levels of the isomer with all acyl chains on the pyranose ring were much lower than the isomer with an acyl chain on the furanose ring, consistent with the much lower levels of the ID 69 acylsugar in 141425-042 vs the ID 62 acylsugar. Together this data suggests that the ID 62 acylsugar has an acyl chain on the furanose ring while the ID 69 acylsugar has all acyl chains on the pyranose ring. The presence/absence of these acylsucrose isomers suggests that AG4QTL, potentially through action of Sp-ASAT2, can generate acylsucroses distinct from those of CU071026.
Another pair of acylsucroses (ID 2 and ID 4) are exclusively accumulated in the entries containing the AG11QTL (141425-112, 141425-120, 141425-185, and 141425-188) and not detected in the 141425-042 samples or CU071026. The likely explanation for this pattern is that the AG11QTL introgression overlaps with the previously identified ASAT3 acyltransferase gene (Schilmiller et al. 2015), which controls the location of an acyl chain attachment. Specifically, Schilmiller et al. (2015) showed that Sl-ASAT3 functions in S. lycopersicum to esterify an acyl chain to the furanose (fructose) ring of a di-acylsucrose substrate, whereas the Sp-ASAT3 adds an acyl chain to the pyranose ring of a mono-acylsucrose substrate. Two acylsucroses (IDs 53 and 65) that are comparable to the ID 2 acylsucrose in mass and mass spectra are highly accumulated in the 141425-042 (lacking AG11QTL) plants and lowly accumulated in the 141425-112 (heterozygous AG11QTL) plants, but not detectable in the 141425-120, 141425-185, and 141425-188 (homozygous AG11QTL) plants. This same pattern holds true for the acylsucrose with ID 4 and an acylsucrose with ID 39. Therefore, it is likely that the difference between the ID 2 and ID 53/65 acylsucroses and the ID 4 and 39 acylsucroses is that the ID 2 and 4 acylsucroses have all acyl chains on the pyranose (glucose) ring. These conclusions are supported by the entirely dissimilar profiles of CU071026 and plants homozygous for AG11QTL, strongly suggesting any acylsucroses in 141425-120, 141425-185, and 141425-188 have all acyl chains on the pyranose ring.
HCA results also show that the 141425-120, 141425-185, and 141425-188 plants, homozygous for AG11QTL, did not accumulate appreciable levels of tetra-acylsucroses, or acylglucoses/acylsucroses with an acetate (C = 2) acyl group. The likely explanation for this phenotype again is that the Sp-ASAT3 acyltransferase acylates the pyranose ring, specifically at the R2 position (Schilmiller et al. 2015), preventing acylation (acetate group) at this same position by the tomato allele of the ASAT4 acyltransferase (Sl-ASAT4) (Schilmiller et al. 2012).
Across entries, the LC-MS data demonstrated the major effect of the AG11QTL to control the relative proportion of acylsucroses versus acylglucoses and tetra-acylsucroses vs tri-acylsucroses in the acylsugar profile. This effect was most pronounced when the AG11QTL was homozygous, but also evident when AG11QTL was heterozygous (Fig. 1). The data also showed that in the absence of the AG11QTL plants could still accumulate low to moderate levels of a few acylglucoses, highlighting the likelihood that the other necessary QTL (AG3 and AG4) are sufficient for some acylglucose accumulation, but that combination with AG11QTL is required for high levels of acylglucoses. Additionally, the data demonstrated the likely effect of AG4QTL and AG11QTL to control accumulation of unique acylsucroses. Finally, the profile of the plant (141425-112) heterozygous for AG11QTL revealed a distinct composition of acylsugars, including three acylsugars unique to 141425-112. This suggests a potential role for hybrid deployment of the AG11QTL, and or other acylglucose QTL, to capitalize on the allelic interactions that convey accumulation of unique acylsugar isomers.
Fine-mapping of the acylglucose QTL and gene candidates
Fine-mapping of the AG3QTL was possible using selections and recombinants from the BC1F3 population. Previously, Leckie et al. (2013) had delineated the AG3QTL region to be located between C2_At1g79840 (70,450,223–70,457,873) and TG244 (72,179,021–72,184,054) (Tomato SL3.0 ITAG3.1 Solgenomics.net). A selection from our study allowed the location of AG3QTL to be further defined as a ca. 1.5 Mbp region between markers C2_At2g42110 (70,672,281–70,673,549) and TG244 (Supplementary Fig. S4) (Tomato SL3.0 ITAG3.1 Solgenomics.net). There are 189 annotated genes within this region, none of which have been implicated in acylsugar biosynthesis (Supplementary Table S7).
Fine-mapping of the AG4QTL was also possible using recombinant and non-recombinant plants from the BC1F3 population. Previously, Leckie et al. (2013) had delineated the AG4QTL region to be located between C2_At2g20390 (3,961,733–3,968,830) and TG182 (4,833,026–4,838,070) (Tomato SL3.0 ITAG3.1 Solgenomics.net). The recombinant plants from our study allowed the location of AG4QTL to be further defined as a 1.9 Mbp region between C2_At3g19895 (2,776,549–2,785,771) and C2_At5g50720 (4,663,049–4,664,350) (Supplementary Fig. S5) (Tomato SL3.0 ITAG3.1 Solgenomics.net) overlapping that from Leckie et al. (2013). Together, the recombinants from this study and data from Leckie et al. (2013) allowed delineation of the AG4QTL down to a ca. 700 Kbp region between C2_At2g20390 and C2_At5g50720. The recently characterized (ASAT2) (Fan et al. 2016) acyltransferase, Solyc04g012020, is located between 4,353,166–4,354,506 bp (Tomato SL3.0 ITAG3.1 Solgenomics.net) on chromosome 4, which is within the fine-mapped location of AG4QTL, and is likely the gene underlying this acylglucose QTL.
Fine-mapping of AG11QTL was possible using GBS analysis of recombinant and non-recombinant plants from the BC1F4 population. Leckie et al. (2013) had delineated the AG11QTL region to be located between C2_At4g01560 (52,303,644–52,309,660) and C2_At2g27290 (53,712,003–53,714,416) (Tomato SL3.0 ITAG3.1 Solgenomics.net). Comparison of GBS, acylsugar level, and acylglucose accumulation data from BC1F4 plants in our study allowed finer resolution of AG11QTL to a ca. 500 Kbp region between GBS SNP 3 (ca. 53.2 Mbp) and GBS SNP 4 (ca. 53.7 Mbp) (Supplementary Fig. S6). The recently identified ASAT3 acyltransferase gene, Solyc11g067270 (Schilmiller et al. 2015) is located between 53,307,619–53,308,911 bp (Tomato SL3.0 ITAG3.1 Solgenomics.net) on chromosome 11, which is within this fine-mapped location of AG11QTL, and is likely the gene underlying the AG11QTL. Sp-ASAT3 has been shown to lower acylsugar levels in IL11-3 and to acylate the pyranose (glucose) ring of acylsucroses in IL11-3 and S. pennellii LA716 (Schilmiller et al. 2015).
Potential for ASAT1 to ASTA4 and other acylsugar genes to affect acylglucose biosynthesis and the phenotypes of BC1F1 to BC1F5 plants
A series of recent studies have elucidated four BAHD acyltransferases (ASAT1 to ASAT4) that largely control the acylation of acylsucroses in tomato and wild relatives (Schilmiller et al. 2012, 2015; Fan et al. 2016). Data from our study in conjunction with knowledge of the ASAT genes helps clarify the likely impact of these acyltransferases on the acylsugar and acylglucose accumulation observed in the BC1F1 to BC1F5 populations.
ASAT1
The ASAT1 acyltransferase (Solyc12g006330) (ca. 849 Kbp) controls the first acylation step in acylsucrose biosynthesis and Fan et al. (2016) showed that the tomato (Sl-ASAT1) and S. pennellii (Sp-ASAT1) alleles have a similar function. It is unlikely ASAT1 had a significant impact on acylsugar or acylglucose accumulation in our study due to similar allele function between Sp-ASAT1/Sl-ASAT1 and the observation of high acylsugar and acylglucose-accumulating entries with either allele.
ASAT2 and ASAT3
Fan et al. (2016) also identified ASAT2, the second acylation step in acylsucrose biosynthesis in tomato. They showed that the tomato allele (Sl-ASAT2) catalyzed transfer of a variety of acyl chain lengths and orientations at the R3 position on the pyranose ring. Sp-ASAT2 was also shown to have little activity in vitro with a common mono-acylsucrose substrate utilized by Sl-ASAT2. Due to limited activity with mono-acylsucroses, it is likely that in S. pennellii, this enzyme is not the second step in acylsucrose biosynthesis. In fact, evidence for a different order of enzymes in S. pennellii acylsucrose biosynthesis is provided by Schilmiller et al. (2015), where they demonstrated in vitro that the S. pennellii allele of a third ASAT acyltransferase, Sp-ASAT3, acylates mono-acylsucroses on the pyranose ring at the R2 position. This is in contrast to the tomato allele, Sl-ASAT3, which acylates di-acylsucroses at the R3’ position on the furanose ring.
ASAT2 and ASAT3 together likely explain much of the variation for acylsugar level and could explain some of the variation for acylglucose accumulation seen in Leckie et al. (2013) and our study. Homozygosity for the Sp-ASAT2 allele with the Sl-ASAT3 allele consistently led to lower total acylsugar levels in the BC1F4 selections from our study homozygous for the AG4QTL and lacking the AG11QTL compared to selections with the same AG11QTL allele but heterozygous for AG4QTL. This same trend was observed in a BC1F2 population from Leckie et al. (2013), and in the IL4-1/IL4-2 plants tested in Schilmiller et al. (2010). Similarly, homozygosity for Sp-ASAT3 with Sl-ASAT2 led to significant decline in total acylsugar levels, for our study in the BC1F2 and BC1F3 plants homozygous for AG11QTL and lacking the AG4QTL. This trend was also observed by Leckie et al. (2013) as well as in evaluation of IL11-3 in Schilmiller et al. (2010, 2015).
There are a few possible explanations for these changes in acylsugar levels. One explanation for the low acylsugar level of plants with the homozygous combination of Sl-ASAT2 and Sp-ASAT3 is competition for the order of acylation. Sl-ASAT2 is the second acylation step in acylsucrose biosynthesis, and Sp-ASAT3 is the second step in the proposed biosynthesis of acylsucroses in S. pennellii (Schilmiller et al. 2015). The Sl-ASAT2 activity could be inhibited if Sp-ASAT3 acylates the R2 position first. Evidence for ASAT activity being affected by acylation order was shown in Schilmiller et al. (2015), who observed that pre-existing acylation at the R2 position in a di-acylsucrose inhibited Sl-ASAT3 acylation of the R3’ position on the furanose ring. Likewise, it is likely Sp-ASAT3 acylation would be inhibited if Sl-ASAT2 acylates the acylsucrose prior to Sp-ASAT3 activity (Schilmiller et al. 2015). If either Sl-ASAT2 or Sp-ASAT3 is inhibited, then it is likely more di-acylsucroses would result. Di-acylsucroses are not commonly observed in LC-MS profiles (Schilmiller et al. 2010, 2015; Fan et al. 2016; Smeda et al. 2016, 2017), which suggests they are unstable. The instability of di-acylsucroses is also suggested in Schilmiller et al. (2015). Therefore, Sl-ASAT2 combined with Sp-ASAT3 could contribute to lower acylsugar levels through production of unstable di-acylsucroses rather than the more stable tri-acylsucroses/tetra-acylsucroses. This same mechanism could explain low acylglucose accumulation in plants with these allele combinations.
A likely explanation for the decreased total acylsugar and trace acylglucose levels observed in plants homozygous for Sp-ASAT2 and Sl-ASAT3 involves lack of di-acylsucrose substrate for Sl-ASAT3 and lack of acylation of acylglucoses. Specifically, Sp-ASAT2 was shown to have lower affinity for particular mono-acylsucroses in vitro (Fan et al. 2016), which would likely lead to prevalence of mono-acylsucrose substrates with limited amounts of the preferred di-acylsucroses substrate for Sl-ASAT3 usage. This disruption in the acylsucrose acylation pathway likely contributed to the lower acylsugar levels observed in this and other studies. The simple explanation for the likely necessity of Sp-ASAT3 for acylglucose accumulation is that Sl-ASAT3 acylates the furanose ring, which is not present in an acylglucose. Therefore, if Sp-ASAT3 is involved in acylating acylglucoses, lack of this allele would likely result in mono or di-acylglucoses, which were not observed in our study and are likely unstable and hydrolyzed. Our conclusions about the impact of ASAT2/ASAT3 are also supported by recent work (Fan et al. 2017) demonstrating the power of allelic variation for ASAT2/ASAT3 to give rise to a “flipped pathway” which largely modulates the acylsugar diversity in S. pennellii and Solanum habrochaites.
ASAT4
The ASAT4 acyltransferase on chromosome 1 (Schilmiller et al. 2012) likely interacted with Sp-ASAT3 in our study to affect the LC-MS profile of the BC1F5 selections. SpASAT3 acylates the R2 position of the pyranose ring which is the same position that Sl-ASAT4 acylates (Schilmiller et al. 2015). All plants in our study were homozygous for the tomato allele (Sl-ASAT4) and therefore the combination of Sl-ASAT4 and Sp-ASAT3 likely prevented plants homozygous for these alleles (141425-120, 141425-185, and 141425-188) from accumulating tetra-acylsucroses (Fig. 1).
Additional genes
In addition to the ASATs, other studies have proposed components of acylglucose biosynthesis that could play a role in the phenotypes observed in our study and in Leckie et al. (2013). Glucosyltransferase activity was proposed as an alternative to the thioester acyl-CoA mediated transfer of acyl groups to acylglucoses (Ghangas and Steffens 1993). Kuai et al. (1997) demonstrated separation and partial purification of two UDP-glucose:fatty acid glucosyltransferases from S. pennellii LA1376 that could catalyze the proposed UDP-Glucose-dependent activation of fatty acids as 1-O-acyl-β-glucoses. Additionally, a serine carboxypeptidase-like acyltransferase (termed a glucose acyltransferase) on chromosome 10 was identified and shown in vitro to catalyze the disproportionation of two molecules of 1-O-acyl-b-glucose to generate di-acylglucose and a free glucose (Ghangas and Steffens 1993; McNally and Mutschler 1997; Li et al. 1999; Li and Steffens 2000). These proposed elements of acylglucose biosynthesis have not been shown in vivo and the glucose acyltransferase was not present in the BC1F1 and BC1F2 populations in our study, and would not have been segregating in the BC1F3, BC1F4, and BC1F5 populations from our study. Therefore, any variation for acylsugar level, acylglucose accumulation, and LC-MS profile observed in the populations and selections in our study is not likely due to the glucose acyltransferase. The additional S. pennellii LA716 introgressions in the AG3/AG4/AG11/AS line, beyond those present in CU071026, contain a number of genes annotated with UDP-glucosyltransferase or glycosyltransferase-like activity; these genes are found in additional introgressions Add-int-4a, Add-int-4b, Add-int-10, Add-int-11, and Add-int-12 (Introgressions defined in Supplementary Table S4, genes contained within all non-CU071026-introgressions in AG3/AG4/AG11/AS are in Supplementary Table S7). Comprehensive knowledge of the genes that control acylsugar level, moiety, and structure would greatly facilitate the use of the acylglucose producing tomato breeding lines for cultivar development, such as guiding selection of plants with recombinations that maintain necessary genes while eliminating linkage drag.
Impact of acylglucose germplasm on insect and virus resistance
A prior study using purified acylsugars from CU071026 and four accessions of S. pennellii demonstrated the differential efficacy of acylsugars to control insect oviposition (Leckie et al. 2016), which leads to various hypotheses regarding structure and function relationships of acylsugars as insect deterrent compounds. Elucidation of the impacts of components of acylsugar chemistry in the Leckie et al. (2016) study was hampered by multiple chemistry differences between purified acylsugar fractions and blends, as discussed in Smeda et al. (2017). In our study, we pursued preliminary evaluation of the AG3/AG4/AG11/AS line against western flower thrips (WFT) (F. occidentalis) and the AG3/AG4/AG11/AS line and related germplasm against beet armyworm (S. exigua) larvae. Our aim was to determine if the AG3/AG4/AG11/AS line or related germplasm were more effective than the trace acylsugar tomato entries and CU071026 at reducing insect feeding, oviposition and virus inoculation.
Beet armyworm weight gain and survival
The plants used to determine acylsugar level, acylglucose accumulation and fatty acid profile in Table 2 were also used in the beet armyworm assay. The survival of the beet armyworm larvae was equally high across all entries with no differences in survival among the entries over 5 days (Table 3). Conversely, while larval weight gain was similar among all of the experimental entries, we observed that weight gain of the beet armyworm larvae over 5 days was much lower on the normal tomato control, NC84173 than the other experimental entries. A set of beet armyworm larvae from the same egg clutch as those raised on plants were raised on media; the weight gain of the larvae grown on media demonstrated that weight gain on all entries was less than that on media (data not shown). The lower weight gain on the tomato control compared to the entries with low to moderate levels of acylsugars was unexpected, and appears to contradict the results of Juvik et al. (1994), where decreased growth and survival of beet armyworm larvae was associated with the acylglucose acylsugars of S. pennellii LA716. One explanation for the lack of observed impact of acylsugar on beet armyworm larvae in this study is that the concentration of acylsugars on the leaflets of tested entries was too low. Juvik et al. (1994), however, observed an impact of acylglucoses sprayed on leaflets at a concentration of 60 μg/cm2, which is comparable to the levels of acylsugars accumulated by most entries in our experiment. A second possible explanation is that the entries containing introgressions with the acylsugar QTL could have disrupted an existing tomato defense system due to the loss of the tomato gene(s) replaced by the introgressions in the acylsugar lines. All entries except the tomato control NC84173 possess the five introgressions from CU071026, which cover 10% of the tomato genome (Leckie et al. 2012), and could contain genes coding for components of tomato defenses previously implicated in reducing beet armyworm growth, such as chlorogenic acid and polyphenol oxidase (Felton et al. 1992). Additional work could help determine if the acylsugar entries have deficiencies in one or more previously studied tomato defense systems. The presence of acylsugars could not be considered to increase larval weight gain since entry AG3/subAG11/AS (analogous to AG3/AG11/AS in Table 2), which only accumulates trace levels of acylsugars due to the lack of the AG4QTL, supports similar larval weight gains as the higher acylsugar producing entries (Table 3).
Western flower thrips oviposition and tomato spotted wilt orthotospovirus infection
Additional bioassays were conducted using leaf disk choice tests that evaluated the impact of the AG3/AG4/AG11/AS line, CU071026 and two trace-acylsugar tomato entries on oviposition by non-viruliferous WFT, and inoculation of Tomato spotted wilt orthotospovirus (TSWV) by viruliferous thrips. We observed that unwashed leaf disks of moderate-acylsugar accumulating entries AG3/AG4/AG11/AS and CU071026 displayed a much lower probability of oviposition compared to the washed leaf disks, whereas for the trace-acylsugar entries probability of oviposition was similar between the washed and unwashed leaf disks. When comparing the probability of WFT oviposition on unwashed leaf disks across entries, oviposition was much more likely on the leaf disks of the trace-acylsugar entries (M82 and Celebrity), compared to CU071026 and AG3/AG4/AG11/AS (Fig. 2a).
Probability of western flower thrips (WFT) oviposition on unwashed leaf disks (a) and probability of tomato spotted wilt virus infection on both washed and unwashed leaf disks (b) for leaf disks from the AG3/AG4/AG11/AS line, CU071026, and two standard tomato varieties (trace acylsugar). Probability of oviposition and tomato spotted wilt virus infection are estimated probabilities derived from the log odds, which are comparable to the observed probability of oviposition and virus infection. For the probability of TSWV infection (b), the asterisk represents a significant difference (T test, df = 38, p = 0.0027) for infection rates between washed and unwashed leaf disks of the AG3/AG4/AG11/AS line; infection rates between washed and unwashed leaf disks were not significant for the other entries. Entry probabilities not connected by the same letter represent significant differences for probability of oviposition (a) and probability of TSWV infection (b) across entries for unwashed leaf disks. Log odds were utilized to generate least square means for statistical separation. Error bars represent 95% confidence intervals
The choice assay results indicate that removing the trichome droplets, which are predominantly acylsugars (Burke et al. 1987), increased WFT oviposition which strongly suggests that the acylsugars are modulating WFT response; this is strongly supported by the results of Leckie et al. (2016). The acylsugars of CU071026 and AG3/AG4/AG11/AS are similar, but not identical, for fatty acid composition, differ for sugar moiety (sucrose vs largely glucose) and for location of acyl chain attachment (Table 2, Fig. 1). However, both CU071026 and AG3/AG4/AG11/AS have similar impacts on WFT oviposition, suggesting that the differences in their acylsugar chemotypes did not demonstrably alter their efficacy against WFT. The similar efficacy of both CU071026 and AG3/AG4/AG11/AS acylsugars indicates that more than one acylsugar chemotype can be similarly effective at reducing WFT oviposition.
Consistent with the oviposition data, there was generally a higher incidence of TSWV infection on washed leaf disks compared to unwashed leaf disks for the moderate acylsugar-accumulating CU071026 and AG3/AG4/AG11/AS entries, although this difference was significant only for AG3/AG4/AG11/AS (Fig. 2b). The standard tomatoes Celebrity and M82 (trace-acylsugar) entries did not display a difference between infection rates between washed and unwashed leaf disks. When comparing the probability of TSWV infection across entries for unwashed leaf disks, CU071026 displayed a similar probability of infection as Celebrity and M82. In contrast, there was a clear decrease in the probability of TSWV infection on unwashed leaf disks of AG3/AG4/AG11/AS compared to unwashed leaf disks of the other entries.
That some factor controlling the differential infection rates was washed off the leaf disks is demonstrated by the lower TSWV infection rate in washed leaf disks (in which acylsugar droplets removed) versus unwashed leaf disks for the moderate acylsugar-accumulating entries. This observation, and the lack of infection difference between washed and unwashed leaf disks of the Celebrity and M82 entries, provides strong support for the role of acylsugars in mediating the incidence of TSWV infection. The difference observed in virus infection of unwashed leaf disks of CU071026 versus AG3/AG4/AG11/AS implies the two are not fully equivalent in their impacts, and suggests that the acylsugars of AG3/AG4/AG11/AS could be more effective than those of CU071026 at inhibiting TSWV infection. It is also possible that other QTL could play a role in the reduced probability of TSWV infection in the AG3/AG4/AG11/AS leaf disks, however those QTL would have to affect some aspects of external leaf chemistry removable by the water washing. The WFT assay results strongly suggest though that acylsugars are mediating the observed variation in TSWV infection and that acylsugar chemotype differences play a significant role in this trait. As discussed above, CU071026 and AG3/AG4/AG11/AS differ for several aspects of acylsugar chemotype, most prominently for acylsugar moiety and the location of acyl chain attachments. Targeted comparison of more closely related germplasm coupled with observation of WFT behavior could further determine the importance of acylsugar moiety and acyl chain location in inhibiting TSWV infection and the mechanism of resistance, such as whether the acylsugars of more effective genotypes alter the feeding or behavior of the vector insects, disrupting TSWV transmission.
Conclusions
This study presents the result of the combination of QTL involved in mediating the sugar moiety of specialized metabolites, acylsugars, to create new acylsugar lines and a comprehensive evaluation of the action of these QTL in the resulting lines to impact acylsugar chemotype and resistance to insects and an insect-transmitted virus. The development and characterization of these acylglucose lines and hybrids complements and builds upon the current platform of acylsugar-accumulating tomato lines and increases our knowledge concerning acylsugar biosynthesis and chemistry. This platform of germplasm could be used to elucidate remaining gaps in our understanding of acylsugar biosynthesis, such as the gene or genes underlying the AG3QTL, the QTL underlying the increase in n-C10/decrease in ai-C5 fatty acids, and whether the acylation patterns and acylglucose accumulation trait are pleiotropically controlled by interaction between ASAT2 and ASAT3.
Development of lines and hybrids utilizing the acylglucose germplasm presented in this study has the potential to reduce insect feeding, damage and virus incidence, as well as the possibility to reduce or eliminate spray regimes in tomato production. The research platform provided by acylsugar producing lines could also be utilized for additional research in entomology, virology and ecology to further elucidate the functionality of acylsugar chemotype in insect deterrence, which is still largely unknown. This study is, to our knowledge, the first demonstration of the impact of acylsugar chemotype in planta to modulate infection of leaf disks by an insect-transmitted virus. Since Leckie et al. (2016) suggest that increased acylsugar diversity could lead to greater insect efficacy through increased synergistic interactions, breeding has already begun to combine the acylglucose QTL with the fatty acid QTL (Smeda et al. 2016, 2017) to increase the diversity of both acylsugar moiety/acyl chain location and acyl chain length/orientation.
Abbreviations
- ai-C5:
-
2-Methylbutanoate (anteiso branched 5-carbon acyl group)
- i-C4:
-
2-Methylpropanoate (iso branched 4-carbon acyl group)
- i-C5:
-
3-Methylbutanoate (iso branched 5-carbon acyl group)
- i-C11:
-
9-Methyldecanoate (iso branched 11-carbon acyl group)
- n-C10:
-
n-Decanoate (straight chain 10-carbon acyl group)
- n-C12:
-
n-Dodecanoate (straight chain 12-carbon acyl group)
References
Akhtar Y, Isman MB (2003) Binary mixtures of feeding deterrents mitigate the decrease in feeding deterrent response to antifeedants following prolonged exposure in the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Chemoecology 13(4):177–182. https://doi.org/10.1007/s00049-003-0246-0
Backus EA, Hunter WB, Arne CN (1988) Technique for staining leafhopper (Homoptera: Cicadellidae) salivary sheaths and eggs within un-sectioned plant tissue. J Econ Entomol 81(6):1819–1823. https://doi.org/10.1093/jee/81.6.1819
Blauth SL, Churchill GA, Mutschler MA (1998) Identification of quantitative trait loci associated with acylsugar accumulation using intraspecific populations of the wild tomato, Lycopersicon pennellii. Theor Appl Genet 96(3-4):458–467. https://doi.org/10.1007/s001220050762
Blauth SL, Steffens JC, Churchill GA, Mutschler MA (1999) QTL analysis of acylsugar fatty acid constitutents using intraspecific populations of the wild tomato Lycopersicon pennellii. Theor Appl Genet 99(1-2):373–381. https://doi.org/10.1007/s001220051247
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23(19):2633–2635. https://doi.org/10.1093/bioinformatics/btm308
Burke B, Goldsby G, Mudd JB (1987) Polar epicuticular lipids of Lycopersicon pennellii. Phytochemistry 26(9):2567–2571. https://doi.org/10.1016/S0031-9422(00)83879-0
Castellanos I, Espinosa-García FJ (1997) Plant secondary metabolite diversity as a resistance trait against insects: a test with Sitophilus granarius (Coleoptera: Curculionidae) and seed secondary metabolites. Biochem Syst Ecol 31(7):591–602. https://doi.org/10.1016/S0305-1978(97)00045-8
Duffey SS, Stout MJ (1996) Antinutritive and toxic components of plant defense against insects. Arch Insect Biochem Physiol 32(1):3–7. https://doi.org/10.1002/(SICI)1520-6327(1996)32:1<3::AID-ARCH2>3.0.CO;2-1
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PloS one 6(5):e19379. https://doi.org/10.1371/journal.pone.0019379
Eshed Y, Zamir D (1994) A genomic library of Lycopersicon pennellii in L. esculentum: a tool for fine mapping of genes. Euphytica 79(3):175–179. https://doi.org/10.1007/BF00022516
Eshed Y, Zamir D (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 141(3):1147–1162
Fan P, Last RL et al (2016) In vitro reconstruction and analysis of evolutionary variation of the tomato acylsucrose metabolic network. Proc Natl Acad Sci 113(2):E239–E248. https://doi.org/10.1073/pnas.1517930113
Fan P, Miller AM, Liu X, Jones AD, Last RL (2017) Evolution of a flipped pathway creates metabolic innovation in tomato trichomes through BAHD enzyme promiscuity. Nat Comm. https://doi.org/10.1038/s41467-017-02045-7
Fancelli M, Vendramim JD, Frighetto RTS, Lourencao AL (2005) Glandular exudate of tomato genotypes and development of B. tabaci (Genn.) (Sternorryncha: Aleyrodidae) biotype B. Neotrop Entomol 34(4):659–665. https://doi.org/10.1590/S1519-566X2005000400017
Felton GW, Donato KK, Broadway RM, Duffey SS (1992) Impact of oxidized plant phenolics on the nutritional quality of dietar protein to a noctuid herbivore, Spodoptera exigua. J Insect Physiol 38(4):277–285. https://doi.org/10.1016/0022-1910(92)90128-Z
Fernandez-Pozo N, Menda N, Edwards JD, Saha S, Tecle IY, Strickler SR, Bombarely A, Fisher-York T, Pujar A, Foerster H, Yan A (2014) The sol genomics network (SGN)—from genotype to phenotype to breeding. Nucleic Acids Res 43(D1):D1036–D1041. https://doi.org/10.1093/nar/gku1195
Fobes JF, Mudd J, Marsden M (1985) Epicuticular lipid on the leaves of L. pennellii and L. esculentum. Plant Physiol 77(4):567–570. https://doi.org/10.1007/s11032-013-9849-5
Ghangas GS, Steffens JC (1993) UDPglucose: fatty acid trans- glucosylation and transacylation in triacylglucose biosynthesis. Proc Natl Acad Sci U S A 90(21):9911–9915. https://doi.org/10.1073/pnas.90.21.9911
Glaubitz JC, Casstevens TM, Lu F, Harriman J, Elshire RJ, Sun Q, Buckler ES (2014) TASSEL-GBS: a high capacity genotyping by sequencing analysis pipeline. PloS one 9(2):e90346. https://doi.org/10.1371/journal.pone.0090346
Goffreda JC, Mutschler MA (1989) Inheritance of potato aphid resistance in hybrids between Lycopersicon esculentum and L. pennellii. Theor Appl Genet 78(2):210–216. https://doi.org/10.1007/BF00288801
Hawthorne DM, Shapiro JA, Tingey WM, Mutschler MA (1992) Trichome-borne and artificially applied acylsugars of wild tomato deter feeding and oviposition of the leafminer, Liriomyza trifolii. Entomol Exp Appl 65(1):65–73. https://doi.org/10.1111/j.1570-7458.1992.tb01628.x
Juvik J, Shapiro JA, Young TE, Mutschler MA (1994) Acyl-glucoses of the wild tomato Lycopersicon pennellii alter behavior and reduce growth and survival of Helicoverpa zea and Spodoptera exigua. J Econ Entomol 87(4):482–492. https://doi.org/10.1007/s11032-013-9849-5
Kim J, Kang K, Gonzales-Vigil E, Shi F, Jones D, Barry CS, Last RL (2012) Striking natural diversity in glandular trichome acylsugar composition is shaped by variation at the Acyltransferase2 locus in the wild tomato Solanum habrochaites. Plant Physiol 160(4):1854–1870. https://doi.org/10.1104/pp.112.204735
King RR, Pelletier Y, Singh RP, Calhoun LA (1986) 3,4-Di-O-isobutyryl-6-O-caprylsucrose: the major component of a novel sucrose ester complex from the type B glandular trichomes of Solanum berthaultii hawkes (Pl 473340). J Chem Soc Chem Commun 14(14):1078–1079. https://doi.org/10.1039/C39860001078
King RR, Calhoun LA, Singh RP (1988) 3,4-DI-O- and 2,3,4-tri-O- acylated glucose esters from the glandular trichomes of nontuberous solanum species. Phytochemistry 27(12):3765–3768. https://doi.org/10.1016/0031-9422(88)83014-0
Kuai JP, Ghangas GS, Steffens JC (1997) Regulation of tri-acylglucose fatty acid composition—uridine diphosphate glucose fatty acid glucosyltransferases with overlapping chain-length specificity. Plant Physiol 115(4):1581–1587. https://doi.org/10.1104/pp.115.4.1581
Leckie BM, DeJong DM, Mutschler MA (2012) Quantitative trait loci increasing acylsugars in tomato breeding lines and their impacts on silverleaf whiteflies. Mol Breed 31(4):957–970. https://doi.org/10.1007/s11032-012-9746-3
Leckie BM, DeJong DM, Mutschler MA (2013) Quantitative trait loci regulating sugar moiety of acylsugars in tomato. Mol Breed 31(4):957–970. https://doi.org/10.1007/s11032-013-9849-5
Leckie BM, Halitschke R, De Jong DM, Smeda JR, Kessler A, Mutschler MA (2014) Quantitative trait loci regulating the fatty acid profile of acylsugars in tomato. Mol Breed 34(4):1201–1213. https://doi.org/10.1007/s11032-013-9849-5
Leckie BM, Mutschler MA et al (2016) Differential and synergistic functionality of acylsugars in suppressing oviposition by insect herbivores. PLoS One 11(4):1–19. https://doi.org/10.1371/journal.pone.0153345
Li AX, Steffens JC (2000) An acyltransferase catalyzing the formation of diacylglucose is a serine carboxypeptidase-like protein. Proc Natl Acad Sci U S A 97(12):6902–6907. https://doi.org/10.1073/pnas.110154197
Li AX, Eannetta N, Ghangas GS, Steffens JC (1999) Glucose polyester biosynthesis. Purification and characterization of a glucose acyltransferase. Plant Physiol 121(2):453–460. https://doi.org/10.1104/pp.121.2.453
Liedl BE, Lawson DM, White KK, Shapiro JA, Cohen DE, Carson WG, Trumble JT, Mutschler MA (1995) Acylglucoses of the wild tomato Lycopersicon pennellii alters settling and reduces oviposition of Bemisia argentifolii. J Econ Entomol 88(3):742–748. https://doi.org/10.1093/jee/88.3.742
Long W, Li Y, Zhou W, Ling HQ, Zheng S (2013) Sequence-based SSR marker development and their application in defining the introgressions of LA0716 (Solanum pennellii) in the background of cv. M82 (Solanum lycopersicum). PLoS One 8(12):e81091. https://doi.org/10.1371/journal.pone.0081091
McNally KL, Mutschler MA (1997) Use of introgression lines and zonal mapping to identify RAPD markers linked to QTL. Mol Breed 3(3):203–212. https://doi.org/10.1023/A:1009657122293
Mutschler MA, Doerge RW, Liu J, Kuai JP, Liedl B, Shapiro Y (1996) QTL analysis of pest resistance in the wild tomato, Lycopersicon pennellii: QTL controlling acylsugar level and composition. Theor Appl Genet 92(6):709–718. https://doi.org/10.1007/BF00226093
Ning J, Last RL et al (2015) A feedback insensitive isopropylmalate synthase affects acylsugar composition in cultivated and wild tomato. Plant Physiol 169(3):1821–1835. https://doi.org/10.1104/pp.15.00474
Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP (2006) GenePattern 2.0. Nat Genet 38(5):500–501. https://doi.org/10.1038/ng0506-500
Rodriguez AE, Tingey WM, Mutschler MA (1993) Acylsugars produced by type IV trichomes of Lycopersicon pennellii (Corr.)D’Arcy deter settling of the green peach aphid, Myzus persicae (Sulzer) (Homoptera: Aphididae). J Econ Entomol 86(1):34–39. https://doi.org/10.1093/jee/86.1.34
SAS Institute Inc (2015) JMP® 12 JSL syntax reference. SAS Institute Inc., Cary
Schilmiller AL, Shi F, Kim J, Charbonneau A, Holmes D, Jones AD, Last RL (2010) Mass spectrometry screening reveals widespread diversity in trichome specialized metabolites of tomato chromosomal substitution lines. Plant J 62(3):391–403. https://doi.org/10.1111/j.1365-313X.2010.04154.x
Schilmiller AL, Charbonneau AL, Last RL (2012) Identification of a BAHD acetyltransferase that produces protective acyl sugars in tomato trichomes. Proc Natl Acad Sci U S A 109(40):16377–16382. https://doi.org/10.1073/pnas.1207906109
Schilmiller AL, Moghe GD, Fan P, Ghosh B, Ning J, Jones AD, Last RL (2015) Functionally divergent alleles and duplicated loci encoding an acyltransferase contribute to acylsugar metabolite diversity in Solanum trichomes. Plant Cell 27(4):1002–1017. https://doi.org/10.1105/tpc.15.00087
Schilmiller AL, Gilgallon K, Ghosh B, Jones AD, Last RL (2016) Acylsugar acylhydrolases: carboxylesterase catalyzed hydrolysis of acylsugars in tomato trichomes. Plant Physiol 170(3):1331–1344. https://doi.org/10.1104/pp.15.01348
Severson RF, Johnson AW, Jackson DM (1985) Cuticular constituents of tobacco: factors affecting their production and their role in insect and disease resistance and smoke quality. Recent Adv Tob Sci 11:105–174
Shapiro J, Steffens J, Mutschler MA (1994) Acylsugars of the wild tomato Lycopersicon pennellii in relation to its geographic distribution. Biochem Syst Ecol 22(6):545–561. https://doi.org/10.1016/0305-1978(94)90067-1
Slocombe SP, Schauvinhold I, McQuinn RP, Besser K, Welsby NA, Harper A, Aziz N, Li Y, Larson TR, Giovannoni J, Dixon RA, Broun P (2008) Transcriptomic and reverse genetic analyses of branched-chain fatty acid and acyl sugar production in Solanum pennellii and Nicotiana benthamiana. Plant Physiol 148(4):1830–1846. https://doi.org/10.1104/pp.108.129510
Smeda JR, Schilmiller AL, Last RL, Mutschler MA (2016) Introgression of acylsugar chemistry QTL modifies the composition and structure of acylsugars produced by high-accumulating tomato lines. Mol Breed 36(12):160. https://doi.org/10.1007/s11032-016-0584-6
Smeda JR, Schilmiller AL, Kessler A, Mutschler MA (2017) Combination of QTL affecting acylsugar chemistry reveals additive and epistatic genetic interactions to increase acylsugar profile diversity. Mol Breed 37(8):104. https://doi.org/10.1007/s11032-017-0690-0
Ullman DE, Cho JJ, Mau RF, Westcot DM, Custer DM (1992) A midgut barrier to tomato spotted wilt virus acquisition by adult western flower thrips. Phytopathol N Y Baltim St Paul 82:1333–1333
Walters DS, Steffens JC (1990) Branched-chain amino acid metabolism in the biosynthesis of Lycoperiscon pennellii glucose esters. Plant Physiol 93(4):1544–1551. https://doi.org/10.1104/pp.93.4.1544
Weinhold A, Baldwin IT (2011) Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. PNAS 108(19):7855–7859. https://doi.org/10.1073/pnas.1101306108
Weinhold A, Ullah C, Dressel S, Schoettner M, Gase K, Gaquerel E, Xu S, Baldwin IT (2017) O-acyl sugars protect a wild tobacco from both native fungal pathogens and a specialist herbivore. Plant Physiol pp-01904. doi: https://doi.org/10.1104/pp.16.01904
Whitehead SR, Bowers MD (2014) Chemical ecology of fruit defence: synergistic and antagonistic interactions among amides from piper. Funct Ecol 28(5):1094–1106. https://doi.org/10.1111/1365-2435.12250
Acknowledgments
We thank Jennifer Thaler, of Cornell University, for valuable discussions on the implications of metabolite diversity as it relates to acylsugar-mediated insect control, use of lab space for the S. exigua assays, and critical reading of the manuscript.
Funding
This project was supported in part by Agriculture and Food Research Initiative Competitive Grant no. 2013-67013-21135 from the USDA National Institute of Food and Agriculture, and by a Coordinated Agricultural Project grant 2012-68004-20166 and by the USDA National Institute of Food and Agriculture, Hatch project NYC-149440 (to M.A.M). Smeda was supported in part by an NSF GRFP graduate fellowship, as well as for one semester on by Agriculture and Food Research Initiative Competitive Grant no. 2010-85117-20551.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 24 kb).
Rights and permissions
About this article
Cite this article
Smeda, J.R., Schilmiller, A.L., Anderson, T. et al. Combination of Acylglucose QTL reveals additive and epistatic genetic interactions and impacts insect oviposition and virus infection. Mol Breeding 38, 3 (2018). https://doi.org/10.1007/s11032-017-0756-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-017-0756-z