Abstract
Melanaphis sacchari causes serious damage to sorghum (Sorghum bicolor (L.) Moench) growth, development and productivity in many countries. A dominant gene (RMES1) conferring resistance to M. sacchari has been found in the grain sorghum variety Henong 16 (HN16), but fine mapping of the RMES1 locus remains to be reported. In this study, genetic populations segregating for RMES1 were prepared with HN16 and BTx623 as parental lines. The latter had been used for sorghum genome sequencing but was found to be susceptible to M. sacchari in this work. A total of 11 molecular markers were mapped to the short arm of chromosome 6 harboring RMES1. The closest markers flanking the RMES1 locus were Sb6m2650 and Sb6rj2776, which delimited a chromosomal region of about 126 kb containing five predicted genes. The utility of the newly identified DNA markers for tagging RMES1 in molecular breeding of M. sacchari resistance, and further efforts in cloning RMES1, are discussed.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Among the major cereal crops cultivated worldwide, sorghum (Sorghum bicolor (L.) Moench) is unique in being used efficiently for food, fuel, feed and fibre production (Paterson et al. 2009). These multiple usages, together with its highly efficient C4 photosynthesis system, strong tolerance to abiotic stresses and high yield potential, make sorghum an increasingly important crop for many countries in dealing with shortages of natural resources and climate changes (Palmer 1992; Jackson et al. 2008; Paterson et al. 2009). Consequently, more and more genetics and genomics studies are being conducted on sorghum, with an aim of further improving the productivity of this crop through molecular breeding (Carpita and McCann 2008; Li et al. 2009; Mace et al. 2009; Paterson et al. 2009; Dugas et al. 2011; Vermerris 2011; Zheng et al. 2011; Bouchet et al. 2012; Zou et al. 2012).
One of the most potent threats to further raising sorghum yield potential is insect damage, as more than 150 insect species have been found to negatively affect worldwide sorghum production (Young and Teetes 1977; Sharma 1993). The major insect pests of sorghum include three aphid species, Melanaphis sacchari, Schizaphis graminum and Rhopalosiphum maidis. While M. sacchari causes serious damage to sorghum and sugarcane (van den Berg 2002; Singh et al. 2004), Schizaphis graminum is destructive of both wheat and sorghum production (Teetes 1980; Eddleman et al. 1999; Blackman and Eastop 2000; Kindler et al. 2002; Punnuri et al. 2012). In order to control the damage from M. sacchari, a number of investigations have been conducted to identify sorghum genes conferring resistance to this pest (Lu and Dahlberg 2001; Singh et al. 2004; Chang et al. 2006, 2012). A dominant resistance gene against M. sacchari, designated as RMES1 (Resistance to Melanaphis sacchari) hereafter, was found in the Chinese grain sorghum variety Henong 16 (HN16) (Chang et al. 2006). Using the segregating populations derived from HN16 and another grain sorghum variety Qianshan that was susceptible to M. sacchari infestation, RMES1 was genetically mapped to chromosome 6 (Chang et al. 2006, 2012). However, the genetic distance values of the mapped microsatellite and amplified fragment length polymorphism markers to RMES1 were generally quite large (≥6 cM), and their efficiencies in selecting aphid resistant progenies in segregating sorghum populations were generally less than 90 % (Chang et al. 2012).
For more effective use of RMES1 in molecular breeding, it is necessary to identify additional markers with closer genetic distances to RMES1, and to finally isolate the gene through map-based cloning. Thus, the major objective of this study was to fine-map RMES1 by developing new DNA markers. Considering that the genome in the sorghum line BTx623 has been sequenced (Paterson et al. 2009), we tested whether this line could be employed for efficiently mapping RMES1. Towards this end, the response of BTx623 to M. sacchari infestation was investigated, and the inheritance character of RMES1 in the BTx623 × HN16 cross and derivative populations was investigated. New DNA markers were developed, which permitted the mapping of RMES1 to a region of about 126 kb on chromosome 6. Finally, the efficiencies of two mapped markers (Sb6m2650 and Sb6rj2776) for selecting RMES1 and aphid resistance in BC2F2 progenies were investigated.
Materials and methods
Plant materials and M. sacchari culture
HN16 possessing RMES1 has been described previously (Chang et al. 2006, 2012). BTx623 is the line used for international sorghum genome sequencing (Paterson et al. 2009). By crossing BTx623 with HN16 and selfing F1 and F2 progenies, 312 F3 families were obtained in October, 2010. The F3 families, together with 571 F4 seedlings derived from five F3 plants (F3-92, 131, 147, 183, 211) heterozygous for RMES1, provided the main materials for mapping RMES1. M. sacchari was cultured on the seedlings of the susceptible variety Qianshan in a growth chamber at 30 °C with a 16-h light/8-h dark photoperiod.
Inoculation of M. sacchari and phenotyping
For investigating the response of BTx623 to M. sacchari, the seeds of BTx623 and HN16 were germinated and grown in vermiculite in a greenhouse with the temperature set at 30 °C with a 16-h light/8-h dark photoperiod. At 7 days after sowing, 30 uniform seedlings (at two-leaf stage) were selected from each variety. Each seedling was then inoculated with 10 apterous adult aphids. The number of aphids on each inoculated seedling was recorded daily for 7 days.
To verify the dominance of RMES1, 30 uniform seedlings were raised for each of the two parental lines (BTx623 and HN16) and the F1, followed by mass inoculation with 300–400 nymphs and apterous adults. These aphids came from 3 to 5 detached leaves of Qianshan seedlings that had been co-cultivated with M. sacchari. The responses of the three genotypes to aphid infestation were recorded at 10 days post-inoculation (DPI). For evaluating the responses of individual F3 families to M. sacchari, 30–35 uniform seedlings were raised for each family, and were inoculated with 300–400 nymphs and apterous adults as described above. The responses to aphid infestation were recorded at 10 DPI. Using the same method, the 571 F4 seedlings were also tested for their responses to M. sacchari. Prior to the test, leaf samples were collected from each of the 571 F4 seedlings for subsequent DNA extraction and mapping experiments.
Extraction of genomic DNA samples and PCR conditions
Genomic DNA samples were extracted from the relevant sorghum materials as described previously (Saghai-Maroof et al. 1984). PCR was conducted as described by Chang et al. (2012). The main cycling conditions included a pre-denaturation step at 94 °C for 5 min, 30 cycles of denaturation (94 °C for 45 s), primer annealing, and extension (72 °C for 1 min), and a final extension at 72 °C for 10 min. The temperature for primer annealing was adjusted for individual markers.
Marker development and evaluation
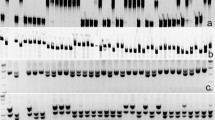
Previous study suggested that the microsatellite marker Xtxp006 was located 8.7 cM away from RMES1 on the short arm of chromosome 6 (Chang et al. 2006). Thus, the chromosomal region containing Xtxp006 (from 1.90 to 4.90 Mb) was downloaded from the sorghum genomic database (www.phytozome.net/sorghum), and was searched for microsatellites using the SSRIT program (www.gramene.org/gramene/searches/ssrtool). The primer pairs flanking each microsatellite motif were designed with the program Primer Premier 5.0 (Premier Biosoft International, CA, USA). Aided by the program RJPrimers (http://probes.pw.usda.gov/RJPrimers/; You et al. 2010), a series of repeat junction (RJ) markers were also developed using the downloaded sequence. All primer pairs were first screened with the two parental lines (BTx623 and HN16). The polymorphic and co-dominant markers were selected for the mapping experiment.
The sorghum genetic markers have been named in different ways by different studies (Mace et al. 2009 and references therein). In this work, we designed an alternative scheme for naming sorghum markers. For example, Li et al. (2009) mapped five microsatellite markers (sam72772, sam71839, sam71307, sam46174 and sam43054) in the chromosomal region (1.90–4.90 Mb) harboring RMES1 on the short arm of chromosome 6. In our scheme, the five markers were renamed as Sb6m1954, Sb6m2600, Sb6m3291, Sb6m4667 and Sb6m4892, respectively. The prefix “Sb6m” stands for Sorghum bicolor chromosome 6 microsatellite, whereas the suffix indicates the approximate physical position of the marker on the specific chromosome. For naming the RJ markers developed in this study, the prefix was accordingly changed to “Sb6rj”.
Genetic mapping
The 64 F3 families homozygously susceptible to M. sacchari, together with the 571 F4 seedlings segregating for the response to M. sacchari, were employed for fine mapping of RMES1. The mapping data were analyzed using MAPMAKER/Exp version 3.0b (Lincoln et al. 1993). The map positions of the markers were visualized using the software Mapchart version 2.1 (Voorrips 2002).
Analysis of selection efficiency for RMES1 by the markers flanking RMES1
The efficiency of selecting RMES1 by the markers flanking, and with the shortest genetic distances to, the resistance gene locus was investigated using BC2F2 progenies. Briefly, a backcrossing program was conducted using BTx623 as recurrent parent and HN16 as pollen donor. Twenty BC2F1 plants were selfed to produce the BC2F2 population. Two random samples of BC2F2 seedlings were genotyped using the left and right flanking markers, respectively. Subsequently, the seedlings with HN16 marker allele (putatively containing RMES1) were subject to inoculation with M. sacchari as described above. The selection efficiency for a given marker was calculated using the following formula: (the number of BC2F2 seedlings resistant to M. sacchari/the number of BC2F2 seedlings with HN16 marker allele) × 100 %.
Statistical analysis
Statistical analysis of the data (mean ± SD) depicted in Fig. 1 was conducted using PASW statistics 18 for Windows (SPSS Inc., Chicago, IL, USA). The Chi squared goodness-of-fit test was conducted as described previously (Chernoff and Lehmann 1954).
Results
Response of BTx623 to M. sacchari infestation
In both BTx623 and HN16, the mean number of nymphs and adults per plant increased for the first 5 days after M. sacchari inoculation, but declined on days 6 and 7 (Fig. 1). However, the scale of the increase was significantly larger in BTx623, and the mean number of aphids per plant was consistently and substantially higher in BTx623 than in HN16 from day 1 to day 7 (Fig. 1). By day 7, the mean number of aphids per plant was approximately six times more than the initial inoculum in BTx623, whereas the number of aphids feeding on HN16 was only twice of the initial inoculum (Fig. 1). Moreover, after 7 days of M. sacchari infestation, BTx623 seedlings, but not those of HN16, became wilted and later died. The resistant response of HN16 seedlings to aphid inoculation observed here agreed well with its strong resistance to M. sacchari at adult stage in the field (Chang et al. 2006, 2012). The experiment depicted in Fig. 1 was repeated five times, obtaining very similar results.
Inheritance of RMES1 in the BTx623 × HN16 cross
Following the experiment above, F1 to F4 populations were developed by crossing BTx623 with HN16. After mass inoculation of M. sacchari, the F1 plants behaved like HN16 in being resistant to aphid infestation (Fig. 2). On the other hand, the inoculated BTx623 seedlings all died after 7 DPI (Fig. 2). Systematic screening of F3 families from self-pollinated F2 plants by mass inoculation of aphids identified 82 families homozygously resistant, and 64 families homozygously susceptible, to M. sacchari, with the remaining 166 families containing both aphid resistant and susceptible progenies (Table 1). The Chi squared test indicated that the responses to M. sacchari of the F3 families fitted the segregation ratio of 1:2:1, suggesting that RMES1 derived from HN16 segregated as a single dominant nuclear gene. Further to the above test, 571 F4 seedlings, germinated from a randomly selected sample of the F4 seeds of five self-pollinated heterozygous F3 plants, were examined for responses to M. sacchari. Among the 571 F4 seedlings, 411 and 160 were found to be resistant and susceptible to M. sacchari, respectively. The Chi squared test showed that the segregation of resistant and susceptible seedlings in this F4 population occurred at a ratio of 3–1, again suggesting that RMES1 behaved as a single dominant nuclear gene.
Genetic mapping of RMES1
As a first step in the mapping experiment, we investigated the polymorphisms of the molecular markers that resided in the target chromosomal region (chromosome 6, 1.90–4.90 Mb) between HN16 and BTx623. Of the five microsatellite markers previously mapped (Li et al. 2009), three were polymorphic and co-dominant (Table 2). Concomitantly, six new polymorphic and co-dominant microsatellite markers were identified by this work (Table 2). Furthermore, two polymorphic and co-dominant RJ markers were discovered (Table 2). After screening the F3 and F4 populations with known phenotypes to M. sacchari with the 11 markers, the genotype and phenotype data were analyzed by the MAPMAKER program. The results showed that the 11 markers covered a genetic distance of 49 cM on the short arm of chromosome 6 (Fig. 3). Sb6m2650 and Sb6rj2776 were the two closest markers flanking RMES1, and their genetic distances to RMES1 were 2 and 1 cM, respectively (Fig. 3).
According to the genomic sequence of chromosome 6 (Paterson et al. 2009; www. phytozome.net/sorghum), the 11 mapped markers covered approximately 2,940 kb, with about 126 kb found between Sb6m2650 and Sb6rj2776 (Fig. 4). Five genes, namely Sb06g001620, Sb06g001630, Sb06g001640, Sb06g001645 and Sb06g001650, had been predicted between Sb6m2650 and Sb6rj2776 by the sorghum genome sequencing project (Fig. 4).
A diagram illustrating the five predicted genes (Sb06g001620, Sb06g001630, Sb06g001640, Sb06g001645 and Sb06g001650) present in the 126-kb genomic segment bordered by Sb6m2650 and Sb6rj2776. The grey box represents the open reading frame (ORF) of the gene. The approximate physical positions of two flanking markers (Sb6m2650 and Sb6rj2776) and the start codons in the ORFs of the five genes on sorghum chromosome 6 are indicated. The products deduced for Sb06g001620 and Sb06g001630 are WD domain-containing and ribosome L18/L5e proteins, respectively. Those deduced for Sb06g001640, Sb06g001645 and Sb06g001650 are leucine-rich repeat proteins
Efficiencies of Sb6m2650 and Sb6rj2776 in tagging RMES1 in BC2F2 progenies
After genotyping 435 BC2F2 seedlings with the co-dominant marker Sb6m2650, 320 were found to contain the HN16 marker allele. Of the 320 seedlings, 317 survived after M. sacchari attack. Thus, the selection efficiency (accuracy) for RMES1 by Sb6m2650 was about 99.1 %. For the co-dominant marker Sb6rj2776, a different sample of BC2F2 seedlings (440 in total) was genotyped, leading to the finding of 318 individuals with the HN16 marker allele. After inoculating the 318 seedlings with M. sacchari, 316 individuals survived. Therefore, the efficiency (accuracy) for selecting RMES1 by Sb6rj2776 was approximately 99.4 %.
Discussion
Previous studies suggest that RMES1 in HN16 is a major dominant gene conferring effective resistance to M. sacchari (Chang et al. 2006, 2012). In this study, we confirmed previous findings using a different genetic cross and derivative populations. More importantly, we succeeded in further mapping RMES1 to a discrete region on chromosome 6. Genetically, the region harboring RMES1 is now filled with 11 molecular markers, with the nearest flanking markers (Sb6rj2776 and Sb6m2650) being 1 and 2 cM, respectively, away from RMES1. Physically, it is now clear that RMES1 resides in a chromosomal segment of about 126 kb containing only five predicted genes. Compared to previous studies (Chang et al. 2006, 2012), this work represents a significant advance on understanding the genetic and physical features of the RMES1 locus.
The major reason behind the efficient mapping of RMES1 by this work is the use of BTx623 as a parent line for developing the genetic populations. The strong susceptibility of BTx623 to M. sacchari facilitated the identification of F3 and F4 progenies lacking RMES1. The draft genome sequence of BTx623 aided the finding of new molecular markers required for fine mapping of RMES1. Both microsatellite and RJ markers were useful for delineating the genetic position of RMES1. The usefulness of microsatellite markers in mapping sorghum genes has been well documented (for example, Chang et al. 2006; Magalhaes et al. 2007; Apotikar et al. 2011; Kawahigashi et al. 2011; Lin et al. 2012). By contrast, the deployment of RJ primers in plant genetic mapping studies has been reported only recently (Paux et al. 2010; You et al. 2010), and this work represents the first demonstration that RJ markers can be used successfully for mapping target genes in sorghum.
In previous mapping studies in sorghum, the genetic markers were named in several different ways (reviewed by Mace et al. 2009), none of which took into account the physical position of the marker on the sorghum chromosome sequence. To facilitate cross-comparisons of future mapping data, we propose a new scheme for naming sorghum genetic markers, which provides information on the approximate physical location of the marker on the specific chromosome. This scheme is likely efficient for designating microsatellite, RJ and indel markers after appropriate modifications.
The efficiencies for selecting M. sacchari-resistant plants by Sb6m2650 and Sb6rj2776 were all above 99 %. These high efficiencies are consistent with their close genetic linkages with RMES1. Therefore, Sb6m2650 and Sb6rj2776 are more efficient than previously reported markers in tagging RMES1 (Chang et al. 2012), which should accelerate the use of this important gene in marker-assisted selection for breeding M. sacchari resistance in sorghum.
Despite the availability of a draft genome sequence, the understanding of the molecular genetic basis underlying sorghum traits is currently lagging behind that of other model plants (e.g., Arabidopsis and rice). Map-based cloning, frequently and efficiently used for investigating important genes in Arabidopsis and rice, has been employed only recently for studying sorghum genes controlling aluminum tolerance (Magalhaes et al. 2007), resistance to the fungal pathogen Bipolaris sorghicola (Kawahigashi et al. 2011), or seed shattering (Lin et al. 2012). Further to the work reported here, we are now in the process of developing more markers both within and among the five predicted genes in the 126-kb region hosting RMES1, which should finally lead to the isolation of RMES1 by map-based cloning.
References
Apotikar DB, Venkateswarlu D, Ghorade RB, Wadaskar RM, Patil JV, Kulwal PL (2011) Mapping of shoot fly tolerance loci in sorghum using SSR markers. J Genet 90:59–66
Blackman RL, Eastop VF (2000) Aphids on the world crop pests: an identification and information guide. Wiley, London, pp 1–8
Bouchet S, Pot D, Deu M, Rami JF, Billot C, Perrier X, Rivallan R, Gardes L, Xia L, Wenzl P, Kilian A, Glaszmann JC (2012) Genetic structure, linkage disequilibrium and signature of selection in sorghum: lessons from physically anchored DArT markers. PLoS ONE 3:1–15
Carpita NC, McCann MC (2008) Maize and sorghum: genetic resources for bioenergy grasses. Trends Plant Sci 13:415–520
Chang JH, Xia XY, Zhang L, Li RG, Liu GQ, Luo YW (2006) Analysis of the resistance gene to the sorghum aphid, Melanaphis sacchari, with SSR marker in Sorghum bicolor. Acta Pratacult Sin 115:113–118 (in Chinese)
Chang JH, Cui JH, Xue W, Zhang QW (2012) Identification of molecular markers for an aphid resistance gene in sorghum and selective efficiency using these markers. J Integr Agric 11:1086–1092
Chernoff H, Lehmann EL (1954) The use of maximum likelihood estimates in χ2 tests for goodness-of-fit. Ann Math Stat 25:579–586
Dugas DV, Monaco MK, Olsen A, Klein RR, Kumari S, Ware D, Klein PE (2011) Functional annotation of the transcriptome of Sorghum bicolor in response to osmotic stress and abscisic acid. BMC Genomics 12:514
Eddleman BR, Chang CC, McCarl BA (1999) Economic benefits from grain sorghum variety improvement in the United States. In: Wiseman BR, Webster JA (eds) Economic, environmental, and social benefits of resistance in field crops. Thomas Say Publications, Maryland, pp 35–37
Jackson DR, Arthur MF, Davis M, Kresovich S, Lawhon WT, Lipinsky ES, Price M, Rudolph A (2008) Research report on development of sweet sorghum as an energy crop. Battelle Press, Columbus, pp 277–290
Kawahigashi H, Kasuga S, Ando T, Kanamori H, Wu J, Yonemaru J, Sazuka T, Matsumoto T (2011) Positional cloning of ds1, the target leaf spot resistance gene against Bipolaris sorghicola in sorghum. Theor Appl Genet 123:131–142
Kindler SD, Elliott NC, Royer TA, Giles KL, Tao F, Fuentes R (2002) Effect of greenbugs on winter wheat yield. J Econ Entomol 95:89–95
Li ML, Yuyama N, Luo L, Hirata M, Cai HW (2009) In silico mapping of 1758 new SSR markers developed from public genomic sequences for sorghum. Mol Breed 24:41–47
Lin ZW, Li XR, Shannon LM, Yeh CT, Wang ML, Bai GH, Peng Z, Li JR, Tric HN, Clemente TE, Doebley J, Schnable PS, Tuinstra MR, Tesso TT, White F, Yu JM (2012) Parallel domestication of the Shattering1 genes in cereals. Nat Genet 44:720–724
Lincoln SE, Daly MJ, Lander ES (1993) Constructing linkage maps with MapMaker/Exp version 3.0. A tutorial reference manual, 3rd edn. Whitehead Institute for Medical Research, Cambridge
Lu QS, Dahlberg JA (2001) Chinese sorghum genetic resources. Econ Bot 55:401–425
Mace ES, Rami JF, Bouchet S, Klein PE, Klein RR, Kilian A, Wenzl P, Xia L, Halloran K, Jordan DR (2009) A consensus genetic map of sorghum that integrates multiple component maps and high-throughput Diversity Array Technology (DArT) markers. BMC Plant Biol 9:13
Magalhaes JV, Liu J, Guimaraes CT, Lana UGP, Alves VM, Wang YH, Schaffert RE, Hoekenga OA, Pineros MA, Shaff JE, Klein PE, Carneiro NP, Coelho CM, Trick HN, Kochian LV (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39:1156–1161
Palmer GH (1992) Sorghum-food, beverage and brewing potentials. Process Biochem 27:145–153
Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, Schmutz J, Spannagl M, Tang H, Wang X, Wicker T, Bharti AK, Chapman J, Feltus FA, Gowik U, Grigoriev IV, Lyons E, Maher CA, Martis M, Narechania A, Otillar RP, Penning BW, Salamov AA, Wang Y, Zhang L, Carpita NC, Freeling M, Gingle AR, Hash CT, Keller B, Klein P, Kresovich S, McCann MC, Ming R, Peterson DG, Mehboob-ur-Rahman WD, Westhoff P, Mayer KFX, Messing J, Rokhsar DS (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457:551–556
Paux E, Faure S, Choulet F, Roger D, Gauthier V, Martinant JP, Sourdille P, Balfourier F, Le Paslier MC, Chauveau A, Cakir M, Gandon B, Feuillet C (2010) Insertion site-based polymorphism markers open new perspectives for genome saturation and marker-assisted selection in wheat. Plant Biotechnol J 8:196–210
Punnuri S, Huang Y, Steets J, Wu Y (2012) Developing new markers and QTL mapping for greenbug resistance in sorghum [Sorghum bicolor (L.) Moench]. Euphytica. doi:10.1007/s10681-012-0755-4
Saghai-Maroof MA, Soliman KM, Jorgesen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley Mendelian inheritance, chromosomal location and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Sharma HC (1993) Host-plant resistance to insects in sorghum and its role in integrated pest management. Crop Prot 12:11–34
Singh BU, Padmaja PG, Seetharama N (2004) Biology and management of the sugarcane aphid, Melanaphis sacchari (Zehntner) (Homoptera: Aphididae), in sorghum: a review. Crop Prot 23:739–755
Teetes GL (1980) Breeding sorghums resistant to insects. In: Maxwell FG, Jennings PR (eds) Breeding plants resistant to insects. Wiley, New York, pp 457–485
van den Berg J (2002) Status of resistance of sorghum hybrids to the aphid, Melanaphis sacchari (Zehntner) (Homoptera: Aphididae). J Plant Soil (South Africa) 19:151–155
Vermerris W (2011) Survey of genomics approaches to improve bioenergy traits in maize, sorghum and sugarcane. J Integr Plant Biol 53:105–119
Voorrips RE (2002) MapChart, software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
You FM, Wanjugi H, Huo NX, Lazo GR, Luo MC, Anderson OD, Dvorak J, Gu YQ (2010) RJPrimers: unique transposable element insertion junction discovery and PCR primer design for marker development. Nucleic Acids Res 38:313–320
Young WR, Teetes GL (1977) Sorghum entomology. Annu Rev Entomol 22:193–218
Zheng LY, Guo XS, He B, Sun LJ, Peng Y, Dong SS, Liu TF, Jiang S, Ramachandran S, Liu CM, Jing HC (2011) Genome-wide patterns of genetic variation in sweet and grain sorghum (Sorghum bicolor). Genome Biol 12:R114
Zou G, Zhai G, Feng Q, Yan S, Wang A, Zhao Q, Shao J, Zhang Z, Zou J, Han B, Tao YZ (2012) Identification of QTLs for eight agronomically important traits using an ultra-high-density map based on SNPs generated from high-throughput sequencing in sorghum under contrasting photoperiods. J Exp Bot. doi:10.1093/jxb/ers205
Acknowledgments
This study was supported by grants from Hebei Province Natural Science Foundation (C2010000758) and the State Key Laboratory of Plant Cell and Chromosome Engineering (PCCE-2008-KF-02). We thank Professor Yinghua Huang (USDA-ARS Plant Science Research Laboratory, USA), Drs Ruiheng Du and Guoqing Liu (Institute of Millet Crops, Hebei Academy of Agricultural and Forestry Sciences, China), Jianghui Cui (Agricultural University of Hebei, China), and Wei Xue (Baoding Vocational and Technical College, China) for constructive suggestions on our work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Faming Wang and Songmin Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, F., Zhao, S., Han, Y. et al. Efficient and fine mapping of RMES1 conferring resistance to sorghum aphid Melanaphis sacchari . Mol Breeding 31, 777–784 (2013). https://doi.org/10.1007/s11032-012-9832-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-012-9832-6