Abstract
The papaya ring spot virus (PRSV) causes significant fruit yield loss in cucurbit crops. Understanding of the inheritance and molecular mapping of PRSV resistance will facilitate development of resistant varieties to control this disease. In the present study, an F2 population was developed from the cross between susceptible ‘65G’ and resistant ‘02245’ cucumber inbred lines. Genetic analysis of PRSV resistance in 144 F2:3-derived F3 families showed that resistance is controlled by a single recessive gene which was designated as prsv 02245. Simple sequence repeat (SSR) markers were employed in polymorphism screening between PRSV-susceptible and resistant DNA pools. The PRSV resistance gene, prsv 02245, was mapped on chromosome 6 that was flanked by two SSR markers, SSR11-177 and SSR11-1, which was 1.1 and 2.9 cM away from the prsv 02245 locus, respectively. The physical distance between the two markers was approximately 600 kb. The accuracy rate of marker-assisted selection of PRSV resistance among 35 cucumber lines using the marker, SSR11-177 was more than 80 %. Results from this study provide a valuable tool for fine mapping, gene cloning, and marker-assisted breeding for PRSV resistance in cucumber.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papaya ring spot virus (PRSV) belongs to the genus Potyvirus in the family Potyviridae. This viral pathogen, which is mainly transmitted by aphids, is found in many crop plants throughout the tropics and subtropical regions of the world (Bateson et al. 2002). Two major biotypes of PRSV have been identified based on differences between host plants, the papaya infecting type (PRSV-P) which affects both papaya and cucurbits, and the cucurbit infecting type (PRSV-W) which affects cucurbits but not papaya (Bateson et al. 1994; Attasart et al. 2002). These two biotypes are serologically indistinguishable (Tomlinson 1987). PRSV-P was isolated first from papaya in Hawaii (Jensen 1949) and was a major limiting factor for papaya production (Purciful et al. 1984). The virus can be transmitted experimentally to cucurbits, but not usually under field condition (Gonsalves 1998). PRSV-W does not infect papaya (Tomlinson 1987), but can readily infect cucurbit crops and cause significant damage. Cucurbit plantings are particularly vulnerable from the end of summer through early autumn, when increased aphid vector populations promote virus epidemics that eventually induce severe losses in fruit yield (Kosaka et al. 2006). No chemical treatment has yet been found to effectively control the virus in infected plants, and thus genetic resistance against the virus is the optimal method for PRSV-W control.
PRSV-W is an important disease in cucumber (Cucumis sativus L.) production in China. PRSV-W resistance has been identified in several cucumber lines such as ‘Surinam,’ a cultivar from South America (Wang et al. 1984), ‘TMG-1,’ an inbred line derived from a single plant selection from the Taiwanese cultivar ‘Taichung Mou Gua’ (Provvidenti 1985), and ‘Dina-1,’ an inbred line derived from self-pollinations of Dutch hybrid ‘Dina’ (Kabelka and Grumet 1997). Wang et al. (1984) reported the inheritance of PRSV-W resistance. AFLP (amplified fragment length polymorphism) markers for PRSV resistance was also reported (Park et al. 2000), but its chromosomal location is unknown. The specific objectives of this study were to better depict the genetic architecture of the PRSV-W resistance, and to identify molecular markers for this resistance that would be useful for marker-assisted selection (MAS) breeding program aimed at introgressing this resistance into commercial cucumber cultivars.
Materials and method
Experimental materials
The two parental plant materials used in the present study included the PRSV-susceptible inbred line ‘65G’ and the resistance inbred line ‘02245’. These were provided by the Cucumber Research Group of the Institute of Vegetable and Flowers Chinese Academy of Agricultural Science (IVF CAAS) and were sequenced by Qi et al. (2013). For study of the inheritance of PRSV resistance and linkage mapping, F1, F2, and F2-derived F3 populations from the two parental lines were used. For screening of PRSV resistance, 144 F2:3 families were used, and at least 15 plants of each family were used for responses to PSRV inoculation.
To validate molecular markers linked to the PRSV resistance locus, 35 cucumber inbred lines or hybrids developed by the Cucumber Research Group of the Institute of Vegetables and Flowers of the Chinese of Academy of Agricultural Sciences were examined for PRSV inoculation test and marker analysis (Table 1).
Virus cultures and inoculation
For the inheritance studies, the PRSV inoculum sources were maintained on squash plants. In order to achieve uniform germination, seeds were treated with 1 % hydrogen peroxide for 2 h, placed on moist filter paper in culture dishes and incubated at 28 °C for 1–2 days. At least 15 plants each of the P1, P2, F1 and 144 F2:3 families were inoculated. Phenotypic data collection for plant responses to PRSV inoculation followed guidelines set by the Ministry of Agriculture of the People’s Republic of China (2010).
In brief, viral inoculum was prepared by blending systemically infected leaves (1:10 w/v) in 0.03 M phosphate buffer (pH = 7.0) and filtering through a double layer of cheesecloth. Seedlings at the first true leaf stage were dusted with carborundum and inoculated by gentle rubbing with a pestle dipped in the viral inoculum. A second inoculation was performed 3 days later to ensure uniform infection. After inoculation, plants were rinsed with water and grown in an artificial climate chamber at 25–28 °C with supplemental lights.
Evaluation of PRSV resistance
Scoring of responses was conducted on each test plant approximately 2 weeks after inoculation according to a six-step disease rating scale ranging from 0 to 9, where 0 = no symptoms; 1 = apical leaves with dispersed vein clearing or slight mottle; 3 = pronounced mosaic in the apical leaves; 5 = mild mosaic and mild leaf distortion in the three youngest leaves; 7 = mosaic and leaf distortion in the three or four youngest leaves; and 9 = severe mosaic and leaf distortion on all leaves or even death. Disease index (DI) was calculated for all test materials following Zhang et al. (2010), where DI = Σ [(s × n)/(S × N)] × 100 %, with s = disease rating scale, n = number of plants with each disease rating, N = total number of plants, and S = highest disease rating scale. The PRSV resistance for each F3 family was evaluated based on its DI value: Families with DI ≤5 % were considered to be high resistant, those with 5 % < DI ≤ 20 % were considered resistant, those with 20 % < DI ≤ 40 % were considered moderate resistance, those with 40 % < DI ≤ 64 % were considered intermediate susceptible, and those with DI > 64 % were considered high susceptible (Ministry of Agriculture of the People’s Republic of China 2010).
Serological procedures
The presence of virus in plants was confirmed using a double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA). DAS-ELISA was carried out with PRSV-specific antiserum purchased from Agdia (Elkhart, Indiana, USA). Absorbance values (405 nm) were monitored using an iMark Microplate Absorbance reader. Well in which color developed indicated positive results, those in which there was no significant color development indicated negative results. Test results were valid only if positive control wells gave a positive result and buffer-only wells remained colorless after incubating for 60 min. The ELISA threshold signal was calculated as the mean of the absorbance ± standard deviations. Samples were considered positive if the measured absorbance was more than twice that of healthy plants.
Molecular marker analysis
The modified CTAB method (Wang et al. 2006) was used for genomic DNA extraction from P1, P2, F1, and each plant of F2 population. The concentration and quality of extracted DNA in samples were detected by electrophoresis on a 1 % (w/v) agarose gel and diluted with distilled water to get a working dilution of 15 ng/μL.
Two DNA bulks, the resistant bulk and the susceptible bulk were constructed by pool equal amount of DNA from seven resistant and seven susceptible F2 plants based on the DI mean of respective F3 families. Microsatellite (SSR) markers selected from the cucumber genetic map by Ren et al. (2009) were used to screen two parental lines and bulks; polymorphic markers were applied to the F2 population for linkage analysis.
The PCR system (10 μL) included 3 μL of DNA (15 ng/μL), 1 μL of both forward and reverse primers (50 ng/μL), and 5 μL of Go Taq Green Master Mix (Promega, USA), and PCR amplification was carried out using the following program: denaturation at 94 °C for 4 min; 35 cycles of denaturation at 94 °C for 15 s, annealing at 55 °C for 15 s, extension at 72 °C for 30 s; and followed by 72 °C for 5 min. Amplified products were separated on 6.0 % non-denaturing polyacrylamide gel at 150 V for 1 h, and the bands visualized and photographed after silver staining.
Linkage analysis
The genotypic data from 144 F2 plants were used to perform linkage analyses using JoinMap 4.0. The Calculate command was first used to calculate the relevant parameters, followed by grouping of linkage group with the Groupings (tree) command with LOD ≥3.0. Finally, the map was drawn with Create Groups for the Mapping and Map commands, and the genetic and physical distance of resistance gene regions were calculated.
Results
Inheritance analysis of PRSV resistance in cucumber
Approximately 2 weeks after inoculation, all the inoculated plants of ‘65G’ were susceptible to PRSV and showed severe mosaic and leaf distortion, whereas all plants of ‘02245’ showed no symptoms. Plants of the F1 were susceptible, showing mosaic and leaf distortion (Fig. 1a). Consistent with this observation, ELISA values obtained for plants of ‘02245’ were significantly lower than those for both ‘65G’ and the F1 plants (Fig. 1b). Chi-square test of segregation ratios among F2 plants with DI means of F3 families indicated that progeny of the F2 generation exhibited a ratio of homozygous dominant (susceptible), heterozygous dominant (susceptible), homozygous recessive (resistant) of approximately 1:2:1 (Table 2), confirming that PRSV resistance in ‘02245’ is conferred by a single recessive gene, which was designated as prsv 02245 hereinafter.
Development of a framework map for PRSV resistance gene prsv 02245
Among 1,288 SSR markers tested, 296 (23.0 %) were polymorphic between ‘65G’ and ‘02245’, of which, 10 were polymorphic between the resistant and susceptible bulks. A linkage map was developed with the 10 SSR markers and 144 F2 plants, which is shown in Fig. 2a, b. This linkage group covered a genetic distance of 44.4 cM, with an average of 4.4 cM/marker. Based on the marker information (Zhang et al. 2012), the prsv 02245 locus was mapped in cucumber chromosome 6 flanked with SSR18405 and SSR33284 that were 7.1 and 10.6 cM away from the target gene, respectively.
Further mapping of the prsv 02245 gene
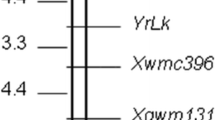
In the genomics region delimited by SSR18405 and SSR33283, 184 new SSR makers were designed based on the 9,930 draft genome sequence (Huang et al. 2009). Six of these showed distinct polymorphisms between the parents. Thus, 16 pairs (Supplemental Table 1) were selected to analyze the F2 mapping population based on the polymorphisms produced. Linkage analysis identified two closer markers, SSR11-177 and SSR11-1 flanking the prsv 02245 locus with genetic distances of 1.1 and 2.9 cM, respectively (Fig. 2c). The physical distance between the two markers was approximately 600 kb.
Validation of molecular markers linked to the prsv 02245 gene for MAS breeding
The SSR marker SSR11-177 that was the closest to prsv 02245 locus was tested among 35 cucumber inbred lines and hybrids, in which 24 were resistant and 11 susceptible to PRSV. Consistent results between phenotypes and genotypes were obtained for 29 of the 35 (Table 1; Fig. 3), suggesting an accuracy rate for SSR11-177 of 82.9 %.
Discussion
There have been contrasting results in previous studies with regard to the inheritance of PRSV resistance in cucumber. Based on the cross between resistant Surinam Local and susceptible Wisconsin 2757 Wang et al. (1984) found that PRSV resistance was controlled by a single recessive gene, prsv-1. Wai and Grumet (1995) suggested that a single dominant or incompletely dominant gene (Prsv-2) determined resistance to PRSV-W in the cucumber line TMG-1. However, Wai et al. (1997) showed that Prsv-2 from TMG-1 and prsv-1 from Surinam Local were at the same locus. Zhang et al. (2005) reported that PRSV resistance was controlled by a major gene and some minor genes by studying a recombinant inbred line (RIL) population derived from No. 8 European (a susceptible line) and Qiupeng (a resistant line). In the present study, based on the PRSV inoculation tests of 144 F2:3 families, we found that PRSV resistance in the cucumber inbred line ‘02245’ is conferred by a single recessive gene, prsv 02245.
Sequential inoculation experiments, using F3 families derived from TMG-1, showed that the gene for PRSV resistance was either at the same locus, or was very tightly linked to, the gene for resistance to Zucchini yellow mosaic virus (ZYMV) (Kabelka 1996). Wai et al. (1997) detected linkages between resistance to ZYMV and Watermelon mosaic virus (WMV), between resistance to PRSV-W (prsv-2), ZYMV (zym), and bitterfree cotyledon (bi). The ZYMV resistance gene, zym, has been recently fine mapped, and a candidate gene was proposed (Amano et al. 2013). Based on the SSR markers linked with prsv 02245 resistance gene in ‘02245’ from the present study (Fig. 2), it seems the prsv 02245 locus in ‘02245’ is close to the zym locus but not the same. This finding confirms that PRSV resistance is linked closely to ZYMV, but that the two loci are at different locations on this chromosome.
In recent years, several recessive plant virus resistance genes have been characterized to be mutated genes of eukaryotic translation initiation (Maule et al. 2007). Meyer et al. (2008) constructed a fosmid library of cucumber (TMG-1) and comparatively analysis the eIF4E and eIF(iso)4E regions from cucumber and melon, whereas the results indicated that eIF4E and eIF(iso)4E were not linked to the cluster of recessive potyvirus resistance loci in TMG-1. Besides, we predicated genes by Softberry (http://linux1.softberry.com/) within our mapping region, no genes were found encoding translation initiation factors typical of recessive resistance genes against plant viruses. Therefore, the translation initiation factors of 4E family may be one of the factors to mediate plant virus resistance. In cucumber, there also exist some other factors associated with virus replication in plants to confer resistance. The pathogenesis-related proteins (PRs) that may be effective in inhibiting pathogen growth, multiplication and spread, and be responsible for the state of systemic acquired resistance have been reported (Van Loon and Van Kammen 1970; Ryals et al. 1996). In our mapping region, we found a gene, pathogenesis-related transcriptional factor/ERF DNA-binding (Csa6P133770.1), which can regulate the ethylene synthesis. Ethylene can induce the translation of pathogenesis-related proteins (PRs). Since there are no reports related to PRSV resistant genes in cucumber, we can only speculate that some genes capable of regulating and producing induced resistant factors may control PRSV resistance in cucumber. To ascertain whether PR transcriptional factor/ERF DNA-binding is relative to PRSV resistance, we will make fine mapping and identify the candidate gene in the further research.
Conclusion
We found that the PRSV resistance in cucumber inbred line ‘02245’ is controlled by a single recessive gene, prsv 02245. The prsv 02245 locus was mapped to chromosome 6 with genetic distances of 1.1 and 2.9 cM from two closely linked SSR markers. Among 35 cucumber lines tested, the closest marker, SSR11-177 to the prsv 02245 locus was associated with PRSV resistance in >80 % lines. These results should be valuable for cucumber breeders in marker-assisted selection of PRSV resistance, which also provide the basis for fine mapping of the PRSV resistant gene in cucumber.
References
Amano M, Mochizuki A, Kawagoe Y, Iwahori K, Niwa K, Svoboda J, Maeda T, Imura Y (2013) High-resolution mapping of zym, a recessive gene for Zucchini yellow mosaic virus resistance in cucumber. Theor Appl Genet 126:2983–2993
Attasart P, Charoensilp G, Kertbundit S, Panyim S, Juricek M (2002) Nucleotide sequence of a Thai isolate of Papaya ringspot virus type W. Acta Virol 46:241–246
Bateson MF, Henderson J, Chaleeprom W, Gibbs AJ, Dale JL (1994) Papaya ringspot potyvirus: isolate variability and the origin of PRSV type P (Australia). J Gen Virol 75:3547–3553
Bateson MF, Lines RE, Revill P, Chaleeprom W, Ha CV, Gibbs AJ, Dale JL (2002) On the evolution and molecular epidemiology of the potyvirus Papaya ringspot virus. J Gen Virol 83:2575–2585
Gonsalves D (1998) Control of papaya ringspot virus in papaya: a case study. Annu Rev Phytopathol 36:415–437
Huang SW, Li RQ, Zhang ZH, Li L, Gu XF, Fan W, Lucas WJ, Wang XW, Xie BY, Ni PX, Ren YY, Zhu HM, Li J, Lin K, Jin WW, Fei ZJ, Li GC, Staub J, Kilian A, van der Vossen EAG, Wu Y, Guo J, He J, Jia ZJ, Ren Y, Tian G, Lu Y, Ruan J, Qian WB, Wang MW, Huang QF, Li B, Xuan ZL, Cao J, San A, Wu ZG, Zhang JB, Cai QL, Bai YQ, Zhao BW, Han YH, Li Y, Li XF, Wang SH, Shi QX, Liu SQ, Cho WK, Kim JY, Xu Y, Heller-Uszynska K, Miao H, Cheng ZC, Zhang SP, Wu J, Yang YH, Kang HX, Li M, Liang HQ, Ren XL, Shi ZB, Wen M, Jian M, Yang HL, Zhang GJ, Yang ZT, Chen R, Liu SF, Li JW, Ma LJ, Liu H, Zhou Y, Zhao J, Fang XD, Li GQ, Fang L, Li YR, Liu DY, Zheng HK, Zhang Y, Qin N, Li Z, Yang GH, Yang S, Bolund L, Kristiansen K, Zheng HC, Li SC, Zhang XQ, Yang HM, Wang J, Sun RF, Zhang BX, Jiang SZ, Wang J, Du YC, Li SG (2009) The genome of the cucumber, Cucumis sativus L. Nat Genet 41:1275–1281
Jensen DD (1949) Papaya virus diseases with special reference to papaya ringspot. Phytopathology 39:191–211
Kabelka E (1996) Allelic characterization and inheritance of potyvirus resistance genes in cucumber. Dissertation, East Lansing, Michigan: Michigan State University
Kabelka E, Grumet R (1997) Inheritance of resistance to the Moroccan watermelon mosaic virus in the cucumber line TMG-1 and cosegregation with zucchini yellow mosaic virus resistance. Euphytica 95:237–242
Kosaka Y, Song RB, Kobori T, Shiomi H, Yasuhara H, Kataoka M (2006) Effectiveness of an attenuated zucchini yellow mosaic virus isolate for cross-protecting cucumber. Plant Dis 90:67–72
Maule AJ, Caranta C, Boulton MI (2007) Sources of natural resistance to plant viruses: status and prospects. Mol Plant Pathol 8:223–231
Meyer JDF, Deleu W, Garcia-Mas J, Havey MJ (2008) Construction of a fosmid library of cucumber (Cucumis sativus) and comparative analyses of the eIF4E and eIF(iso)4E regions from cucumber and melon (Cucumis melo). Mol Genet Genomics 279:473–480
Ministry of Agriculture of the People’s Republic of China (2010) Rules for evaluation of cucumber for resistance to diseases. Part 7: rule for evaluation of cucumber for resistance to cucumber mosaic virus (NY/T 1857.7—2010 Beijing). (In Chinese)
Park YH, Sensoy S, Wye C, Antonise R, Peleman J, Havey MJ (2000) A genetic map of cucumber composed of RAPDs, RFLPs, AFLPs, and loci conditioning resistance to papaya ringspot and zucchini yellow mosaic viruses. Genome 43:1003–1010
Provvidenti R (1985) Source of resistance to virus in two accessions of Cucumis sativus. Cucurbit Genet Coop Rep 8:12
Purciful DE, Edwardson JR, Hiebert E, Consalves D (1984) Papaya ringspot virus. CMI/AAB Descr Plant Viruses 292:8
Qi JJ, Liu X, Shen D, Miao H, Xie BY, Li XX, Zeng P, Wang SH, Shang Y, Gu XF, Du YC, Li Y, Lin T, Yuan JH, Yang XY, Chen JF, Chen HM, Xiong XY, Huang K, Fei ZJ, Mao LY, Tian L, Städler T, Renner SS, Kamoun S, Lucas W, Zhang ZH, Huang SW (2013) A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nat Genet 45:1510–1515
Ren Y, Zhang ZH, Liu JH, Staub JE, Han YH, Cheng ZH, Li XF, Miao H, Kang HX, Xie BY, Gu XF, Wang XW, Du YC, Jin WW, Huang SW (2009) An integrated genetic and cytogenetic map of the cucumber genome. PLoS ONE 4:e5795
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8:1809–1819
Tomlinson JA (1987) Epidemiology and control of virus diseases of vegetables. Ann Appl Biol 110:661–681
Van Loon LC, Van Kammen A (1970) Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. ‘Samsun’ and ‘Samsun NN’ II. Changes in protein constitution after infection with tobacco mosaic virus. Virology 40:199–211
Wai T, Grumet R (1995) Inheritance of resistance to the watermelon strain of Papaya ringspot virus in the cucumber line TMG-1. HortScience 30:338–340
Wai T, Staub JE, Kabelka E, Grumet R (1997) Linkage analysis of potyvirus resistance alleles in cucumber. J Hered 88:454–458
Wang YJ, Provvidenti R, Robinson RW (1984) Inheritance of resistance in cucumber to Watermelon mosaic virus 1. HortScience 19:587–588
Wang HJ, Wu Y, Gu W, Sun XD, Qin ZW (2006) Extraction of DNA from cucumber by improved CTAB method. Heilongjiang Agric Sci 5:124–125 (in Chinese)
Zhang HY, Miao AJ, Zhang F, Wang YJ, Xu Y (2005) Genetic analysis of four major cucumber diseases. Acta Agric Boreali-Sin 20:100–103 (in Chinese)
Zhang SP, Miao H, Gu XF, Yang YH, Xie BY, Wang XW, Huang SW, Du YC, Sun RF (2010) Genetic mapping of the scab resistance gene in cucumber. J Am Soc Hort Sci 135:53–58
Zhang WW, Pan JS, He HL, Zhang C, Li Z, Zhao JL, Yuan G, Yuan XJ, Zhu LH, Huang SW, Cai R (2012) Construction of a high density integrated genetic map for cucumber (Cucumis sativus L.). Theor Appl Genet 124:249–259
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (863 Program, No. 2012AA100103); the earmarked fund for Modern Agro-industry Technology Research System (CARS-25) and the Key Laboratory of Biology and Genetic Improvement of Horticultural Crops, Ministry of Agriculture, China. The authors would like to thank Dr. Graham Collins, formerly of the University of Adelide, South Australia for proofreading.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guili Tian and Yuhong Yang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, G., Yang, Y., Zhang, S. et al. Genetic analysis and gene mapping of papaya ring spot virus resistance in cucumber. Mol Breeding 35, 110 (2015). https://doi.org/10.1007/s11032-015-0279-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-015-0279-4