Abstract
Stripe rust, caused by Puccinia striiformis f. sp. tritici (Pst), is an important disease of wheat worldwide. Host resistance is the best way to control the disease. Genetic analysis of F2 and F2:3 populations from an Avocet S/Jimai 22 cross indicated that stripe rust resistance in Jimai 22 was conferred by a single dominant gene, tentatively designated YrJ22. A total of 377 F2 plants and 127 F2:3 lines were tested with Chinese Pst race CYR32 and genotyped with simple sequence repeat (SSR) and single nucleotide polymorphism (SNP) markers. A linkage map was constructed with five SSR and two SNP markers. Xwmc658 and IWA1348 flanked YrJ22 at genetic distances of 1.0 and 7.3 cM, proximally and distally, respectively. The chromosomal location was confirmed using Chinese Spring nulli-tetrasomic, ditelosomics and deletion lines. Seedling reactions to 21 Pst races demonstrated differences in specificity between YrJ22 and other resistance genes on chromosome 2AL, indicating that YrJ22 is likely to be a new wheat stripe rust resistance gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat is the major source of calories and protein for the diets of humans and livestock, with over 728.4 million tonnes being harvested globally in 2015 (http://www.fao.org/worldfoodsituation/csdb/en/). Wheat production worldwide is challenged by several diseases. Stripe rust, a fungal disease caused by Puccinia striiformis f. sp. tritici (Pst), is one of the most widespread biotic stresses, and it can reduce the production and quality of wheat. With nearly 24 million ha of wheat production, China is the largest potential stripe rust epidemic region in the world (Stubbs 1988). During the past decade, stripe rust occurred on about 3.6 million ha annually in China caused substantial losses in wheat production [National Agro-technical Extension and Service Center (NAESC) 2015]. Stripe rust remains a significant threat in many wheat-growing regions of the world, with regular crop losses ranging from 0.1 to 5 % and rare serious epidemics causing losses of 5–25 % (Wellings 2011). Resistant cultivars are considered the most effective and economic means to control the disease (Line 2002; Chen 2014).
Stripe rust resistance is often classified into two general types named seedling resistance (or all-stage resistance, ASR) and adult-plant resistance (APR) (Chen 2005). ASR is race specific and effective throughout the entire growth cycle, whereas APR confers partial resistance against a broad range of pathogen races, and is usually more durable (Line 2002; Chen 2005). To reduce the amount of inoculum and develop resistant cultivars, the best breeding strategy is to combine ASR with APR in wheat. Therefore, there is still a requirement for continuing discovery and documentation of stripe rust resistance genes (He et al. 2011; Ren et al. 2012; Chen 2013).
Mapping of resistance genes is an important first step for gene pyramiding, gene deployment and developing multiline cultivars. Stripe rust resistance in wheat has been studied for more than 60 years (Feng et al. 2015). To date, 70 genes at 67 loci conferring resistance to stripe rust (Yr1–Yr67) have been cataloged in common wheat or durum (McIntosh et al. 2013, 2014; Herrera-Foessel et al. 2015; Randhawa et al. 2015). However, only a few genes are effective against the predominant Pst races in China (Ren et al. 2012; Bai et al. 2014). The most recent event in China has been loss of resistance in many cultivars with the resistance gene Yr26 following the emergence of the Pst race v26 group (Ren et al. 2015).
Molecular markers are useful for mapping resistance genes in crop species. Until recently, microsatellites, or simple sequence repeats (SSRs), were favored due to their often codominant inheritance and robustness defined by repeatability and reliability in PCR-based marker systems (Röder et al. 1998). High-density single nucleotide polymorphism (SNP) genotyping arrays are now becoming preferred options in mapping experiments (Wang et al. 2014). The Kompetitive Allele-Specific PCR (KASP) genotyping system is a homogeneous, fluorescent, endpoint genotyping technology that offers the simplest, most cost-effective and flexible way for molecular mapping and breeding (Semagn et al. 2014). Closely linked markers can be used for marker-assisted selection in breeding programs to pyramid resistance genes.
Jimai 22, developed by the Crop Research Institute, Shandong Academy of Agricultural Science, is an elite wheat cultivar with high yield, wide adaptability and resistance to stripe rust and powdery mildew at both the seedling and adult-plant stages (Li et al. 2007). From 2006 to 2015, it was grown on the largest area in China, with an accumulated area of 12 million ha (http://www.sdcrops.cn/newsinfo.asp?id=3062; Prof. Jianjun Liu pers. comm.). Currently, Jimai 22 is widely used as an elite parent in wheat breeding in China. The objectives of the present study were to map the stripe rust resistance genes in Jimai 22 using SSR and SNP markers.
Materials and methods
Plant materials
The stripe rust-resistant parent Jimai 22 was crossed with a highly susceptible line Avocet S. The 377 F2 plants and 127 F2:3 lines were used for genetic analysis and mapping of the stripe rust gene in Jimai 22.
Chinese Spring (CS), CS nulli-tetrasomic (N2AT2D, N2BT2A, N2BT2D, N2DT2A and N2D-T2B), ditelosomic (Dt2AL and Dt2AS) and deletion lines 2AL1-0.85 and 2AS5-0.78 were used for chromosomal arm assignment and bin mapping of molecular markers flanking the stripe rust resistance gene.
Seedling tests
Fifteen F1, 377 F2 plants and 127 F2-derived F2:3 lines were evaluated for stripe rust resistance with the prevalent Pst race CYR32. The original inoculum was kindly provided by Dr. Gangming Zhan, Northwest A & F University, Yangling, Shaanxi Province.
Seedling tests were conducted under controlled greenhouse conditions as described previously (Li et al. 2006). Seeds were planted in plastic pots (9 × 9 × 9 cm) with 15 plants each, and three plants of susceptible cultivar Mingxian 169 were used as a control in each pot. Plants were inoculated with race CYR32 by brushing fresh urediniospores from sporulating leaves on to fully expanded new seedling leaves. Inoculated plants were kept in a dark dew chamber at 10 ± 2 °C and 100 % RH for 24 h, before being moved to a growth chamber with a 16-h light/8-h darkness photoperiod at 15 ± 2 °C. Infection types (ITs) were scored 15–18 days after inoculation based on a 0–4 scale (Bariana and McIntosh 1993), when pustules were fully developed on the susceptible control. Plants with ITs 0–2 were considered to be resistant, whereas those with ITs 3–4 were susceptible. After rust assessment, about 10-cm fresh leaf of each plant was harvested and put into a 2-μl centrifuge tube for DNA extraction.
Twenty-one Pst races were also used to inoculate the resistant parent and differential lines carrying Yr1 and Yr32. These races originating from different countries were maintained by the Institute of Plant Protection, CAAS (Table 2).
SSR analysis
Genomic DNA of parents, F1, F2 plants and F2:3 lines were extracted from young leaves using the CTAB protocol (http://www.diversityarrays.com). The DNA was precipitated by adding isopropanol, followed by washing the pellet with 70 % ice-cold ethanol, and re-suspension in 200 µl of Tris HCl EDTA (pH 8.0). Resistant (Br) and susceptible (Bs) bulks were made of equal amounts of DNA from 15 resistant (IT = 0;) and 15 susceptible (IT = 4) F2 plants, respectively.
A total of 1680 SSR markers covering all 21 wheat chromosomes, comprising 553 BARC (Beltsville Agricultural Research Station primers, Song et al. 2000), 127 Clermont Ferrand D (CFD-genome primers, Sourdille et al. 2004), 35 Clermont Ferrand A (CFA-genome primers, Sourdille et al. 2004), 152 Gatersleben Wheat Microsatellite (GWM primers, Röder et al. 1998) and 813 Wheat Microsatellite Consortium (WMC primers, Gupta et al. 2002) markers, were screened on the two parents and the resistant and susceptible bulks. Markers showing polymorphisms between the resistant and susceptible parents and the respective bulks were used to genotype the entire F2 population for linkage analysis. The Yr1-specific marker stm673acag was used to test the parents to discriminate Yr1 and YrJ22 (Bansal et al. 2009).
PCRs were performed in volumes of 15 μl containing 1.5 μl of 10× PCR buffer (10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2), 300 μM of each dNTP, 8 pmol of each primer, 60 ng of genomic DNA and 1.0 U Taq DNA polymerase. Amplifications were carried out at 94 °C for 5 min, followed by 38 cycles of 94 °C for 1 min, 50–62 °C (depending on primers) for 1 min and 72 °C for 1 min, and a final extension at 72 °C for 10 min. Three microliter of loading buffer was added to PCR products and then denatured at 95 °C for 8 min. PCR products were separated on 6 % denaturing polyacrylamide gels as previously described (Li et al. 2006) and visualized by the silver staining.
Single nucleotide polymorphism (SNP) genotyping
The resistant and susceptible DNA bulks were genotyped with the Illumina Infinium Wheat 90K iSelect BeadChip containing 81,587 SNPs using the Illumina BeadStation and iScan instruments, according to the manufacture’s protocols by CapitalBio Corporation (http://cn.capitalbio.com/). SNP allele clustering and genotype calling were performed using Genome Studio version 2011.1 software (Illumina). The default clustering algorithm was initially used to classify each SNP call into three allelic clusters. Manual data curation was then performed for more accurate genotyping. The wheat consensus SNP map was used to determine the chromosome location of each SNP (Cavanagh et al. 2013; Wang et al. 2014). SNPs showing polymorphism between the resistant and susceptible bulks on chromosome 2AL were used to genotype the 377 F2 plants for linkage analysis by KASP technique (Semagn et al. 2014). A 5-μl reaction volume for the KASP assay included 2.5 μl of 2× reaction mix, 0.056 μl of assay mix (LGC Genomics, Beverly, MA, USA) and 33 ng of genomic DNA. PCR was carried out using a Bio-Rad S1000 Thermal Cycler, and fluorescent endpoint readings were carried out with Biotek Synergy H1 Multi-Mode Reader (Biotek Instruments, Inc., USA) following the manufacturer’s manual (http://www.lgcgroup.com/LGCGroup/media/PDFs/Products/Genotyping/KASP-quick-start-guide.pdf?ext=.pdf).
Chromosome assignment and genetic mapping
Chromosome locations of linked SSR markers were initially based on the consensus map of common wheat (Somers et al. 2004). The map locations were further confirmed using CS nulli-tetrasomic, ditelosomic and deletion lines. Polymorphic markers were mapped to chromosome bins based on the smallest deletion bin possessing them.
Markers polymorphic between the parental lines and the resistant and susceptible DNA bulks were genotyped on the mapping population to develop a linkage map using Joinmap version 4.0 (Stam 1993). The genetic map was constructed with the software Mapdraw version 2.1 (Liu and Meng 2003).
Results
Phenotypic and genetic analyses of stripe rust resistance in Jimai 22
The parents, 15 F1, 377 F2 plants and 127 F2:3 lines from the cross between Avocet S and Jimai 22 were challenged with Pst race CYR32. Jimai 22 was highly resistant (IT 0), whereas Avocet S was highly susceptible (IT 3, 4) (Table 1). The 15 F1 plants were highly resistant (IT 0) indicating dominance of resistance. The F2 plants segregated 285 resistant: 92 susceptible, conforming with a 3:1 ratio (χ 2 = 0.07, P 1df > 0.05). The F2:3 lines segregated 32 homozygous resistant, 57 segregating and 28 homozygous susceptible, fitting a 1:2:1 segregation ratio (χ 2 = 0.35, P 2df > 0.05). These results suggested that stripe rust resistance in Jimai 22 was conferred by a single dominant gene, temporarily designated YrJ22.
Linkage analysis
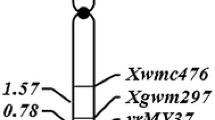
The parents and respective bulks (Br and Bs) were screened by 1680 SSR markers distributed throughout the whole genome. SSR markers Xwmc658, Xgwm382, Xgwm311, Xcfd50 and Xgdm93 showed clear polymorphisms between the resistant and susceptible DNA bulks as well as the parents. Subsequent linkage analysis, based on the phenotypic and genotypic data of 377 F2 plants with the five polymorphic markers, indicated that YrJ22 was linked to these SSR loci. The five linked SSR markers are located on chromosome 2A (Somers et al. 2004; Zhang et al. 2008; http://wheat.pw.usda.gov/cgi-bin/graingenes). The closest linked SSR locus Xwmc658 was located on chromosome 2AL (Somers et al. 2004). Xgwm382 and Xgwm311 were located on chromosome bin 2AL1-0.85-1.00 (Sourdille et al. 2004). Xgdm93 was also located on chromosome 2AL (Zhang et al. 2008). To confirm the chromosomal position of YrJ22, three SSR markers Xgwm311, Xgwm382 and Xwmc658 were amplified in CS and nulli-tetrasomic lines N2A-T2D, N2B-T2A, N2B-T2D, N2D-T2A and N2D-T2B; no PCR products were generated in lines N2A-T2D and deletion line 2AL1-0.85, whereas Xcfd50 was amplified in CS and deletion lines 2AL1-0.85 and 2AS5-0.78. The results further confirmed that Xgwm311, Xgwm382, Xwmc658 were located on bin 2AL1-0.85-1.00 and Xcfd50 was located on bin C-2AL1-0.85. Therefore, the resistance gene YrJ22 was confirmed to be located in the distal part of chromosome 2AL.
KASP assay for mapping of YrJ22
Bulked segregant analysis of susceptible and resistant bulks using the Wheat 90K SNP chip indicated that 323 of 81,587 SNP markers were polymorphic. Among them, 18.9 % were located on chromosome 2A based on the consensus SNP linkage map of hexaploid wheat (Cavanagh et al. 2013; Wang et al. 2014). Two SNPs chosen from chromosome 2AL, IWA1348 and IWB56077 were converted to KASP assays and used to genotype the 377 F2 plants.
Subsequent linkage analysis, based on the phenotypic and genotypic data with the five SSR and two SNP markers, indicated that YrJ22 was linked to these loci (Fig. 1). The two closest loci flanking the resistance gene were IWA1348 (distal) and Xwmc658 (proximal), with genetic distances of 7.3 and 1.0 cM from YrJ22, respectively.
Reactions of wheat lines with YrJ22, Yr1 and Yr32 in multi-race tests
Seedling tests with 21 Pst races (Table 2) showed that Chinese 166 (Yr1) was susceptible (IT 3–4) to eight races from China, Germany, Ecuador and the Netherlands, namely CYR26, CYR29, CYR32, Su-1, 60105, 74187, 78070 and 80551; Carstens V with Yr32 was susceptible to nine races, whereas YrJ22 was resistant (IT 0;–1) to all races except 76093 from Pakistan and Chinese race CYR29. The results indicated that YrJ22 is different in specificity from Yr1 and Yr32.
Discussion
Jimai 22 was developed from cross 935024/935106 by Crop Research Institute, Shandong Academy of Agricultural Sciences, Jinan. Genetic analysis showed that stripe rust resistance to Pst race CYR32 in Jimai 22 was conferred by a single dominant gene (YrJ22) and molecular markers mapped the gene on chromosome 2AL. Pedigree analyses indicated that the stripe rust resistance gene in Jimai 22 was derived from 935024 (Linyuan 7069/Lumai 14). Lumai 14 was a leading cultivar planted in eastern Shandong during the 1990s and allegedly had stripe rust resistance gene Yr9 from Lovrin 13 (Zhuang 2003). Jimai 22 is not 1BL/1RS genotype, since no PCR product was generated when amplified with marker H20 or GluB3j. As YrJ22 is located in chromosome 2A, it cannot be Yr9. Linyuan 7069, highly resistant to the prevalent Chinese Pst races, was selected from the cross of TJB259/87/(Lumaidasui//Xibeifengshou/ST2422-464) at Shanxi Academy of Agricultural Science (Cui et al. 1993; Wu et al. 1997). Lumaidasui was derived from Funo which is susceptible to race CYR32 (Wan et al. 2004); and Xibeifengshou is very susceptible to stripe rust and powdery mildew (Yin et al. 2009). ST2422-464 was also susceptible to race CYR32 (Li et al. 2006). Therefore, the stripe rust resistance in Jimai 22 likely comes from TJB259/87, which was introduced from England and allegedly had durable resistance to stripe rust and powdery mildew (Wang et al. 1992).
SSR markers with relatively low marker densities, compared to sequenced genomes, limit the mapping resolution of resistance genes. In the current study, SSR markers and iSelect 90K SNP chip were both used for BSA and the results proved that both kinds of markers mapped the gene on the same chromosome. Compared with the labor intensity of screening SSR markers, SNP chip is a time-saving choice to determine the chromosome location of resistance genes. Analysis of resistant and susceptible bulks with the 90K SNP array identified clear differences for a group of SNP markers on chromosome 2A, implying that the stripe rust resistance gene was located on 2A. To construct the linkage map, the KASP assay made this approach even more robust. The results also showed that the genotypic data obtained from the KASP assays matched the data based on the Infinium assay, confirming the location of the resistance gene. The combined use of SNP chips and SSR markers reduces the time and effort required for gene mapping. Firstly, resistance genes can be located on chromosomes by analysis of resistant and susceptible bulks using SNP chips. Then the SSR and SNP markers on targeted chromosomes can be used to genotype the entire segregating population for linkage map construction. A 660K wheat SNP chip is now available (Prof. Jizeng Jia, http://wheat.pw.usda.gov/ggpages/topics/Wheat660_SNP_array_developed_by_CAAS.pdf), which also provides a large number of SNP markers for fine mapping of resistance genes and marker-assisted breeding.
In the present study, tests on an Avocet S/Jimai 22 population revealed that YrJ22 was located on chromosome bin 2AL1-0.85-1.00, flanked by Xwmc658 and IWA1348. In the Avocet S/Jimai 22 F2 population, the resistance allele (Jimai 22 allele) at Xwmc658 locus was amplified in all 270 highly resistant F2 plants (IT = 0 or 0;), with 98 homozygous and 172 heterozygous genotypes, respectively, while the susceptible allele (Avocet S allele) was detected in 38 out of 39 highly susceptible plants (IT = 4). At IWA1348 locus, the resistance allele was identified in 267 of 270 highly resistant F2 plants (IT = 0 or 0;), while 39 highly susceptible F2 plants amplified 38 homozygous susceptible genotype (Avocet S genotype) and 1 heterozygous genotype. These suggest that the flanking markers Xwmc658 and IWA1348 could effectively distinguish resistant and susceptible alleles in the population and can be used for the selection of YrJ22 in breeding programs. In addition to YrJ22, four resistance genes have been reported on chromosome 2A; Yr17 and Yr56 located on chromosome 2AS, and Yr1 and Yr32 located on chromosome 2AL (Bansal et al. 2009; Bariana and McIntosh 1993; Eriksen et al. 2004; McIntosh et al. 2014). Based on the pedigree of Jimai 22, none of the parents have Yr1. Bansal et al. (2009) reported that stm673acag closely linked with Yr1 (1.1 cM), and validation of this marker suggested that stm673acag could be used for marker-assisted selection of Yr1. We tested the parents of Avocet S/Jimai 22 population with stm673acag, and the Yr1-specific PCR fragment (120 bp) was not amplified in Jimai 22. In a test of the world and European differential lines and some other cultivars, CYR32 was virulent on Chinese 166 (Yr1) (Wan et al. 2004). Similarly, the multi-pathotype tests using 21 Pst races in the present study indicated that YrJ22 is different from Yr1 and Yr32 (Table 2). The resistance spectrum of Jimai 22 was broader than of Chinese 166 and Carstens V. Eriksen et al. (2004) reported that Yr1 and Yr32 had a genetic distance of 33–35 cM. Based on the wheat SSR consensus map (Somers et al. 2004), the genetic distance between Yr32 and YrJ22 is over 35 cM. All these indicate that YrJ22, Yr1 and Yr32 are different genes.
In conclusion, YrJ22 is likely to be a new stripe rust resistance gene. The current information on chromosomal location of YrJ22 and linked markers should be useful for developing stripe rust-resistant cultivars, preferably having combinations of two or more effective resistance genes.
References
Bai B, Du JY, Lu QL, He CY, Zhang LJ, Zhou G, Xia XC, He ZH, Wang CS (2014) Effective resistance to wheat stripe rust in a region with high disease pressure. Plant Dis 98:891–897
Bansal UK, Hayden MJ, Keller B, Wellings CR, Park RF, Bariana HS (2009) Relationship between wheat rust resistance genes Yr1 and Sr48 and a microsatellite marker. Plant Pathol 58:1039–1043
Bariana HS, McIntosh RA (1993) Cytogenetic studies in wheat. XV. Location of rust resistance genes in VPM1 and their genetic linkage with other disease resistance genes in chromosome 2A. Genome 36:476–482
Cavanagh C, Chao S, Wang S, Huang BE, Stephen S, Kiani S, Forrest K, Saintenac C, Brown-Guedira GL, Akhunova A, See D, Bai G, Pumphrey M, Tomar L, Wong D, Kong S, Reynolds M, Da Silva ML, Bockelman H, Talbert L, Anderson JA, Dreisigacker S, Baenziger S, Carter A, Korzun V, Morrell PL, Dubcovsky J, Morell MK, Sorrells ME, Hayden MJ, Akhunov E (2013) Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc Natl Acad Sci USA 110:8057–8062
Chen XM (2005) Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Can J Plant Pathol 27:314–337
Chen XM (2013) High-temperature adult-plant resistance, key for sustainable control of stripe rust. Am J Plant Sci 4:608–627
Chen XM (2014) Integration of cultivar resistance and fungicide application for control of wheat stripe rust. Can J Plant Pathol 36:311–326
Cui SL, Chen XM, Li XH, Meng FH (1993) The test and analysis of some parental winter wheat. Crops 3:12–13 (in Chinese)
Eriksen L, Afshari F, Christiansen MJ, McIntosh RA, Jahoor A, Wellings CR (2004) Yr32 for resistance to stripe (yellow) rust present in the wheat cultivar Carstens V. Theor Appl Genet 108:567–575
Feng JY, Wang MN, Chen XM, See DR, Zheng YL, Chao SM, Wan AM (2015) Molecular mapping of YrSP and its relationship with other genes for stripe rust resistance in wheat chromosome 2BL. Phytopathology 105:1206–1213
Gupta PK, Balyan HS, Edwards KJ, Isaac P, Korzun V, Röder M, Gautier MF, Joudrier P, Schlatter AR, Dubcovsky J, De la Pena RC, Khairallah M, Penner G, Hayden MJ, Sharp P, Keller B, Wang RCC, Hardouin J, Jack P, Leroy P (2002) Genetic mapping of 66 new microsatellite (SSR) loci in bread wheat. Theor Appl Genet 105:413–422
He ZH, Xia XC, Chen XM, Zhuang QS (2011) Progress and perspective in research of adult-plant resistance to stripe rust and powdery mildew in wheat. Acta Agron Sin 37:202–215 (in Chinese with English abstract)
Herrera-Foessel SA, Singh RP, Lan CX, Huerta-Espino J, Calvo-Salazar V, Bansal UK, Bariana HS, Lagudah ES (2015) Yr60, a gene conferring moderate resistance to stripe rust in wheat. Plant Dis 99:508–511
Li ZF, Zheng TC, He ZH, Li GQ, Xu SC, Li XP, Yang GY, Singh RP, Xia XC (2006) Molecular tagging of stripe rust resistance gene YrZH84 in Chinese wheat line Zhou 8425B. Theor Appl Genet 112:1098–1103
Li HS, Liu JJ, Song JM, Liu AF, Cheng DG, Zhao ZD (2007) Wheat cultivar Jimai 22 with high yield, stable productivity, good disease resistance and wide adaptability. J Triticeae Crops 27:744 (in Chinese with English abstract)
Line RF (2002) Stripe rust of wheat and barley in North America: a retrospective historical review. Annu Rev Phytopathol 40:75–118
Liu RH, Meng JL (2003) MapDraw: a Microsoft Excel macro for drawing genetic linkage maps based on given genetic linkage data. Hereditas 25:317–321
McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers WJ, Morris C, Appels R, Xia XC (2013) Catalogue of gene symbols for wheat. http://shigen.nig.ac.jp/wheat/komugi/genes/symbolClassList.jsp
McIntosh RA, Dubcovsky J, Rogers WJ, Morris C, Appels R, Xia XC, Azul B (2014) Catalogue of gene symbols for wheat: 2013–2014 supplement. http://shigen.nig.ac.jp/wheat/komugi/genes/symbolClassList.jsp
National Agro-technical Extension and Service Center (NAESC) (2015) Epidemic characteristics of wheat stripe rust in China in 2015 and its control strategies. China Plant Prot 41:1–4
Randhawa MS, Bariana HS, Mago R, Bansal UK (2015) Mapping of a new stripe rust resistance locus Yr57 on chromosome 3BS of wheat. Mol Breed 35:65
Ren Y, He Z, Li J, Lillemo M, Wu L, Bai B, Lu Q, Zhu H, Zhou G, Du J, Lu Q, Xia X (2012) QTL mapping of adult-plant resistance to stripe rust in a population derived from common wheat cultivars Naxos and Shanghai3/Catbird. Theor Appl Genet 125:1211–1221
Ren Y, Li SR, Xia XC, Zhou Q, He YJ, Wei YM, Zheng YL, He ZH (2015) Molecular mapping of a recessive stripe rust resistance gene yrMY37 in Chinese wheat cultivar Mianmai 37. Mol Breed 35:97
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Semagn K, Babu R, Hearne S, Olsen M (2014) Single nucleotide polymorphism genotyping using kompetitive allele specific PCR (KASP): overview of the technology and its application in crop improvement. Mol Breed 33:1–14
Somers DJ, Isaac P, Edwards K (2004) A high density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Song QJ, Fickus EW, Cregan PB (2000) Characterization of trinucleotide SSR motifs in wheat. Theor Appl Genet 104:286–293
Sourdille P, Singh S, Cadalen T, Brown-Guedira GL, Gay G, Qi L, Gill BS, Dufour P, Murigneux A, Bernard M (2004) Microsatellite-based deletion bin system for the establishment of genetic-physical map relationships in wheat (Triticum aestivum L.). Funct Integr Genomics 4:12–25
Stam P (1993) Construction of integrated genetic linkage maps by means of a new computer package: JOINMAP. Plant J 5:739–744
Stubbs RW (1988) Pathogenicity analysis of yellow (stripe) rust of wheat and its significance in a global context. Breeding strategies for resistance to the rusts of wheat. CIMMYT, Mexico
Wan A, Zhao Z, Chen X, He Z, Jin S, Jia Q, Yao G, Yang J, Wang B, Li G, Bi Y, Yuan Z (2004) Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002. Plant Dis 88:896–904
Wang JX, Zhang HQ, Guo XC (1992) A tentative study on application of the resistance to stripe rust and powdery mildew from English wheat variety. Acad J Henan Agric Univ 26:174–178 (in Chinese with English abstract)
Wang S, Wong D, Forrest K, Allen A, Chao S, Huang BE, Maccaferri M, Salvi S, Milner SG, Cattivelli L, Mastrangelo AM, Stephen S, Barker G, Wieseke R, Plieske J, International Wheat Genome Sequencing Consortium, Lillemo M, Mather D, Appels R, Dulferos R, Brown-Guedira G, Korol A, Akhunova AR, Feuillet C, Salse J, Morgante M, Pozniak C, Luo MC, Dvorak J, Morell M, Dubcovsky J, Ganal M, Tuberosa R, Lawley C, Mikoulitch I, Cavanagh C, Edwards KJ, Hayden M, Akhunov E (2014) Characterization of polyploid wheat genomic diversity using the high-density 90,000 SNP array. Plant Biotechnol J 12:787–796
Wellings CR (2011) Global status of stripe rust: a review of historical and current threats. Euphytica 179:129–141
Wu JP, Xu GH, Qiu SY, Meng ZP, Xue JZ, Lu LH (1997) Application and heritability analysis of wheat resistant source Linyuan7069. J Triticeae Crops 5:18–20 (in Chinese)
Yin GH, Li GY, He ZH, Liu JJ, Wang H, Xia XC (2009) Molecular mapping of powdery mildew resistance gene in wheat cultivar Jimai 22. Acta Agron Sin 35:1425–1431 (in Chinese with English abstract)
Zhang W, Chao S, Manthey F, Chicaiza O, Brevis JC, Echenique V, Dubcovsky J (2008) QTL analysis of pasta quality using a composite microsatellite and SNP map of durum wheat. Theor Appl Genet 117:1361–1377
Zhuang QS (2003) Wheat improvement and pedigree analysis in Chinese wheat cultivars. China Agriculture Press, Beijing (in Chinese)
Acknowledgments
The authors are grateful to Prof. R.A. McIntosh, Plant Breeding Institute, University of Sydney, for critical review of this manuscript. This study was supported by the National Key Basic Research Program of China (2013CB127700), National Natural Science Foundation of China (31261140370), National 863 Project (2012AA101105) and the China Agriculture Research System (CARS-3-1-3).
Authors’ contributions
CC performed the experiment and wrote the paper. JL, JL and YR participated in stripe rust test in the greenhouse. ZH, CM and XX designed the experiment and wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in regard to this manuscript.
Ethical standards
We declare that these experiments comply with the ethical standards in China, where they were performed.
Rights and permissions
About this article
Cite this article
Chen, C., He, Z., Lu, J. et al. Molecular mapping of stripe rust resistance gene YrJ22 in Chinese wheat cultivar Jimai 22. Mol Breeding 36, 118 (2016). https://doi.org/10.1007/s11032-016-0540-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-016-0540-5